Abstract
While we occasionally observe negative BOLD signals, its physiological basis has remained uncertain. This is in part due to the qualitative use of fMRI where the baseline is conveniently differenced away to reveal focal area(s) of interest. Recently, however, there has been a noticeable trend towards quantitative neuroimaging where changes in oxidative energetics (CMRO2) are quantified by calibrated fMRI. Pasley et al (2006) used calibrated fMRI in conjunction with a novel stimulus paradigm to investigate the neural basis of the negative BOLD signal in awake humans. They hypothesized – based on prior results – that if the baseline was lowered then ΔCMRO2 would have to be larger. While their main findings point to an energetic basis for the negative BOLD signal, their results have far reaching implications for the present definition of baseline as well as for future research investigating the neural and/or energetic basis of baseline.
Keywords: glia, glucose, glutamate, metabolism, neuroimaging
It is now generally agreed that the human brain, which comprises only 2% of the body’s mass but consumes approximately 20% of the body’s energy in the form of oxygen consumption, efficiently uses the energy to support its function (Sokoloff, 1991). The new consensus (Shulman and Rothman, 1998) differs from a prior prevailing view (Creutzfeldt, 1975) that a negligible fraction of cerebral energy was used for function. An important consequence of these recent experimental findings is that the resting brain – with no definable mental activities or driven physical actions – is not inactive or static but is churning with cellular signaling requiring, amongst other costly energetic processes, continual coordinated release and cycling of neurotransmitters and restoration of ion gradients.
Superimposed on this assessment of the high resting brain activity are results of functional imaging methods like fMRI and PET that register – usually by inference – changes in cerebral energy consumption, when the baseline state is perturbed by sensory, motor, or cognitive stimuli. Early functional imaging reports focused on stimulus-induced increments, although as discussed below, stimulus-induced decrements have become increasingly apparent and proven to be important. Attempts to relate both positive and negative stimulus-induced changes – measured readily by differencing away the baseline – to absolute values of functional energy consumption have been moved forward in a study by Pasley et al (2006) in this issue.
Central to the assumption about differencing in functional imaging is the following question: When the brain is perturbed by a stimulus, is the energy supporting the functional processes associated with the perturbation represented by just the increment from the baseline or by the total (i.e., baseline + increment) in the new, perturbed state? This question has been focused by calibrated fMRI studies which deconvolute or calibrate the positive BOLD increment (van Zijl et al, 1998; Davis et al, 1998; Hoge et al, 1999; Kim et al, 1999; Kida et al, 1999) into separate contributions from changes in oxidative energy consumption (ΔCMRO2) and/or cerebral blood flow (ΔCBF) – the dominant parameters that modulate the fMRI signal (Hyder, 2004). Hence incremental evaluations of ΔCMRO2 (from calibrated fMRI) and independent measurements of the absolute values of CMRO2 (from 13C MRS) for different conditions are commensurate (Kida et al, 2000) – no longer apples and oranges – and each has been considered as the relevant energy needed to support the particular condition of brain activity (Hyder et al, 2001).
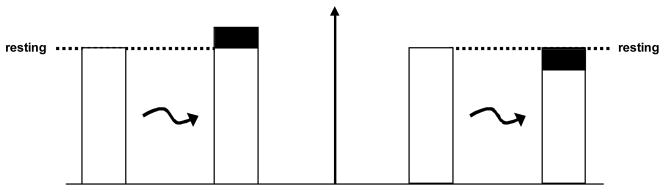
In the view of the last generation’s assessments regarding energetic costs of signaling – where the brain’s energy requirement at rest was considered to be negligible and cerebral activity could be turned on only by the stimulus – the incremental energy could fully support the incremental function (Shuman et al, 1999). On the other hand, if the brain requires a significant amount of energy even in the unstimulated, resting state then introducing a stimulus merely moves the brain into a different state with a characteristic energy – higher or lower – as demanded by the activity of the new, perturbed state (Fig. 1). In this case, however, the total energy needed would be characteristic of that new, perturbed state and the difference in energy would be merely the difference between the stimulated and unstimulated states. For the case of an increment the total energy in the stimulated state would be larger than baseline, whereas for a decrement the total energy would be smaller. Two recent calibrated fMRI studies – one for forelimb stimulation in the anesthetized rat (Smith et al, 2002) and another for visual stimulation in the awake human (Uludag et al, 2004) – have shown that the magnitude of the positive BOLD signal, and the associated ΔCMRO2 depended upon the baseline, being greater when starting from a lower baseline state. While these results showed that the positive BOLD signal represented an increase in total energy, the energy contributions of the negative BOLD signal had only been partially answered previously (Smith et al, 2002). The study by Pasley et al (2006) delves into this issue further.
Figure 1.
Schematic representations of possible increase (left) or decrease (right) in activity upon stimulation (black jagged arrow) from the resting, unperturbed baseline (dotted line). The modular activity, obtained by differencing, is represented by the dark rectangles and is normally used to reveal focally activated (left) or deactivated (right) regions. The remaining activity, which is represented by white rectangles, is ignored by the differencing method, which is the resting, unperturbed baseline. Modified from Shulman et al (1999).
Negative BOLD signals have been observed for more than a decade, but largely ignored. The negative BOLD signals presented no paradox if the resting energy was high because they merely showed that the stimulus decreased the energy in certain brain regions. However lingering ideas that the brain consumed negligible energy at rest raised concerns as to how cerebral energy could decrease if it already did not exist (Raichle, 1998). Adding to these uncertainties, recent studies (Harel et al, 2002; Shmuel et al, 2002; Devor et al, 2005) interpreting the negative BOLD signal suggested that there could be a large hemodynamic component (e.g., vascular steal), so that the energy contributions remained uncertain (Shmuel et al, 2006).
Pasley et al (2006) have answered this question by a creative experimental paradigm (see below) that builds upon the previous findings (Smith et al, 2002) that a stimulated region reaches nearly the same total energy regardless of the baseline (Hyder et al, 2002). Accepting the previous results, they hypothesized that if the baseline were lowered, then the increment, measured as ΔCMRO2 from calibrated fMRI, would have to be larger. The study by Pasley et al (2006) not only resolved questions about energetic contributions to the negative BOLD signal, but also adds to the growing body of evidence that total energy is required for function (Ginsberg et al, 1987; Ueki et al, 1988; 1992; Hyder et al, 1996; 1997; Tsukada et al, 1997; Cholet et al, 1997; Smith et al, 2002; Uludag et al, 2004; Kida et al, 2006) where the increments are less variable and usually larger from a lower baseline (Adrian, 1941; King, 1956; Angel et al, 1973; Winters, 1976; Chapin et al, 1981; Chapin and Lin, 1984; Chapin, 1986; Armstrong-James and Fox, 1987; Simons et al, 1992; Lindauer et al, 1993; Smith et al, 2002; Chen et al, 2005).
First, from the resting baseline (i.e., with eyes open), Pasley et al (2006) presented a peripheral annular stimulus which produced a positive BOLD signal in the visual cortex. Second, to lower the baseline they presented a foveal annular stimulus and measured a negative BOLD signal in the same region. Third, with the foveal stimulus present, they simultaneously presented the peripheral stimulus and found a positive BOLD signal again, although this time much larger than previously. Because they also measured CBF for all these conditions, using calibrated fMRI they calculated that ΔCMRO2 was larger from the lower baseline. The comparative magnitudes of ΔCMRO2/ΔCBF for the two positive BOLD signals revealed that the flow-metabolism coupling was nearly the same from the high and low baselines. Since the low baseline was achieved by a stimulus that caused a negative BOLD signal, Pasley et al (2006) concluded that the oxidative energetic “component accounts for essentially the entire negative response…”
With the assurance that a particular level of CMRO2 characterizes a state and knowing that negative and positive BOLD signals reflect energy changes, we now can ask how the incremental (or decremental) and total energies can help to understand, and perhaps define, brain function. If we focus only on increments (or decrements), there is a tendency to ignore the existence of the total, which requires physiological investigations – as demonstrated by Pasley et al (2006) – to understand it. Morcom and Fletcher (2006) in a recent commentary in NeuroImage have accepted that the “resting state is an active one from the neuronal point of view.” Nevertheless they describe the high baseline activity as physiological, and therefore not relevant for cognition because their “concern is with what impact, if any, the physiological changes have on the study of cognitive processing.” However by defining the high baseline, and presumably any total activity, as physiological they have deemed it not relevant for brain function including cognition. While we agree that the total activity under any circumstance has no special status in its relation to function, we question their premise for a privileged link of neural correlates to cognition by “identifying differences in activity between tasks that differ only in terms of that function”. Morcom and Fletcher (2006) are not alone in this simplistic and overreaching view of cognitive psychology where the physiology of cognition is completely determined by differencing. While many limitations of this paradigmatic view of cognitive psychology have been expressed earlier (Shulman, 1996; Friston, 1998; Fodor, 2000; Shulman, 2001), here we are suggesting the total activity might be explored more fully by physiology, as discussed below.
We must start by reviewing the relationship between energy consumption and neuronal activity. The most direct and quantitative relationship between cerebral energy from glucose oxidation (CMRglc(ox)) and neurotransmitter release by neuronal firing (Vcyc) came from 13C MRS experiments in rats and humans (Shulman et al, 2002). The results, over a wide range of activity in rats, measured a one-to-one relationship (Sibson et al, 1998) between the incremental metabolism of glucose in the neuronal tri-carboxylic acid cycle (i.e., for oxidative ATP generation) and the incremental cycling of neurotransmitters (i.e., glutamate + GABA), released by neuronal firing and cycled through astrocytes by way of glutamine (Hyder et al, 2006). In the resting state, a small fraction of cerebral energy is used for non-signaling “housekeeping” functions so that the larger fraction – as much as 80% – of total energy consumption is associated with the processes of neuronal firing. The relative oxidative energy consumption (Laughlin, 2001) amongst the different synaptic functions (e.g., ion pumping to restore gradients, glutamate and GABA release, cycling and storage, as well as its distribution amongst synaptic structures such as pre- or post-synaptic neurons, glia or the smaller non-signaling or “housekeeping” functions) are subjects of ongoing research (Lennie, 2003).
The one-to-one relationship between ΔCMRglc(ox) and ΔVcyc was extended further by electrical recordings of multi-unit activity (in the rat) where absolute firing rates (ν) and their changes (Δν) were measured and compared with ΔCMRO2 obtained by calibrated fMRI (Smith et al, 2002). The nearly one-to-one correspondence observed between Δν and ΔCMRO2 during sensory stimulation revealed that changes in energy consumption track neuronal firing and are indeed a valid assessment of changes in neuronal activity. These studies used high impedance extracellular electrodes (Geddes and Roeder, 2001) which can measure both the multi-unit activity and slower field oscillations (Mitzdorf, 1985). However Smith et al (2002) extracted both increases and decreases in the firing rates because these changes offer a criterion for determining the baseline levels in absolute units.
What does all this mean for baseline activity, which has become of considerable interest (Gusnard and Raichle, 2001), in part because of its somewhat apparently paradoxical energetic basis, and in part because of the incomplete assignments of the energetic contributions to the negative BOLD signal, now resolved by the study of Pasley et al (2006). We propose, therefore, that any brain state can be considered a baseline by virtue of it being the comparative state for a perturbation of brain activity which we choose to designate and introduce. In all baseline states, of course, the brain is actively consuming energy which is primarily dedicated to neuronal firing, including contributions of excitatory (i.e., glutamate) and inhibitory (i.e., GABA-like) activity. Furthermore during an external perturbation – like a sensory or mental task – the small modular energies measure the difference between the energy needed during the task and during the baseline phase, but do not express the energy needed to perform the task – that is described by the total energy (Shulman et al, 1999; Hyder et al, 2002). But the typical differences in human studies, as shown by Pasley et al (2006), are at most 20% of the total energy even in the visual cortex of the awake human, so that the purpose for the majority of the energy in the baseline and perturbed states is still not understood.
The relationships established between energy and neuronal firing leads us to consider the relevance of the present energetic results extended by Pasley et al (2006) for understanding the baseline activity, whose subjective functions are usually too diffuse for specification. While it may seem that energetic results are too coarse to elucidate the most subtle, most nuanced of all biological functions namely of the individualized human brain, still energetics are firmly based upon thermodynamics, the fundamentals of quantitative science, and can be compared directly to neuronal activity (e.g., as in Smith et al, 2002). The contrary popular view, as expressed by Thomas Nagel, is that existing physical studies must leave the subjective human perspective behind. Physical science cannot answer the important questions because it provides too “impoverished and reductive idea of objectivity” (Nagel, 1986). For this reason contemporary explorations of baseline activity usually turn to non-physical assumptions such as from cognitive psychology, networks theory, rational choice, game theory or the contingencies of Mind and Consciousness. However appreciating that we are not addressing these large unsolved questions, we can still explore a schematic understanding of neuronal activity supported by baseline energy, bolstered ever so subtly by the present results of Pasley et al (2006).
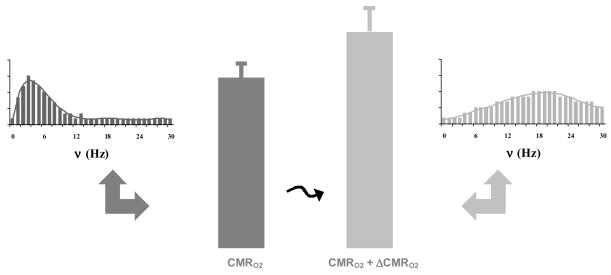
On the microscopic level of the neuronal ensemble, describable by a histogram of neuronal firing rates, some or many of the individual neurons change their firing rates, and during a task the total regional activities may increase or decrease (Shulman et al, 2004). Since the changes are small, the high level of total activity or energy for the perturbed state is still on the order of the baseline (Fig. 2). In a previous α-chloralose anesthetized rat study (Smith et al, 2002), histograms portraying the voting mannerisms of a large number of neurons in the somatosensory cortex showed nearly identical distributions and total energies during sensory stimulation from two baseline levels, achieved by anesthetic depth. These results suggested that the nearly comparable redeployments of the ensemble for the sensory task from the two baselines demanded almost equivalent amounts of total energy (Hyder et al, 2002). In the Pasley et al (2006) study, a truly bold step has been taken towards achieving more practical ways of varying baseline in the awake human using sensory stimuli (see also Marx et al, 2004; Uludag et al, 2004; Gardener et al, 2005). If the findings of Pasley et al (2006) of similar total energies from the two baselines are extended based on the rat results, we expect similar histograms of firing from the neuronal population in the visual cortex. However the enticing possibility that baseline can be varied with (e.g., Pasley et al, 2006) and without (e.g., Smith et al, 2002) stimuli is evocative of an “eclectic” definition of baseline (see above) and strongly advocates, therefore, that while it is quite convenient to ignore baseline enroute to localizing activations in fMRI data, the baseline is far too important to remain dark and mysterious. Besides the encouraging and promising uses of calibrated fMRI that the Pasley et al (2006) study demonstrates, it endorses more carefully designed studies in the future to further explore the neuronal and energetic basis of baseline activity.
Figure 2.
Changes in energy consumption (CMRO2) and histogram of spiking rate (ν). Upon stimulation (black jagged arrow) from the resting, unperturbed baseline (left) the distribution of firing rates shift in congruence with the energy required for the new, perturbed state (right). Modified from Smith et al (2002).
Acknowledgments
We are grateful for support from National Institutes of Health R01 grants (DC-03710 to FH; MH-67528 to FH; NS-037527 to DLR; NS-051854 to DLR) and appreciate engineers of MRRC (mrrc.yale.edu) and staff of QNMR (qnmr.yale.edu).
Abbreviations
- BOLD
blood-oxygenation level dependent
- CBF
cerebral blood flow
- CMRglc(ox)
cerebral metabolic rate of glucose oxidation
- CMRO2
cerebral metabolic rate of oxygen consumption
- fMRI
functional magnetic resonance imaging
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- PET
positron emission tomography
- ν
neuronal spiking rate
- Vcyc
rate of total neurotransmitter cycling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian ED. Afferent discharges to the cerebral cortex from peripheral sense organs. J Physiol (Lond) 1941;100:159–191. doi: 10.1113/jphysiol.1941.sp003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel A, Berridge DA, Unwin J. The effect of anaesthetic agents on primary cortical evoked responses. Br J Anaesth. 1973;45:824–836. doi: 10.1093/bja/45.8.824. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol. 1987;263:265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Chapin JK. Laminar differences in sizes, shapes, and response profiles of cutaneous receptive fields in the rat SI cortex. Exp Brain Res. 1986;62:549–559. doi: 10.1007/BF00236033. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Waterhouse BD, Woodward DJ. Differences in cutaneous sensory response properties of single somatosensory cortical neurons in awake and halothane anesthetized rats. Brain Res Bull. 1981;6:63–70. doi: 10.1016/s0361-9230(81)80069-x. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol. 1984;229:199–213. doi: 10.1002/cne.902290206. [DOI] [PubMed] [Google Scholar]
- Chen LM, Friedman RM, Roe AW. Optical imaging of SI topography in anesthetized and awake squirrel monkeys. J Neurosci. 2005;25:7648–7659. doi: 10.1523/JNEUROSCI.1990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholet N, Seylaz J, Lacombe P, Bonvento G. Local uncoupling of the cerebrovascular and metabolic responses to somatosensory stimulation after neuronal nitric oxide synthase inhibition. J Cereb Blood Flow Metab. 1997;17:1191–201. doi: 10.1097/00004647-199711000-00008. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD. Neurophysiological correlates of different functional states of the brain. In: Ingvar DH, Lassen NA, editors. Alfred Benzon Symposium VII. Academic Press; New York: 1975. pp. 21–46. [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci USA. 2005;102:3822–3827. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor J. The mind doesn’t work that way: The scope and limits of computational psychology. MIT Press; Cambridge: 2000. [Google Scholar]
- Friston KJ. Imaging neuroscience: Principles or maps? Proc Natl Acad Sci USA. 1998;95:796–802. doi: 10.1073/pnas.95.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Sun P, Waggoner RA, Ueno K, Tanaka K, Cheng K. Contrast adaptation and representation in human early visual cortex. Neuron. 2005;47:607–620. doi: 10.1016/j.neuron.2005.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes LA, Roeder R. Measurement of the direct-current (faradic) resistance of the electrode-electrolyte interface for commonly used electrode materials. Ann Biomed Eng. 2001;29:181–186. doi: 10.1114/1.1349699. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Dietrich WD, Busto R. Coupled forebrain increases of local cerebral glucose utilization and blood flow during physiologic stimulation of a somatosensory pathway in the rat: Demonstration by double-label autoradiography. Neurology. 1987;37:11–19. doi: 10.1212/wnl.37.1.11. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F. Neuroimaging with calibrated fMRI. Stroke. 2004;35(Suppl 1):2635–2641. doi: 10.1161/01.STR.0000143324.31408.db. [DOI] [PubMed] [Google Scholar]
- Hyder F, Chase JR, Behar KL, Mason GF, Siddeek M, Rothman DL, Shulman RG. Increased tri-carboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C] NMR. Proc Natl Acad Sci USA. 1996;93:7612–7617. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Mason GF, Rangarajan A, Behar KL, Shulman RG. Oxidative glucose metabolism in rat brain during single forepaw stimulation: A spatially localized 1H[13C] NMR study. J Cereb Blood Flow Metab. 1997;17:1040–1047. doi: 10.1097/00004647-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: Towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc Natl Acad Sci USA. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Kida I, Hyder F, Kennan RP, Behar KL. Towards absolute quantitation of BOLD functional MRI. Adv Exp Med Biol. 1999;471:681–689. doi: 10.1007/978-1-4615-4717-4_78. [DOI] [PubMed] [Google Scholar]
- Kida I, Kennan RP, Rothman DL, Behar KL, Hyder F. High-resolution CMRO2 mapping in rat cortex: A multi-parametric approach to calibration of BOLD image contrast at 7 Tesla. J Cereb Blood Flow Metab. 2000;20:847–860. doi: 10.1097/00004647-200005000-00012. [DOI] [PubMed] [Google Scholar]
- Kida I, Smith AJ, Blumenfeld H, Behar KL, Hyder F. Lamotrigine suppresses neurophysiological responses to somatosensory stimulation in the rodent. NeuroImage. 2006;29:216–224. doi: 10.1016/j.neuroimage.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB. Determination of relative CMRO2 from CBF and BOLD changes: Significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med. 1999;41:1152–1161. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- King EE. Differential action of anesthetics and interneuron depressants upon EEG arousal and recruitment responses. J Pharmacol Exp Ther. 1956;116:404–417. [PubMed] [Google Scholar]
- Laughlin SB. Energy as a constraint on the coding and processing of sensory information. Curr Opin Neurobiol. 2001;11:475–480. doi: 10.1016/s0959-4388(00)00237-3. [DOI] [PubMed] [Google Scholar]
- Lennie P. The cost of cortical computation. Curr Biol. 2003;13:493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Villringer A, Dirnagl U. Characterization of CBF response to somatosensory stimulation: model and influence of anesthetics. Am J Physiol. 1993;26:H1223–H1228. doi: 10.1152/ajpheart.1993.264.4.H1223. [DOI] [PubMed] [Google Scholar]
- Marx E, Deutschlander A, Stephan T, Dieterich M, Wiesmann M, Brandt T. Eyes open and eyes closed as rest conditions: Impact on brain activation patterns. Neuroimage. 2004;21:1818–1824. doi: 10.1016/j.neuroimage.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. NeuroImage. 2006 doi: 10.1016/j.neuroimage.2007.06.019. (in press) [DOI] [PubMed] [Google Scholar]
- Nagel T. The view from nowhere. Oxford University Press; Oxford: 1986. [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. NeuroImage. 2006 doi: 10.1016/j.neuroimage.2006.09.015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Behind the scenes of functional brain imaging: A historical and physiological perspective. Proc Natl Acad Sci USA. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shulman RG. Interview with Robert G. Shulman. J Cogn Neurosci. 1996;8:474–480. doi: 10.1162/jocn.1996.8.5.474. [DOI] [PubMed] [Google Scholar]
- Shulman RG. Functional imaging studies: Linking mind and basic neuroscience. Amer J Psychiat. 2001;158:11–20. doi: 10.1176/appi.ajp.158.1.11. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL. Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc Natl Acad Sci USA. 1998;95:11993–11998. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. Stimulated changes in localized cerebral energy consumption under anesthesia. Proc Natl Acad Sci USA. 1999;96:245–3250. doi: 10.1073/pnas.96.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Biophysical basis of brain activity: Implications for neuroimaging. Q Rev Biophys. 2002;35:287–325. doi: 10.1017/s0033583502003803. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE, Hershey AE, Bryant DP. Responses of barrel cortex neurons in awake rats and effects of urethane anesthesia. Exp Brain Res. 1992;91:259–272. doi: 10.1007/BF00231659. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Relationship between functional activity and energy metabolism in the nervous system: whether, where and why? In: Lassen NA, Ingvar DH, Raichle ME, Friberg L, editors. Brain work and mental activity. Munksgaard; Copenhagen: 1991. pp. 52–64. [Google Scholar]
- Tsukada H, Kakiuchi T, Ando I, Shizuno H, Nakanishi S, Ouchi Y. Regulation of cerebral blood flow response to somatosensory stimulation through the cholinergic system: A positron emission tomography study in unanesthetized monkeys. Brain Res. 1997;749:10–17. doi: 10.1016/s0006-8993(96)01028-1. [DOI] [PubMed] [Google Scholar]
- Ueki M, Linn F, Hossman KA. Functional activation of cerebral blood flow and metabolism and after global ischemia of rat brain. J Cereb Blood Flow Metab. 1988;8:486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- Ueki M, Mies G, Hossmann KA. Effect of α-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand. 1992;36:318–322. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- Uludag K, Dubowitz DJ, Yoder EJ, Restom K, Liu TT, Buxton RB. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. NeuroImage. 2004;23:148–155. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Winters WD. Effects of drugs on the electrical activity of the brain: Anesthetics. Annu Rev Pharmacol Toxicol. 1976;16:413–426. doi: 10.1146/annurev.pa.16.040176.002213. [DOI] [PubMed] [Google Scholar]
- van Zijl PCM, Efefe SM, Ulatowski JA, Oja JME, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nature Med. 1998;4:159–167. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]