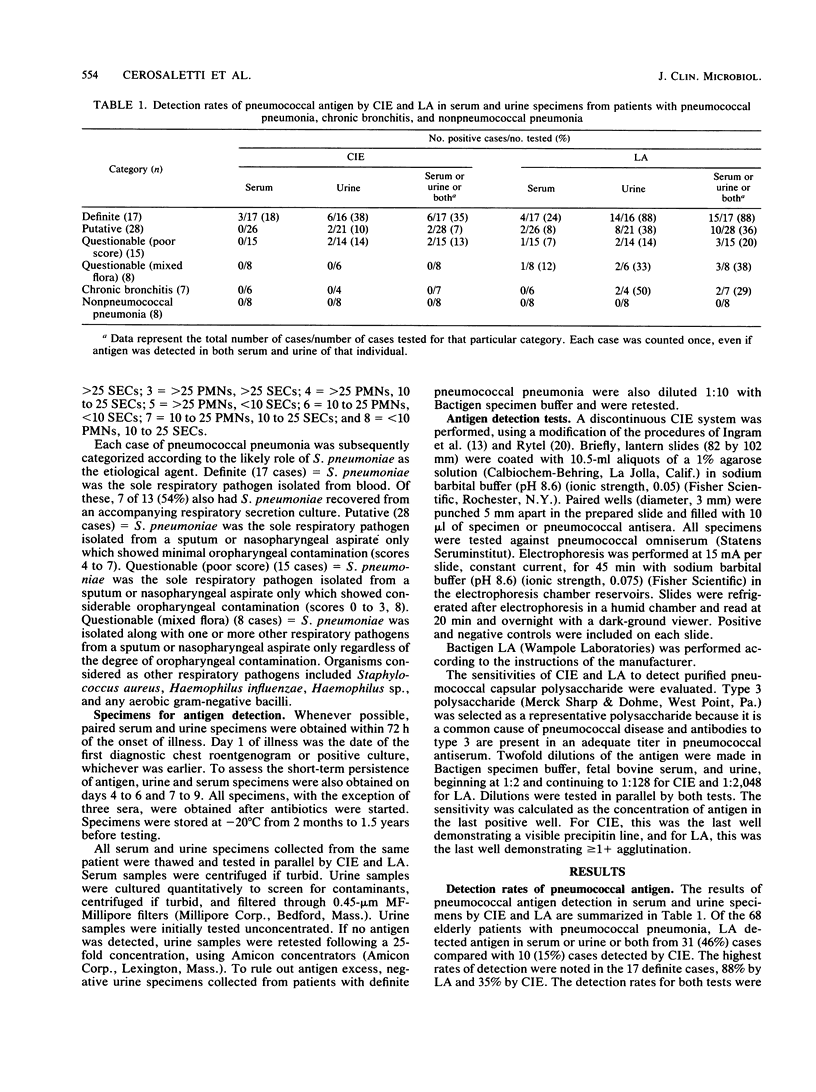
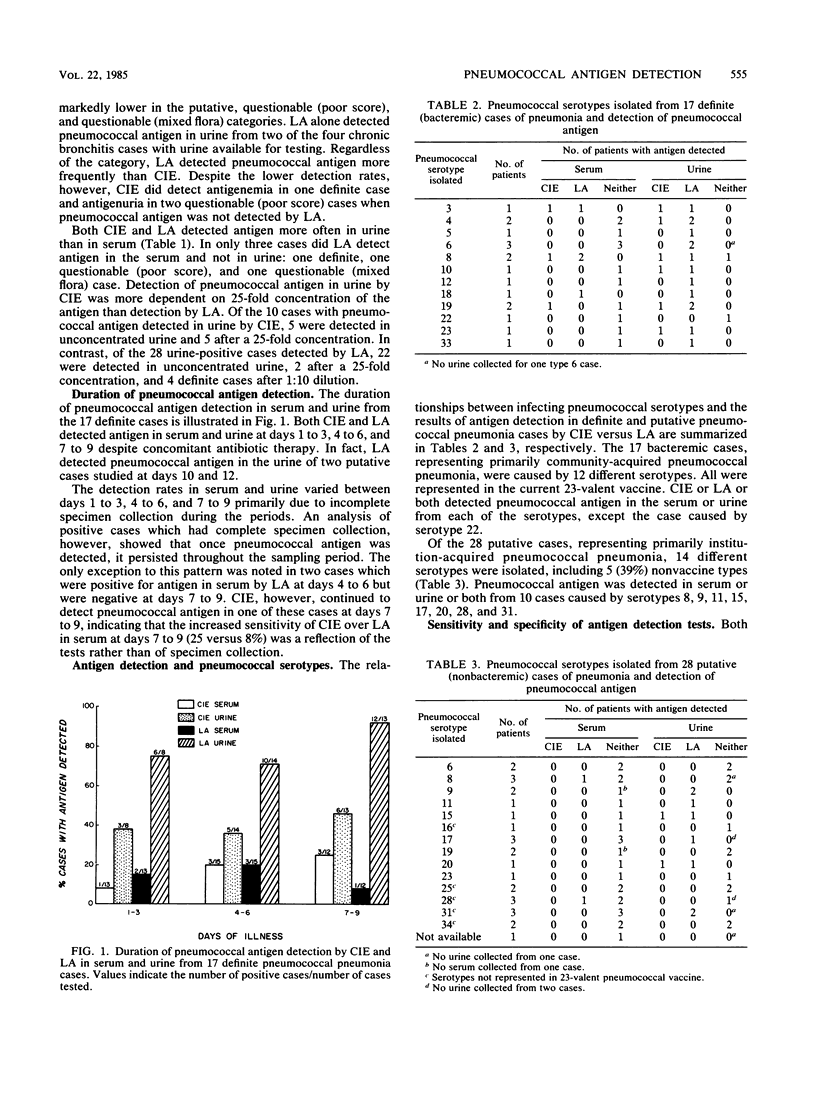
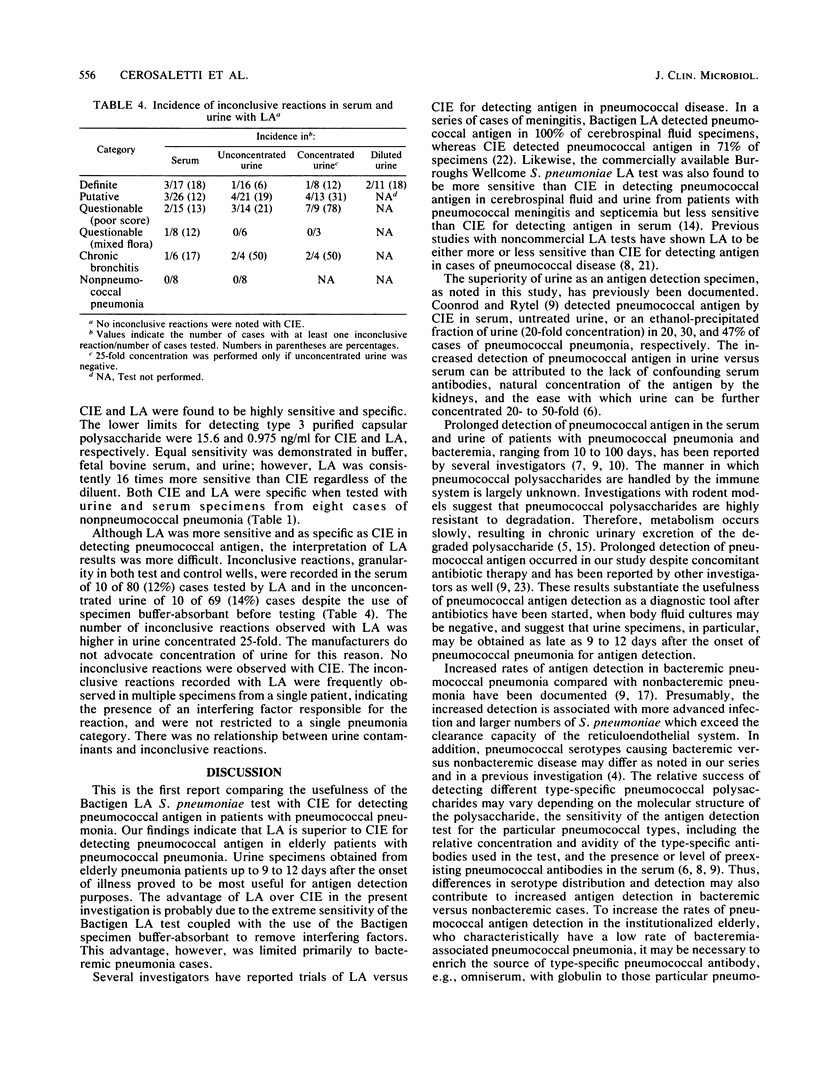
Abstract
A Streptococcus pneumoniae latex agglutination (LA) test (Bactigen; Wampole Laboratories, Div. Carter-Wallace, Inc., Cranbury, N.J.) and counterimmunoelectrophoresis (CIE) were compared for the detection of pneumococcal antigen in serum and urine specimens from 68 elderly patients with pneumococcal pneumonia. The cases were categorized according to the presumptive role of S. pneumoniae: definite, putative, questionable (poor score), or questionable (mixed flora). Serum and urine samples were collected on days 1 to 3, 4 to 6, and 7 to 9 of illness and screened in parallel by LA and CIE. LA detected pneumococcal antigen in the serum or urine or both from 31 (46%) of the 68 pneumococcal pneumonia cases compared with 10 (15%) of cases detected by CIE. The highest rates of detection were noted in the 17 definite (bacteremic) cases: 88% by LA and 38% by CIE. The detection rates for both tests were lower in the other nonbacteremic pneumonia categories. Pneumococcal antigen was detected more often in urine specimens than in serum specimens by LA and CIE and was detected in the urine of 92 and 46% of definite cases, respectively, after 7 to 9 days of illness despite antibiotic therapy. Both tests were specific when tested with nonpneumococcal pneumonia cases, but LA detected pneumococcal antigen in two of seven chronic bronchitis cases. This study suggests that LA is as specific and more sensitive than CIE and is useful for detecting antigen in the elderly with proven bacteremic pneumococcal pneumonia. LA is less sensitive for detecting nonbacteremic pneumococcal pneumonia and, therefore, would be of limited value in the care and study of the institutionalized elderly.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austrian R. Maxwell Finland Lecture. Random gleanings from a life with the pneumococcus. J Infect Dis. 1975 Apr;131(4):474–484. doi: 10.1093/infdis/131.4.474. [DOI] [PubMed] [Google Scholar]
- Austrian R. The quellung reaction, a neglected microbiologic technique. Mt Sinai J Med. 1976 Nov-Dec;43(6):699–709. [PubMed] [Google Scholar]
- Bentley D. W., Ha K., Mamot K., Moon D., Moore L., Poletto P., Springett A. Pneumococcal vaccine in the institutionalized elderly: design of a nonrandomized trial and preliminary results. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S71–S81. doi: 10.1093/clinids/3.supplement_1.s71. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D., Drennan D. P. Pneumococcal pneumonia: capsular polysaccharide antigenemia and antibody responses. Ann Intern Med. 1976 Mar;84(3):254–260. doi: 10.7326/0003-4819-84-3-254. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D. Evidence of breakdown of pneumococcal polysaccharides in vivo. Proc Soc Exp Biol Med. 1979 Nov;162(2):249–253. doi: 10.3181/00379727-162-40659. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D., Rylko-Bauer Latex agglutination in the diagnosis of pneumococcal infection. J Clin Microbiol. 1976 Aug;4(2):168–174. doi: 10.1128/jcm.4.2.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Rytel M. W. Detection of type-specific pneumococcal antigens by counterimmunoelectrophoresis. II. Etiologic diagnosis of pneumococcal pneumonia. J Lab Clin Med. 1973 May;81(5):778–786. [PubMed] [Google Scholar]
- Coonrod J. D. Urine as an antigen reservoir for diagnosis of infectious diseases. Am J Med. 1983 Jul 28;75(1B):85–92. doi: 10.1016/0002-9343(83)90077-3. [DOI] [PubMed] [Google Scholar]
- Edwards E. A., Coonrod J. D. Coagglutination and counterimmunoelectrophoresis for detection of pneumococcal antigens in the sputum of pneumonia patients. J Clin Microbiol. 1980 May;11(5):488–491. doi: 10.1128/jcm.11.5.488-491.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy H. M., Wentworth B., Kenny G. E., Kloeck J. M., Grayston J. T. Pneumococcal isolations from patients with pneumonia and control subjects in a prepaid medical care group. Am Rev Respir Dis. 1975 May;111(5):595–603. doi: 10.1164/arrd.1975.111.5.595. [DOI] [PubMed] [Google Scholar]
- Ingram D. L., Anderson P., Smith D. H. Countercurrent immunoelectrophoresis in the diagnosis of systemic diseases caused by Hemophilus infleunzae type b. J Pediatr. 1972 Dec;81(6):1156–1159. doi: 10.1016/s0022-3476(72)80252-x. [DOI] [PubMed] [Google Scholar]
- Ingram D. L., Pearson A. W., Occhiuti A. R. Detection of bacterial antigens in body fluids with the Wellcogen Haemophilus influenzae b, Streptococcus pneumoniae, and Neisseria meningitidis (ACYW135) latex agglutination tests. J Clin Microbiol. 1983 Nov;18(5):1119–1121. doi: 10.1128/jcm.18.5.1119-1121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Prescott B. Kinetics of the antibody response to type III pneumococcal polysaccharide (SSS-III). I. Use of 125I-labeled SSS-III to study serum antibody levels, as well as the distribution and excretion of antigen after immunization. J Immunol. 1976 Jan;116(1):41–51. [PubMed] [Google Scholar]
- Kaldor J., Asznowicz R., Buist D. G. Latex agglutination in diagnosis of bacterial infections, with special reference to patients with meningitis and septicemia. Am J Clin Pathol. 1977 Aug;68(2):284–289. doi: 10.1093/ajcp/68.2.284. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Wentworth B. B., Beasley R. P., Foy H. M. Correlation of circulating capsular polysaccharide with bacteremia in pneumococcal pneumonia. Infect Immun. 1972 Oct;6(4):431–437. doi: 10.1128/iai.6.4.431-437.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Washington J. A. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975 Jun;50(6):339–344. [PubMed] [Google Scholar]
- Oseasohn R., Skipper B. E., Tempest B. Pneumonia in a Navajo community: a two-year experience. Am Rev Respir Dis. 1978 Jun;117(6):1003–1009. doi: 10.1164/arrd.1978.117.6.1003. [DOI] [PubMed] [Google Scholar]
- Thirumoorthi M. C., Dajani A. S. Comparison of staphylococcal coagglutination, latex agglutination, and counterimmunoelectrophoresis for bacterial antigen detection. J Clin Microbiol. 1979 Jan;9(1):28–32. doi: 10.1128/jcm.9.1.28-32.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. C., Dias F., Ryan R. W. Comparative evaluation of three commercial products and counterimmunoelectrophoresis for the detection of antigens in cerebrospinal fluid. J Clin Microbiol. 1984 Aug;20(2):231–234. doi: 10.1128/jcm.20.2.231-234.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugwell P., Greenwood B. M. Pneumococcal antigen in lobar pneumonia. J Clin Pathol. 1975 Feb;28(2):118–123. doi: 10.1136/jcp.28.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]


