Abstract
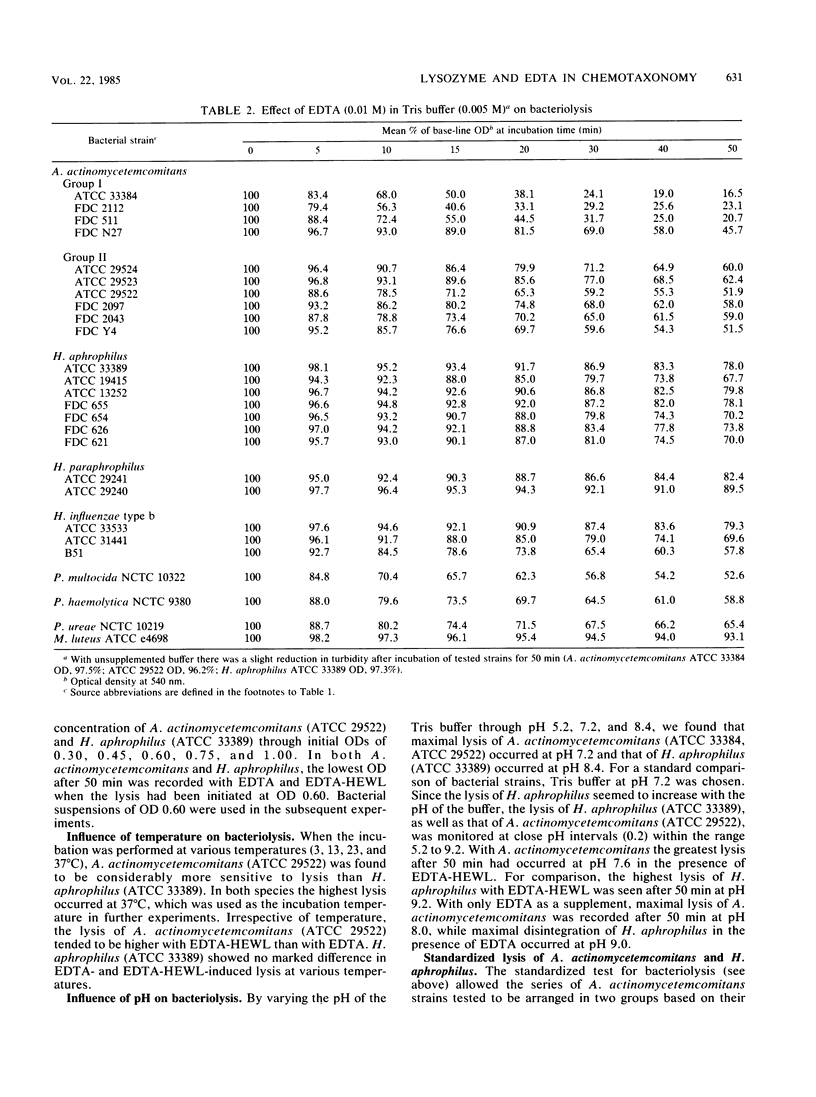
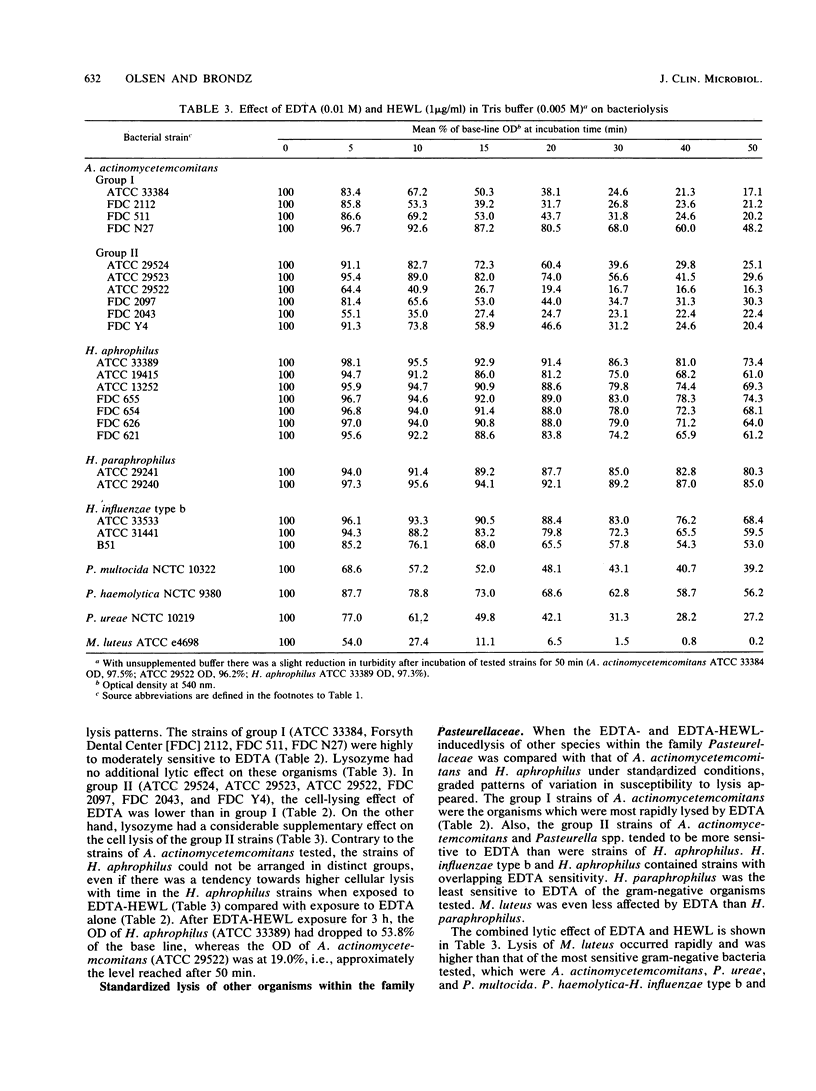
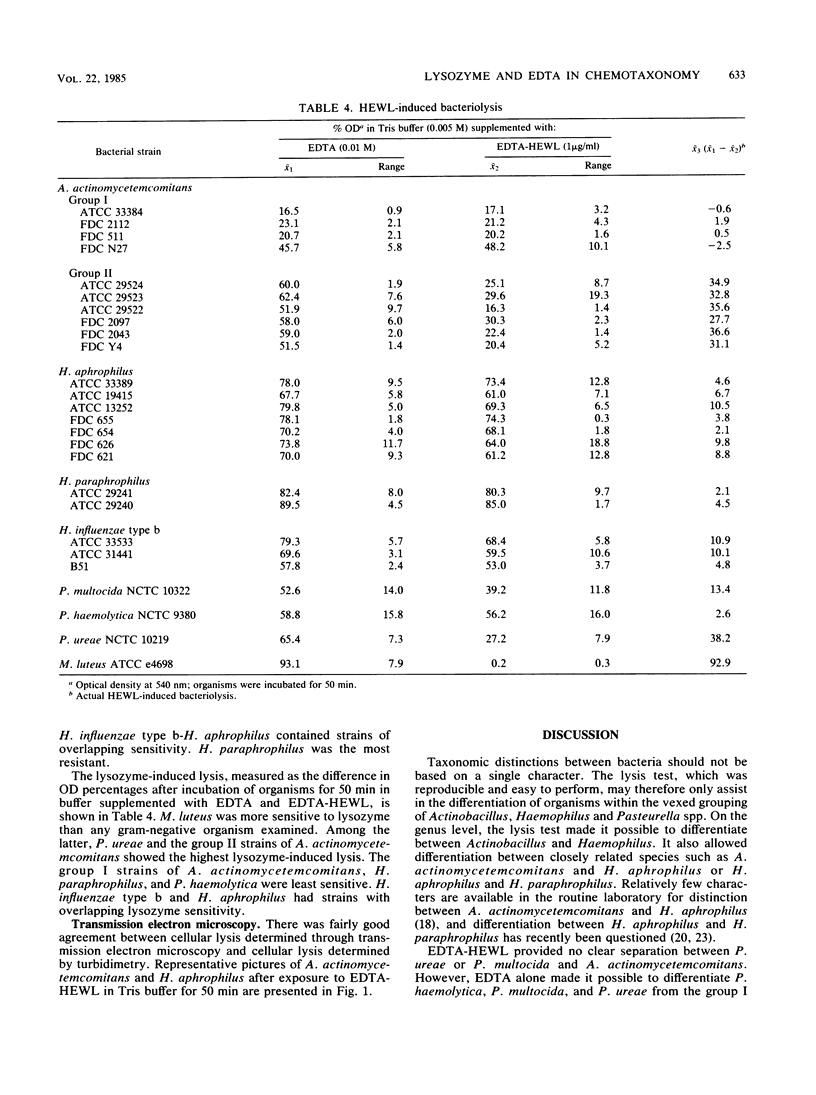
Bacteriolysis in Tris-maleate buffer (0.005 M, pH 7.2) supplemented with EDTA (0.01 M) and hen egg white lysozyme (HEWL, 1.0 microgram/ml) was set up to assist differentiation between the taxonomically closely related Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. A. actinomycetemcomitans was more sensitive to lysis in this system than H. aphrophilus. The standard method for bacteriolysis separated the 10 tested strains of A. actinomycetemcomitans into two groups (I and II) based on their lysis patterns, whereas the 7 strains of H. aphrophilus examined were homogeneous. In group I of A. actinomycetemcomitans, EDTA displayed a considerable lytic effect, which was not increased by supplementation with HEWL. In group II, the lytic effect of EDTA was much less, but HEWL had a considerable supplementary lytic effect. When the turbidity of A. actinomycetemcomitans (ATCC 29522) or H. aphrophilus (ATCC 33389) suspended in Tris buffer was monitored at close pH intervals (0.2) from pH 5.2 to 9.2, maximal lysis of ATCC 29522 occurred with EDTA at pH 8.0 and with EDTA-HEWL at pH 7.6, while ATCC 33389 lysed with EDTA at pH 9.0 and with EDTA-HEWL at pH 9.2. When other members of the family Pasteurellaceae (Haemophilus influenzae type b, Haemophilus paraphrophilus, Pasteurella multocida, Pasteurella haemolytica, and Pasteurella ureae) were included for comparison, the group I strains of A. actinomycetemcomitans were the most rapidly lysed by EDTA. H. paraphrophilus was the least sensitive of the gram-negative strains tested, but not as resistant as Micrococcus luteus (control). M. luteus was the organism most sensitive to lysozyme, followed by P. ureae and the group II strains of A. actinomycetemcomitans, while the group I strains of A. actinomycetemcomitans, H. paraphrophilus, and P. haemolytica were the least sensitive organisms.
Full text
PDF

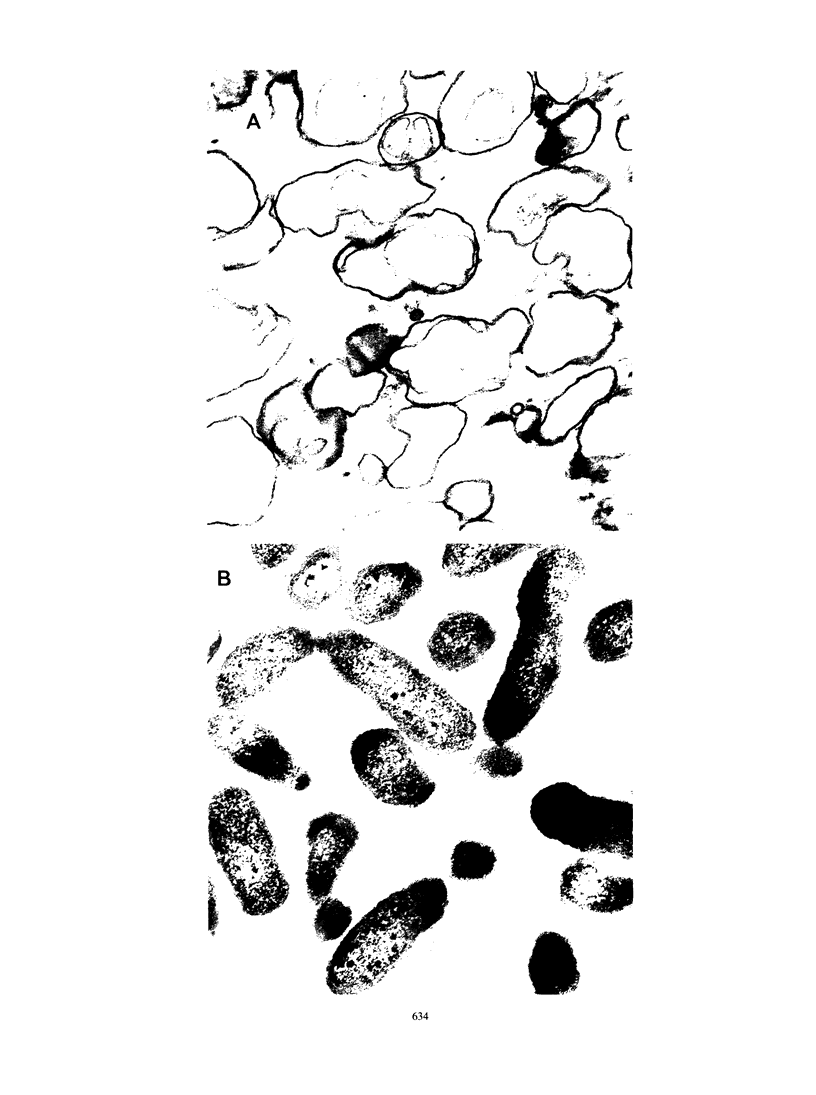


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brondz I., Olsen I. Differentiation between Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus based on carbohydrates in lipopolysaccharide. J Chromatogr. 1984 Oct 12;310(2):261–272. doi: 10.1016/0378-4347(84)80091-2. [DOI] [PubMed] [Google Scholar]
- Brondz I., Olsen I. Differentiation between major species of the Actinobacillus--Haemophilus--Pasteurella group by gas chromatography of trifluoroacetic acid anhydride derivatives from whole-cell methanolysates. J Chromatogr. 1985 Jul 12;342(1):13–23. doi: 10.1016/s0378-4347(00)84485-0. [DOI] [PubMed] [Google Scholar]
- Hardaway K. L., Buller C. S. Effect of ethylenediaminetetraacetate on phospholipids and outer membrane function in Escherichia coli. J Bacteriol. 1979 Jan;137(1):62–68. doi: 10.1128/jb.137.1.62-68.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Tanner A. C., Socransky S. S. Morphology and ultrastructure of oral strains of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. Infect Immun. 1980 Nov;30(2):588–600. doi: 10.1128/iai.30.2.588-600.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., Boldt P. R., MacKay B. J., Cho M. I., Pollock J. J. Lytic sensitivity of Actinobacillus actinomycetemcomitans Y4 to lysozyme. Infect Immun. 1983 May;40(2):773–784. doi: 10.1128/iai.40.2.773-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Costerton J. W. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J Bacteriol. 1981 Mar;145(3):1397–1403. doi: 10.1128/jb.145.3.1397-1403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOLLES P., OWALL H., JAUREGUI-ADELL J., JOLLES J. [New chromatographic method of preparation of lysozyme]. J Chromatogr. 1962 Jul;8:363–368. doi: 10.1016/s0021-9673(01)99271-4. [DOI] [PubMed] [Google Scholar]
- Leive L., Kollin V. Controlling EDTA treatment to produce permeable Escherichia coli with normal metabolic processes. Biochem Biophys Res Commun. 1967 Jul 21;28(2):229–236. doi: 10.1016/0006-291x(67)90434-2. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- NOLLER E. C., HARTSELL S. E. Bacteriolysis of Enterobacteriaceae. I. Lysis by four lytic systems utilizing lysozyme. J Bacteriol. 1961 Mar;81:482–491. doi: 10.1128/jb.81.3.482-491.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. The structure of a disaccharide liberated by lysozyme from the cell walls of Micrococcus lysodeikticus. Biochem J. 1960 Jan;74:182–186. doi: 10.1042/bj0740182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Reynolds H. S., Genco R. J. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun. 1980 Sep;29(3):1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Salient Biochemical Characters of Actinobacillus actinomycetemcomitans. Arch Microbiol. 1982 Feb;131(1):60–67. doi: 10.1007/BF00451500. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A. C., Visconti R. A., Socransky S. S., Holt S. C. Classification and identification of Actinobacillus actinomycetemcomitans and haemophilus aphrophilus by cluster analysis and deoxyribonucleic acid hybridizations. J Periodontal Res. 1982 Nov;17(6):585–596. doi: 10.1111/j.1600-0765.1982.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Voss J. G. Effects of organic cations on the gram-negative cell wall and their bactericidal activity with ethylenediaminetetra-acetate and surface active agents. J Gen Microbiol. 1967 Sep;48(3):391–400. doi: 10.1099/00221287-48-3-391. [DOI] [PubMed] [Google Scholar]
- von Graevenitz A. Fastidious gram-negative, facultatively anaerobic rods: a renewed interest. Eur J Clin Microbiol. 1984 Jun;3(3):223–224. doi: 10.1007/BF02014889. [DOI] [PubMed] [Google Scholar]