Abstract
Drug delivery to the brain is hindered by the presence of the blood-brain barrier (BBB). Although the BBB restricts the passage of many substances, it is actually selectively permeable to nutrients necessary for healthy brain function. To accomplish the task of nutrient transport, the brain endothelium is endowed with a diverse collection of molecular transport systems. One such class of transport system, known as a receptor-mediated transcytosis (RMT), employs the vesicular trafficking machinery of the endothelium to transport substrates between blood and brain. If appropriately targeted, RMT systems can also be used to shuttle a wide range of therapeutics into the brain in a noninvasive manner. Over the last decade, there have been significant developments in the arena of RMT-based brain drug transport, and this review will focus on those approaches that have been validated in an in vivo setting.
Keywords: blood-brain barrier, transcytosis, brain drug delivery, antibody
INTRODUCTION
The blood-brain barrier (BBB) provides the brain with nutrients, prevents the introduction of harmful blood-borne substances, and restricts the movement of ions and fluid to ensure an optimal environment for brain function. As a consequence of its barrier properties, the BBB also prevents the movement of drugs from the blood into the brain, and therefore acts as an obstacle for the systemic delivery of neurotherapeutics. Unless a therapeutic molecule is lipid-soluble with a molecular weight of 400–600 Da or less, brain penetration is limited (1). Furthermore, efflux transport systems may target the drugs that meet these criteria and export them from the brain. As a result, the BBB excludes many small-molecule pharmaceuticals, and nearly all biopharmaceuticals such as gene and protein medicines fail to penetrate into the brain tissue to an appreciable extent (1). Thus, although the surface area of the human brain microvasculature available for drug transport (~20 m2) is more than adequate for treating the entire brain volume, the barrier properties of the BBB continue to restrict brain drug delivery via the bloodstream (2).
To date, strategies for the delivery of drugs that do not have an appreciable BBB permeability have included both invasive and noninvasive approaches. Direct intracranial injection, intraventricular administration and BBB disruption are examples of invasive delivery techniques that have been reviewed elsewhere (3). Instead, this review will focus on a rapidly developing class of novel, delivery reagents that function in mediating noninvasive blood-to-brain transport by taking advantage of endogenous nutrient transport systems present at the BBB. Nutrients and water-soluble compounds such as ions, amino acids, vitamins, and proteins that are necessary for brain function possess specific transport systems embedded in the plasma membranes of the BBB to allow brain entry. Two main classes of transport systems function at the BBB. The first, carrier-mediated transport, relies on molecular carriers present at both the apical (blood) and basolateral (brain) membranes of the BBB (Figure 1C). These carriers tend to be highly stereospecific and function in the selective transport of small molecules such as ions, energy sources and amino acids. Using carrier-mediated transport systems for noninvasive drug delivery by conjugating therapeutics to the natural substrates has been studied and is reviewed elsewhere in this issue. One factor to take into consideration is that since carrier-mediated transport systems are typically small, stereospecific pores, they are not particularly amenable to the transport of large-molecule therapeutics.
Figure 1.
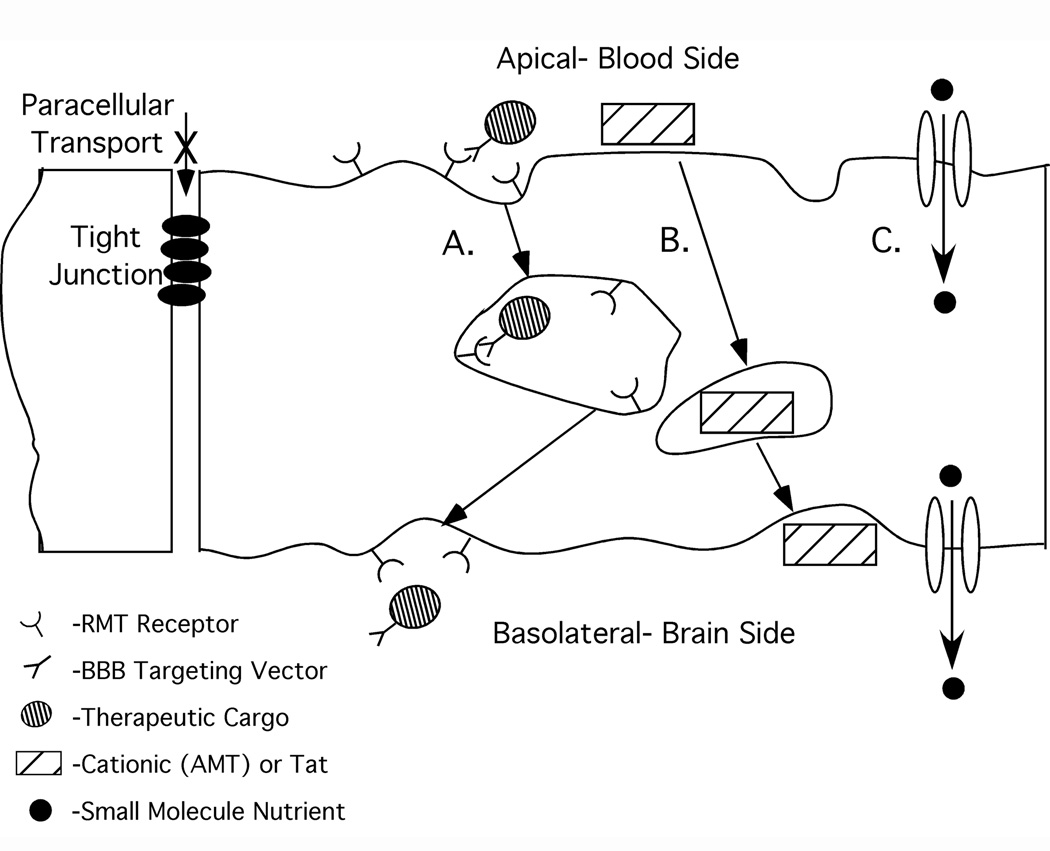
Schematic of transport routes at the BBB. As a result of tight junctions closing down the paracellular space between adjacent endothelial cells, therapeutics must either diffuse through cell membranes or be transported by one of the mechanisms indicated in order to successfully reach brain tissue. A. Receptor-mediated transcytosis. B. Non-specific uptake either by cationization and absorptive-mediated transcytosis or by protein transduction domain. C. Carrier-mediated transport where nutrients enter the brain by traveling serially through transporters present in the apical and basolateral endothelial cell plasma membranes.
Receptor-mediated transport mechanisms are also present at the BBB, and these involve the vesicular trafficking system of the brain endothelium (Figure 1A). Brain influx of nutrients such as iron (4), insulin (5), and leptin (6) occurs by a transcellular, receptor-mediated transport mechanism known as transcytosis. Briefly, a circulating ligand interacts with a specific receptor at the apical plasma membrane of the endothelial cell. Once bound to ligand, the process of endocytosis is initiated as the receptor-ligand complexes cluster and membrane invagination leads to the formation of intracellular transport vesicles (7). The transport vesicles are subject to sorting within the cell; and in transcytosis, the vesicles containing receptor-ligand complexes or alternatively, vesicles containing dissociated ligands are sent to the basolateral side of the polarized endothelial cell, where they are released. In this way, molecules can cross the endothelium and enter the brain without disruption of the barrier properties.
While receptor-mediated transcytosis (RMT) systems are selective pathways for trans-BBB transport in that they require the initial binding of a ligand to something on or in the plasma membrane of the endothelial cells that make up the BBB, absorptive-mediated transcytosis relies on nonspecific charge-based interactions (3). Absorptive-mediated transcytosis (AMT) can be initiated by polycationic molecules binding to negative charges on the plasma membrane (8) (Figure 1B). However, this method lacks specific targeting and may lead to widespread absorption (3). Similarly, protein transduction domains such as the HIV TAT peptide lack targeting and have been shown to have a broad biodistribution that may necessitate prohibitive doses (9). Since noninvasive delivery approaches based on cationization (AMT) or the use of protein transduction domains lack targeting specificity, this review will be focused only on those RMT systems that can be explicitly targeted.
In order to exploit endogenous RMT systems for drug delivery, the therapeutic or therapeutic carrier of interest must be conjugated to a molecule that has the capability of targeting an RMT system (RMT delivery vector). This vector could be either the natural ligand or artificial ligands like antibodies or peptides. In either case, the vector-conjugated drug cargo gains access to the brain interstitium by “piggybacking” on the natural RMT system (Figure 1A). In contrast to carrier-mediated transport, size restrictions on therapeutic cargo are greatly diminished when targeting an RMT system since it employs vesicle-based transport rather than a stereoselective carrier. As examples detailed throughout this review support, small molecules, proteins, genes, and drug-loaded particles can all be delivered via RMT mechanisms. Thus, using noninvasive approaches that require nothing more than an intravenous injection can allow for brain delivery of a variety of drug cargoes. This review will first detail several strategies for linking therapeutics to BBB delivery vectors. Then, we will discuss the promising approaches employing RMT systems for in vivo brain delivery. Finally, strategies for secondary targeting of specific brain cell populations will be touched upon.
STRATEGIES FOR COUPLING THERAPEUTICS TO BBB DELIVERY VECTORS
In order for a neuropharmaceutical to be delivered into the brain via the receptor-mediated mechanism depicted in Figure 1, it must first be linked to the BBB delivery vector. The following section briefly reviews several strategies that have been used to link therapeutic cargo with BBB delivery vectors [more extensive reviews include (3, 8, 10–12)]. These include both covalent linkage and non-covalent association between drug and delivery vector. Recently, the use of liposomes and nanoparticles loaded with drug and decorated with a BBB targeting vector has also been reported. Details regarding which linkage method was used for a specific brain delivery study can be found in Table 1.
Table 1.
Studies focused on In vivo validation of RMT systems for brain drug delivery
| BBB Receptor | BBB targeting Vector | Species Used | Linkage | Secondary Target | Payload | Ref. |
|---|---|---|---|---|---|---|
| rat TfR | human Tf | rat | PEGylated albumin nanoparticles | azidothymidine | (34) | |
| mouse TfR | human Tf | mouse | PEGylated liposome | colon 26 tumor | nido-carborane | (90) |
| rat TfR | OX26 MAb | rat | hydrasone linkage | methotrexate | (50) | |
| rat TfR | OX26 MAb | rat | disulfide linkage | recombinant human soluble CD4 | (91) | |
| rat TfR | OX26 MAb | rat | SA/B linkage | vasoactive intestinal peptide analog | (13, 92) | |
| rat TfR | OX26 MAb | rat | SA/B linkage | nerve growth factor | (39) | |
| rat TfR | OX26 MAb | rat | SA/B linkage | β-amyloid peptide Aβ1–40 | (93) | |
| rat TfR | OX26 MAb | rat | SA/B linkage | brain-derived neurotrophic factor | (14, 36, 94) | |
| rat TfR | OX26 MAb | rat | SA/B linkage | C6 glioma cells transfected with a gene encoding the human EGFR | human epidermal growth factor | (95) |
| rat TfR | OX26 MAb | rat | SA/B linkage | C6 glioma cells transfected with a luciferase expression | PNA antisense to luciferase mRNA | (43) |
| rat TfR | OX26 MAb | rat | SA/B linkage | basic fibroblast growth factor | (38, 96) | |
| rat TfR | OX26 MAb | rat | SA/B linkage | C6 and RG2 glial cells | PNA antisense to rat caveolin-1α | (44) |
| rat TfR | OX26 MAb | rat | PEGylated liposome | daunomycin | (16, 40, 41) | |
| rat TfR | OX26 MAb | rat | PEGylated liposome | neurons | expression plasmid encoding β-galactosidase | (17) |
| rat TfR | OX26 MAb | rat | PEGylated liposome | neurons in rat model of Parkinson disease | expression plasmid encoding tyrosine hydroxylase | (84, 85) |
| rat TfR | anti-TfR IgG3-CH3 (OX26 HV region) | rat | Av/B linkage | brain parenchyma | PNA antisense to rev gene of HIV-1 | (45) |
| mouse TfR | 8D3 MAb | mouse | SA/B linkage | neurons in R6/2 transgenic mice | PNA antisense to huntingtin gene | (48) |
| mouse TfR | 8D3 MAb | mouse | SA/B linkage | β-galactosidase | (47) | |
| mouse TfR | 8D3 MAb | mouse | PEGylated liposome | astrocytes | expression plasmid encoding β-galactosidase | (46) |
| mouse TfR | 8D3 MAb | mouse | PEGylated liposome | expression plasmid encoding luciferase | (46) | |
| mouse TfR | 8D3 MAb | mouse | PEGylated liposome | U87 human glial tumors | expression plasmid encoding antisense mRNA to human EGFR | (87) |
| mouse TfR | 8D3 MAb | mouse | PEGylated liposome | U87 human glial tumors | expression plasmid encoding short hairpin RNA directed at human EGFR | (88) |
| human TfR | 128.1 MAb | monkey | disulfide linkage | recombinant human soluble CD4 | (91) | |
| HB-EGF | CRM197 | guinea pig | primary amine | horseradish peroxidase | (71) | |
| human IR | 83-14 MAb | monkey | SA/B linkage | β-amyloid peptide Aβ1–40 | (61) | |
| human IR | 83-14 MAb | monkey | PEGylated liposome | neurons | expression plasmid encoding β-galactosidase or luciferase | (15) |
| human IR (blood-retinal barrier) | 83-14 MAb | monkey | PEGylated liposome | ocular cells | expression plasmid encoding β-galactosidase or luciferase | (62) |
| human IR | chimeric 83-14 | monkey | fusion protein | brain-derived neurotrophic factor | (63) | |
| human IR | humanized 83-14 | monkey | (58) | |||
| M6P | β-glucuronidase | neonatal mice | (64) | |||
| LRP | p97 | mice | cross-linking | adriamycin | (67) | |
| LRP | polysorbate 80 coating | mice | nanoparticle | dalargin | (19, 26, 97, 98) | |
| LRP | polysorbate 80 coating | rat | nanoparticles | doxorubicin | (20–23) | |
| LRP | polysorbate 80 coating | mice | nanoparticles | loperamide | (24, 26) | |
| LRP | polysorbate 80 coating | rat | nanoparticles | methotrexate | (25) | |
| LRP | polysorbate 80 coating | mice | nanoparticles | 5-fluorouracil | (99) | |
| LRP | apolipoprotein E | mice | nanoparticles | loperamide | (100) | |
| LRP | RAP | rat | (68) | |||
| LRP | Tissue-type Plasminogen Activator | rat | (101) | |||
| unknown | FC5 scFv | mouse | (76) |
I. Chemical linkage
The key to any linkage strategy is to ensure that both the transport vector and pharmaceutical protein retain their functionality. Several well-established methods for covalent chemical conjugation have been used to achieve this goal. The most common approach is linkage via primary amines, principally lysine residues, of either the targeting vector or protein therapeutic. Chemical functionalization using Traut’s reagent (2-iminothiolane) yields a thiol that can subsequently be reacted with maleimide-functionalized drug or vector to form a stable thioether bond. Thiolated drug or vector can also be reacted with a free cysteine or reduced disulfide bond to yield a disulfide-bonded drug-vector conjugate (3). To further ensure functionality of the vector and protein, a chemical spacer (CH2)5NHCO(CH2)5NHCO or polyethylene glycol (PEG) moiety can be incorporated into the linkage to reduce steric hindrance (10).
II. Non-covalent streptavidin/biotin linkage
Due to the extremely high binding affinity between streptavidin and biotin (Kd ~ 10−15 M), this non-covalent interaction can be used to couple BBB delivery vectors with therapeutics (3, 10). To achieve this coupling, the therapeutics can be monobiotinylated at lysine residues using N-hydroxysuccinimide (NHS) analogs of biotin, or alternatively, biotin can be attached using biotin hydrazide which reacts with carboxylic acid moieties on glutamate and aspartate residues (10). Having multiple choices of amino acid residues where biotin can be attached can be helpful to ensure that the therapeutic activity is retained upon biotinylation (13). In addition, as a result of streptavidin multivalency, it has been shown that monobiotinylation is necessary to prevent the formation of aggregates, and hence rapid clearance by the reticuloendothelial system (RES) (2). The streptavidin can be coupled to the targeting vector via a thioether linkage using methods described in the previous section. A BBB-targeted therapeutic can then be created simply by mixing the biotinylated therapeutic with the streptavidin-functionalized targeting vector. Again a PEG linkage can be used to better separate the therapeutic and targeting moiety, while also providing improved plasma residence time in some cases (14).
III. Liposomes
Liposomes are spherical phospholipid-based nanocontainers that form spontaneously in an aqueous solution. For the purposes of drug delivery to the brain, controlling liposome size to be around 85 nm in diameter has proven successful (15). The liposomes can be used to encapsulate a large amount of small water-soluble molecules in their aqueous core, absorb lipophilic drugs in their lipid bilayer membrane, or complex with gene-based medicines (12, 16, 17). Early problems with liposomes involved their rapid uptake by the RES and consequent removal from circulating blood (18). However, once the liposomes were sterically stabilized through the incorporation of PEG-distearoylphosphatidylethanolamine (DSPE) moieties into the liposome bilayer, loss via the RES system was substantially reduced (18). In addition, specificity can be added to liposomes by coating their surface with targeting molecules. As an example, “immunoliposomes” can be formed by coating liposomes with a BBB-targeting antibody. This can be accomplished by incorporating PEG-DSPE that possesses a maleimide at the PEG tip to facilitate attachment to a thiolated targeting vector by stable thioether linkage (10, 15, 17).
IV. Nanoparticles
Nanoparticles are solid colloidal particles that can be synthesized using a variety of polymers and techniques (11). Pharmaceuticals can be entrapped in the matrix, encapsulated in the core, or attached to the surface of a nanoparticle (11, 12). The most frequently used nanoparticle formulation for brain drug delivery consists of poly(butyl cyanoacrylate) (PBCA) (19–26). In order to overcome the uptake in RES of the liver and spleen, nanoparticles can be coated with surfactants or, PEGylated like liposomes (12). As described later in this review, coating with the surfactant polysorbate 80 also indirectly promotes brain uptake of the nanoparticle contents (19–25).
RECEPTOR-MEDIATED TARGETS FOR BRAIN ENTRY
The most important aspect of the design of BBB delivery vector-drug conjugates is clearly the choice of BBB delivery vector and its cognate RMT system. In this section, we will detail the current choices for receptor-mediated BBB transport, with a special emphasis towards those systems that have shown promise in vivo. In addition, the delivery attributes of the various BBB RMT systems have been compiled in Table I to facilitate comparison.
I. Transferrin receptor
The transferrin receptor (TfR), perhaps the most studied receptor known to undergo RMT, is highly expressed by brain capillaries to mediate the delivery of iron to the brain (4). The natural ligand for the TfR is the iron binding protein, transferrin (Tf) (27). Human TfR is a transmembrane glycoprotein consisting of two 90 kDa subunits joined by intermolecular disulfide bonds, with each subunit having the capability of binding to one Tf molecule (28, 29). There are some questions as to the actual extent of Tf transcytosis as it has been shown that uptake of iron by the brain exceeds that of Tf (30). Subsequent studies have concluded that only a small amount of Tf is actually transcytosed across the brain capillary endothelial cells and deposited in the brain (31, 32). Despite the questions regarding delivery mechanism, the Tf ligand itself has been used for brain targeting. For example, Shin et al. (33) describe the brain delivery of iodinated IgG3 antibody fused with Tf. The human IgG3 immunoglobulin was used as a model antibody to test the Tf linkage points that led to the most efficient brain delivery. It was found that attachment of Tf to the hinge region of IgG3 yielded the highest brain uptake, 0.3% of the injected dose. More recently, the antiviral drug, azidothymidine (AZT) has been delivered using Tf-targeted, PEGylated albumin nanoparticles (PEG-NP). The percentage of drug recovered in the rat brain was 21.1% using the Tf-targeted PEG-NP, while non-targeted PEG-NP alone showed only a 9.3% accumulation after 4 hours (34).
However, Tf is likely not an ideal brain delivery vector since the TfR is nearly saturated with endogenous Tf that persists in the bloodstream at a concentration of 25 µM, meaning that a Tf-targeted drug would have to compete with the natural ligand (3, 29). As an alternative, there has been much proof-of-concept success in using antibodies against the TfR. The mouse monoclonal antibody (MAb) against the rat TfR, OX26, has been the most thoroughly studied. This antibody binds to an extracellular epitope of the TfR that is distinct from the Tf binding site therefore limiting effects on normal Tf transport, and preventing competition for binding sites between the drug targeting vector and natural ligand (35). The OX26 BBB-targeting vector has been validated quite extensively as a brain delivery agent that, when conjugated to therapeutic cargo, can elicit pharmacologic effects. For example, neuroprotective agents such as brain-derived neurotrophic factor (BDNF) have been tested for amelioration of stroke symptoms (36). BDNF was coupled to biotin (B) via PEG-hydrazide and streptavidin (SA) was coupled to OX26, yielding an OX26-BDNF conjugate as a result of streptavidin/biotin (SA/B) interactions. Unconjugated BDNF or the OX26-BDNF conjugate were intravenously injected into rats subjected to permanent middle cerebral artery occlusion (MCAO). It was found that rats given the OX26-BDNF conjugate had a 243% increase in motor performance relative to BDNF alone (36), indicating significant promise for treatment of stroke symptoms.
Using similar techniques, OX26 has been used to deliver many different drugs to the rat brain in vivo in a noninvasive fashion. A few highlights are described here. Vasoactive intestinal peptide (VIP) participates in the regulation of cerebral blood flow. When an OX26-VIP conjugate (SA/B) was injected into conscious adult rats, there was a 60% increase in hemispheric blood flow, while unconjugated VIP produced no change in blood flow (13). Human basic fibroblast growth factor (bFGF), also known as fibroblast growth factor-2 (FGF-2), is a neurotrophic factor that was found to be neuroprotective in conditions of brain ischemia when administered by intracerebroventricular injection (37, 38). When OX26-conjugated bFGF (SA/B) was injected intravenously into rats with permanent MCAO, an 80% reduction in infarct volume resulted. In contrast, the same dose of unconjugated biotinylated bFGF did not result in significant change (38). Kordower et al. (39) examined the use of OX26 conjugated to NGF in a rat model of Huntington’s disease (HD) and found it prevented the degeneration of cholinergic striatal acetyltransferase-immunoreactive neurons. In addition to delivering protein payloads, OX26 can also be used to deliver plasmid DNA encoding exogenous therapeutic genes. For instance, plasmid DNA encoding β-galactosidase has been delivered noninvasively to the rat brain by encapsulating it in OX26-targeted, PEGylated liposomes. This approach resulted in gene expression deep within the parenchyma of the brain in all cell types, and also in other organs with enriched expression of TfR, like the liver and the spleen (17). Similar approaches have also been used to deliver small molecules like daunomycin (16, 40, 41), antisense oligonucleotides (ODN) and peptide nucleic acids (PNA) as therapeutics (42–45).
Since OX26 does not crossreact with the mouse TfR, another monoclonal antibody, 8D3, has been used as a BBB-targeting vector in the mouse (35). Examples of using 8D3 for mouse brain delivery include the delivery of exogenous plasmid DNA encoding the model enzymes β-galactosidase and luciferase using 8D3-targeted PEGylated liposomes (46). When gene expression was driven by the constitutive simian virus 40 (SV40) promoter, β-galactosidase activity was expressed in the brain and other TfR-rich organs like the spleen, liver, and lung. In contrast, when the brain-specific glial fibrillary acidic protein (GFAP) promoter was instead used to drive enzyme expression, there was no gene expression in the peripheral organs, but high level expression in astrocytes was observed (46). Brain and peripheral organ delivery of β-galactosidase was also successful when the actual enzyme, rather than the gene, was conjugated to 8D3 using an SA/B linkage (47). As another example of nucleic acid delivery, 8D3 was conjugated to a radiolabeled 16-mer antisense PNA using SA/B linkage and used for imaging in a model of HD. The antisense PNA was designed to hybridize to exon 1 of the huntingtin gene (48). The 8D3-PNA conjugate was administered intravenously to R6/2 transgenic mice, a model of HD which expresses exon 1 of the huntingtin gene (48). The 8D3-PNA compound was sequestered, as measured by brain radioactivity, in the brains of the transgenic mice at a 3-fold higher level relative to the littermate control (48). The 8D3 system has also been used to image beta-amyloid plaques in a transgenic mouse model of Alzheimer’s disease. Intravenous injection of 8D3-conjugated, radiolabeled Aβ1–40 into the transgenic mouse model allowed the visualization and quantitation of amyloid deposition (49). Another antibody to the mouse TfR, the monoclonal antibody R17–127, also undergoes RMT through the mouse BBB. The antibody 8D3 has a higher plasma area under the curve (AUC), brain uptake, and percent injected dose/g brain than R17–127; but interestingly R17–127 is more brain selective in that there was no measurable uptake in the mouse kidney or liver (35). It was suggested that the R17–127 may achieve its organ specificity by recognizing an epitope on BBB TfR that is inaccessible in the liver (35). Thus, all targeting vectors do not behave the same way, even when they target the same receptor.
Although the TfR has been substantially studied as an RMT system that allows noninvasive delivery of various therapeutics to the brain using the OX26 and 8D3 antibodies, there are some drawbacks that exist. First, the widespread expression of the TfR on peripheral organs limits its capability for specific brain delivery, and as a result only 0.44% of the injected dose OX26 reaches the rat brain after 24 hours (50). Similarly, approximately 1.5% (3.1 %ID/g, ~0.5 g/brain) and 0.8% (1.6 %ID/g, ~0.5 g/brain) of the injected dose of 8D3 and R17–127, respectively, reached the mouse brain after 1 hour (35). However, pharmacologic effects are clearly observed and using secondary targeting strategies such as a brain-specific promoter to drive cell or tissue-specific gene expression could limit concerns regarding drug side effects in non-brain tissues (46). In addition, although these particular antibodies do not recognize the human TfR, antibodies that do recognize the human transferrin receptor and transport into the primate brain exist (51). However, these antibodies are not of human origin and could cause an immunogenic effect if ever used for human treatment. Strategies to overcome this hurdle will be discussed in reference to the insulin receptor RMT/anti-insulin receptor MAb system in the next section.
II. Insulin receptor and insulin-like growth factor receptor
The insulin receptor, like the TfR, is found on the luminal membrane of brain capillary endothelial cells as well as at the plasma membrane of other brain cells (52, 53), and it can undergo RMT across the BBB endothelium (5). The insulin receptor is a ~300 kDa integral membrane glycoprotein that consist of two α-subunits and two β-subunits (54, 55). The α- and β-subunits are joined by disulfide bonds to form a cylindrical structure, and when insulin binds, a conformation change takes place allowing for tyrosine kinase activity and subsequent receptor internalization (54, 55). Using the endogenous ligand, insulin, as a vector has not been attempted, likely because of two potential problems. First, insulin is rapidly degraded in the bloodstream with a serum half-life of only 10 minutes, and secondly, interfering with the natural insulin balance could cause hypoglycemia (3). However, like the TfR, there are antibodies recognizing the insulin receptor that have been used as BBB-targeting vectors. Extensive research using the 83-14 mouse MAb against the human insulin receptor (HIR) as an RMT delivery vector has been performed. The 83-14 antibody is effective in Old World primates like the Rhesus monkey, so it can be evaluated in a non-human setting (56).
When compared to the transport of an anti-human TfR MAb (0.3% brain uptake at 24 hours) (51), the transport of 83-14 across the primate BBB is approximately 10-fold greater, with uptake nearing 4% (0.04 %ID/g, ~100g/brain) of the injected dose 3 hours after injection (56). Similar to the transferrin antibodies, however, is the fact that 83-14 is a mouse antibody, so it too could lead to immunogenic responses in humans. As a way to address this shortcoming, engineered forms of the 83-14 MAb have been created to increase its human character. Both a chimeric antibody (57) and more recently, a fully humanized form of the 83-14 antibody against the HIR have been created (58). First, a chimeric MAb that is 85% human and 15% mouse was created by grafting the mouse sequences encoding the variable fragment binding regions onto a human immunoglobulin scaffold (57, 59). The chimeric antibody had identical reactivity to the HIR of human brain capillaries and comparable uptake into the Rhesus monkey brain as the parent mouse 83-14 antibody (57, 58). Fully humanized antibodies would likely produce even less immunogenic response than chimeric antibodies (58, 60). To fully humanize the 83-14 antibody, only the CDR loops responsible for antigen binding were grafted onto the variable chain framework regions of a homologous human antibody (B43 IgG heavy chain and the human REI kappa light chain) (58). In order to improve secretion of the humanized antibody in myeloma cells, the variable heavy chain region of the humanized 83-14 was further engineered by the replacement of five human residues with the original murine residues (58). The affinity of the humanized antibody decreased only 27% relative to the murine antibody, and the humanized antibody was still transported to all parts of the Rhesus monkey brain after intravenous injection (58). Thus, the 83-14 MAb not only transports into the brain in animal models, but via humanization could be directly applicable to clinical application in human patients.
Many of the experiments using the differentially humanized forms of the 83-14 MAb to deliver drug cargo to the Rhesus monkey brain were similar to those using the TfR antibodies in mice or rats. A few high impact studies are described below, and a complete listing can be found in Table I. As a potential imaging agent of amyloid burden in vivo, a radiolabeled Aβ1–40 83-14 fusion protein was created. Brain uptake was tested by intravenous injection into Rhesus monkeys, and like the situation observed in the mouse with 8D3 as a delivery vector, an increase in brain concentration of radiolabeled Aβ1–40 was observed when conjugated to 83-14 (61). Furthermore, the Aβ1–40 component of the fusion protein was tested by labeling tissue sections of an autopsied human Alzheimer’s disease brain, where emulsion autoradiography showed the radiolabeled conjugate bound to neuritic plaques (61). As another example, an expression plasmid encoding β-galactosidase was encapsulated in liposomes decorated with 83-14. After intravenous injection, the exogenous gene was delivered across both the BBB and the plasma membrane of neurons where it was expressed at high levels (15). Moreover, when the expression plasmid was controlled by the ocular-specific, opsin promoter, β-galactosidase gene expression could be specifically limited to the primate eye, suggesting a capability for tissue-specific gene targeting (62). More recently, a single polypeptide fusion comprised of human BDNF linked to the chimeric version of the 83-14 MAb was produced; and when injected intravenously into Rhesus monkeys, the fusion protein resulted in a level of BDNF more than 10-fold greater than endogenous levels (63).
The role of the mannose 6-phosphate (M6P) receptor, also known as the insulin-like growth factor II (IGF-II) receptor, in transport across the BBB of neonatal mice has also been studied (64). The M6P receptor functions in binding to the M6P component of lysosomal enzymes which in turn, leads to internalization and their sorting to the lysosome. It had been shown previously that β-glucuronidase (GUS), a 312 kDa enzyme, was accessible to the brain only in the first two weeks of life (64, 65). Deficiency in this enzyme results in the lysosomal storage disorder mucopolysaccharidosis type VII (64). When radiolabeled and phosphorylated-GUS (131I-P-GUS) or nonphosphorylated GUS were administered intravenously to neonatal mice, P-GUS entered the brain, but the uptake of nonphosphorylated GUS was significantly lower (less than ~25% relative to P-GUS). At seven weeks of age, transport of 131I-P-GUS became insignificant. These observations indicated that P-GUS undergoes RMT via the M6P/IGF-II receptor early in postnatal life but not in adults (64). Thus, correction of lysosomal storage disorders may be possible in neonates using this RMT mechanism where the therapeutic is actually the enzyme itself, and the authors suggested that understanding how to induce M6P receptor in adults could lead to a potential route of enzyme administration for adults suffering with lysosomal storage disorders (64).
III. Low density lipoprotein receptor-related protein 1 and low density lipoprotein receptor-related protein 2
Low density lipoprotein receptor-related proteins 1 (LRP1) and 2 (LRP2) have also been utilized for targeted drug delivery. These receptors are structurally similar to the low density lipoprotein (LDL) receptor, and they are multifunctional RMT systems having multiple ligands (54). As such, there are reports of several BBB-targeting vectors that take advantage of the LRP1 and LRP2 RMT systems.
Melanotransferrin, or human melanoma antigen p97, has been found to transcytose using LRP1 (66). About 0.1% of the injected dose of p97 is delivered to the mouse brain (0.25 %ID/g, ~0.5 g/brain) 1 hour after intravenous injection (67). P97 is structurally homologous to Tf and (54) Gabathuler et al. were able to successfully deliver the anticancer drug adriamycin (ADR) to the mouse brain by conjugating it to p97 (67). The p97 targeting moiety was conjugated to ADR via the cross-linking of N-Succinimidyl S-acetylthioacetate (SATA) on p97 with Succinimidyl 4-[N-maleimidomethyl]-cyclohexane-1-carboxylate (SMCC) on ADR (67). In addition, treatment of mice with intracranial rat C6 glioma with the p97 conjugate significantly increased the rate of survival, relative to ADR alone (67).
Receptor-associated protein (RAP) participates in the folding and trafficking of LRP1 and LRP2 (68). Pan et al. tested the hypothesis that RAP could undergo RMT via the LRP2 transporter and consequently be transported into the mouse brain parenchyma. These researchers showed that the BBB permeability of RAP is greater than that found for either Tf or p97 both in vitro and in vivo (68). In mice, between 0.25 and 0.5% of the injected dose of radiolabeled RAP (0.5–1 %ID/g, ~0.5 g/brain) was delivered to the mouse brain in 30 minutes (68). Importantly, RAP has also been shown to remain functional when proteins are fused to either the N- or C-terminal, making it a good candidate for direct fusion to protein therapeutics (68). Although the RAP system has yet to be used to deliver therapeutic cargo, these results suggest that RAP may ultimately prove to be a feasible drug delivery vector. One potential problem is that RAP is cleared by the kidney and the liver limiting its plasma residence time and brain uptake (68).
Nanoparticles coated with the surfactant, polysorbate 80 (Tween 80), have also been used for brain delivery of drugs like dalargin (19), doxorubicin (20–23), loperamide (24), and methotrexate (25) While definitive proof of nanoparticle transcytosis was not shown, drug was clearly entering the brain and eliciting a pharmacologic effect. To investigate the mechanism of brain uptake, the role of apolipoprotein in the transport of drug-laden, polysorbate 80-coated nanoparticles was studied (26). PBCA nanoparticles were loaded with dalargin or loperamide and coated with different apolipoproteins; while other samples were first precoated with polysorbate 80 prior to apolipoprotein coating. The nanoparticles were intravenously injected into mice, and results indicated that nanoparticles that provided an antinociceptive effect required coating with either polysorbate 80, apolipoprotein B or E, or both polysorbate 80 coating and apolipoprotein B/E. Based on these and other results, it was hypothesized that the polysorbate 80-coated nanoparticles adsorb apolipoproteins in the bloodstream and therefore could undergo receptor-mediated endocytosis into the brain capillary endothelial cells like lipoproteins via the LDL receptor family (26). The authors further hypothesized that the drug is then transported by diffusion after release in the endothelial cells or transcytosed into the brain as intact nanoparticles (26). Subsequently, a study by Sun et al. employed analytical electron microscopy (AEM) to observe the polysorbate 80-coated poly-DL-lactide nanoparticles in vivo, and their results indicated the presence of nanoparticles within the brain endothelium and also in the brain parechyma (69). As mentioned earlier, polysorbate coating can reduce the amount of nanoparticle uptake in peripheral organs, particularly those of the RES. However, when polysorbate 80-coated nanoparticles were loaded with doxorubicin and injected into rats, their biodistribution reverted to that found for uncoated nanoparticles, thereby limiting this advantage (22, 23). The percent injected dose of drug-loaded nanoparticles reaching rat brain peaked one hour after intravenous injection at 0.44% (22). The authors believe the positive charge of the doxorubicin-coated nanoparticle compound may have contributed to this reduction (22), and illustrates that care must be taken when loading nanoparticles so as not to significantly change the physical properties of the particle-drug conjugate. There remain questions regarding the mechanism of uptake for surfactant-coated nanoparticles and their safety profile, and further information can be found in a recent review article (70).
IV. Diphtheria toxin receptor/ Heparin binding epidermal growth factor-like growth factor
Gaillard et al. (71) have tested the use of CRM197, a nontoxic mutant of diphtheria toxin, as a targeting vector for drug delivery to the brain. This mutant has been used since the mid-1980s as a carrier protein in human vaccinations (72) and more recently it has shown some degree of antitumor activity in humans (73), so it has a long history of safe use in human treatments. In the Gaillard study, CRM197 was tested for its brain delivery potential as it has been shown to endocytose after binding the membrane-bound precursor of heparin binding epidermal growth factor-like growth factor (HB-EGF), also known as the diphtheria toxin receptor (71). CRM197 was conjugated to the protein tracer, horseradish peroxidase (HRP), and transport capacity was monitored using a bovine brain capillary/rat astrocytes in vitro BBB model. These assays indicated transcytosis, albeit only after an initial delay and at a slow rate on the order of hours. In addition, in the brains of guinea pigs intravenously injected with the CRM197-HRP conjugate, HRP reaction product was primarily observed sequestered in brain blood vessels corroborating the in vitro data that indicated a slow rate of transcytosis. However, a fraction of HRP was also found fully transcytosed into the brain parenchyma (71). The authors point out a potential complication for the CRM197 system is the serum presence of neutralizing antibodies against CRM197 as a result of previous vaccinations (71), but they refer to the anticancer clinical trails which indicated that the neutralizing antibody level actually decreased after repeated treatment (73).
V. Novel BBB transport vectors and RMT targets
Although the aforementioned systems are promising in terms of generating a pharmacologic effect after intravenous injection of BBB-targeting vector-therapeutic conjugates, there are several groups working to identify new targeting vectors and new RMT systems that could be useful in brain drug delivery. The present methods rely on receptors like the transferrin and insulin receptors that are, in general, ubiquitously expressed. This leads to mis-targeting of brain drugs to other tissues where they could have unwanted side effects. In addition, the present methodologies generally result in a low fraction 1–4% of the injected dose actually reaching the brain target as a consequence of this poor selectivity and nonideal BBB permeability (50, 56, 57, 74). It is important to note that the total amount of drug entering the brain is indeed sufficient in these cases and suggests clinical feasibility from a therapeutic standpoint. However, the loss of between 96–99% of the administered therapeutic could hamper the development of these delivery approaches given the cost of drug manufacture. This may be especially true for expensive protein and gene-based medicines that currently comprise nearly 700 drugs in various stages of clinical trials (75). Thus, one of the goals has been to identify a BBB-specific RMT system with a high transport capacity. One approach to identify new BBB vectors and their conjugate RMT systems has been to use the power of combinatorial antibody library technology.
Combinatorial antibody libraries are large pools of antibodies (~108-1012) having diverse specificities. These libraries can be “searched” for antibodies that perform a specific function such as binding to the plasma membranes of BBB endothelium and triggering transcytosis. In this way, a naïve phage display library of llama single-domain antibodies (sdAb) was used to identify sdAb that transcytose across an in vitro model of the human BBB (76) (Figure 2A). Single domain antibodies (sdAb) are merely the variable heavy domain (VHH) of the llama antibodies (77). Since sdAb lack the Fc domain of a full antibody, the nonspecific uptake in organs that highly express Fc receptors is low (76). Furthermore, it has been suggested that llama VHH would have limited immunogenic effect (78). Two sdAb, FC5 and FC44, that selectively bind to and transcytose human cerebromicrovascular endothelial cells (HCEC) were isolated by a subtractive panning method. After intravenous injection into mice, both phage-displayed and soluble FC5 and FC44 accumulated in the brain in addition to accumulating in the kidney and liver. To compare with measures of brain uptake efficiency described earlier, phage-displayed FC5 and FC44 accumulated in mouse brain at levels of 4.5 %ID/g (~2.2 %ID/brain) and 2.9 %ID/g (~1.5 %ID/brain), respectively (76). Subsequent studies to characterize the mechanism of transport strongly suggest that FC5 transcytoses via a receptor-mediated mechanism and recognizes in α(2,3)-sialoglycoprotein on the luminal surface of brain endothelial cells (77).
Figure 2.
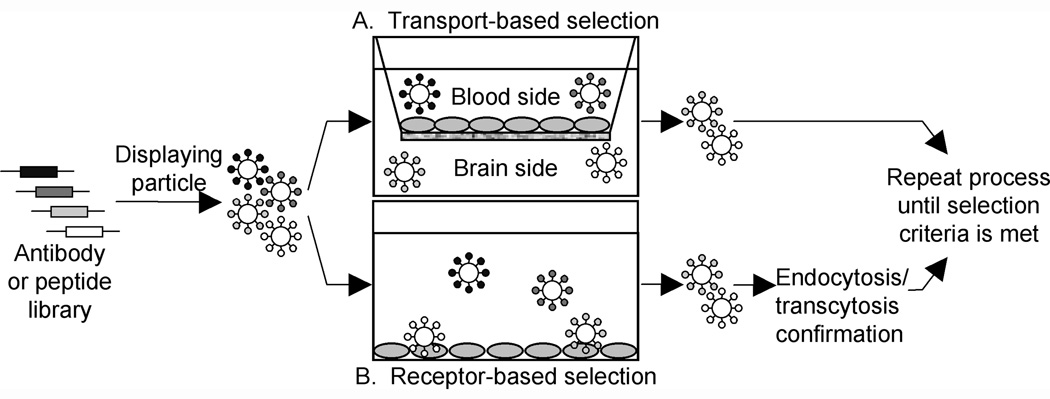
Schematic of strategies used for identifying novel BBB receptors and cognate targeting reagents. A diverse library of 109-1012 genes that encode antibody fragments or peptides can be displayed on the surface of phage, yeast, ribosomes or some other display platform. These libraries can then be screened for antibodies/peptides that bind to receptors on the apical side of the BBB. In the case of antibodies, these libraries often represent a significant fraction of the natural repertoire of the immune system. In contrast to traditional immunization strategies where a known antigen is used to raise antibodies in vivo, the library approach allows for in vitro selections even against antigens or receptors whose identities are not initially known. The figure depicts two such approaches. A. Transport-based selection. Selection using an in vitro model of the BBB grown on a permeable membrane. By simply applying the library to the blood side of the in vitro BBB, one can collect those particles that transport to the opposite chamber or brain side as a result of the antibody/peptide displayed on the particle surface. This process can be repeated until the target population is comprised almost exclusively of transporting particles, at which time the antibodies/peptides mediating the particle transcytosis process can be identified. B. Receptor-based selection. Alternatively, rather than using transport as the selection criterion, one can simply screen for antibodies or peptides that meet the requirement of binding to proteins located at the apical plasma membranes of the BBB endothelium. Subsequently, those binding antibodies or peptides can be screened for their capability to trigger endocytosis or transcytosis. Both approaches have been applied to BBB research as described in the main text.
The antibodies described earlier in this review were either of rodent origin or partially humanized, and this could lead to unwanted immunogenic reactions in human patients. For human therapeutic application, fully human antibodies would be preferable (79). To this end, a nonimmune yeast surface display library of human single-chain antibody fragments (scFv: comprised of the variable light and heavy domains of full antibodies) was recently mined for antibodies that target novel BBB cell-surface receptors (80) (Figure 2B). Thirty-four unique antibody clones that bind to the brain endothelial cell surface were identified, and in some cases, these antibodies targeted an endocytosis system. Interestingly, immunoprecipitation and analysis of the cognate receptor indicated that it was neither the transferrin nor insulin receptor. Although the transcytosis capability of these scFv has yet to be determined, these experiments led to the identification of many novel BBB-targeting antibodies of human origin (80).
Both of these approaches have the potential to identify new targeting reagents and new RMT systems. Of course, there is no guarantee that any new BBB vector-RMT systems will outperform those already known like the transferrin, insulin, or LDL-based systems. Thus, rather than achieving the “holy grail” of brain drug delivery and identifying a highly specific and high permeability BBB vector-RMT system, it is likely that different RMT systems and BBB targeting vectors will have distinct strengths and weaknesses regarding specificity and capacity. The vector-RMT system could then be chosen and tailored based on the desired application. The aforementioned study regarding the 8D3 and R17–127 antibodies exemplifies this principle since these antibodies yielded different biodistributions and brain uptake although they both targeted the TfR (35).
SECONDARY TARGETING TO SITE(S) OF ACTION WITHIN CENTRAL NERVOUS SYSTEM
Once the BBB-targeting vector-therapeutic conjugate has crossed the BBB, there may still be a need to target it to the diseased tissue. For instance, targeting a cytotoxic drug specifically to tumor cells, or targeting gene therapy to a selected subset of the neurons and glia may be necessary. Alternatively, rather than having a specific cell targeting requirement, the drug payload may require internalization into neurons or glia to achieve therapeutic effects. Approaches that have been employed to achieve this second level of targeting/internalization within the central nervous system are discussed below.
I. Transporter present on both BBB and target cell population
A single targeting vector that could function both in mediating transport across the BBB and internalization into target cells would represent the simplest approach for meeting secondary targeting requirements (Figure 3). The TfR, for example, is expressed on neurons (81, 82) and over-expressed in tumor tissue (83). The ubiquitous expression pattern of TfR has been advantageous in developing a BBB RMT-based therapeutic for Parkinson’s disease (84, 85). Deficiency in the striatum of tyrosine hydroxylase (TH), the catalyst in the rate-limiting step of the formation of dopamine, is a result of Parkinson’s disease (86). Zhang et al. (84, 85) encapsulated a nonviral TH expression plasmid in a PEGylated liposome, and targeted it through the BBB using the OX26 antibody. Since the TfR is also expressed by neurons, the gene therapeutic could cross the neuronal plasma membrane by receptor-mediated endocytosis without further modifications. Indeed, after intravenous injection of this targeted liposome into the rat 6-hydroxydopamine model of Parkinson’s disease, striatal TH activity was normalized and apomorphine-induced rotation behavior was significantly reduced (84, 85). A similar approach was used for imaging of diseased tissue by delivering an antisense payload capable of discriminating affected tissue. As described earlier, a radiolabeled PNA was used to image huntingin gene expression in a mouse model of Huntington’s disease (48). When intravenously injected, the radiolabeled PNA-OX26 conjugate transcytosed the BBB and endocytosed into the target brain cells that express the huntingin gene. The antisense PNA then hybridized to the target mRNA in the cytosol and allowed imaging and quantitative autoradiography (48).
Figure 3.
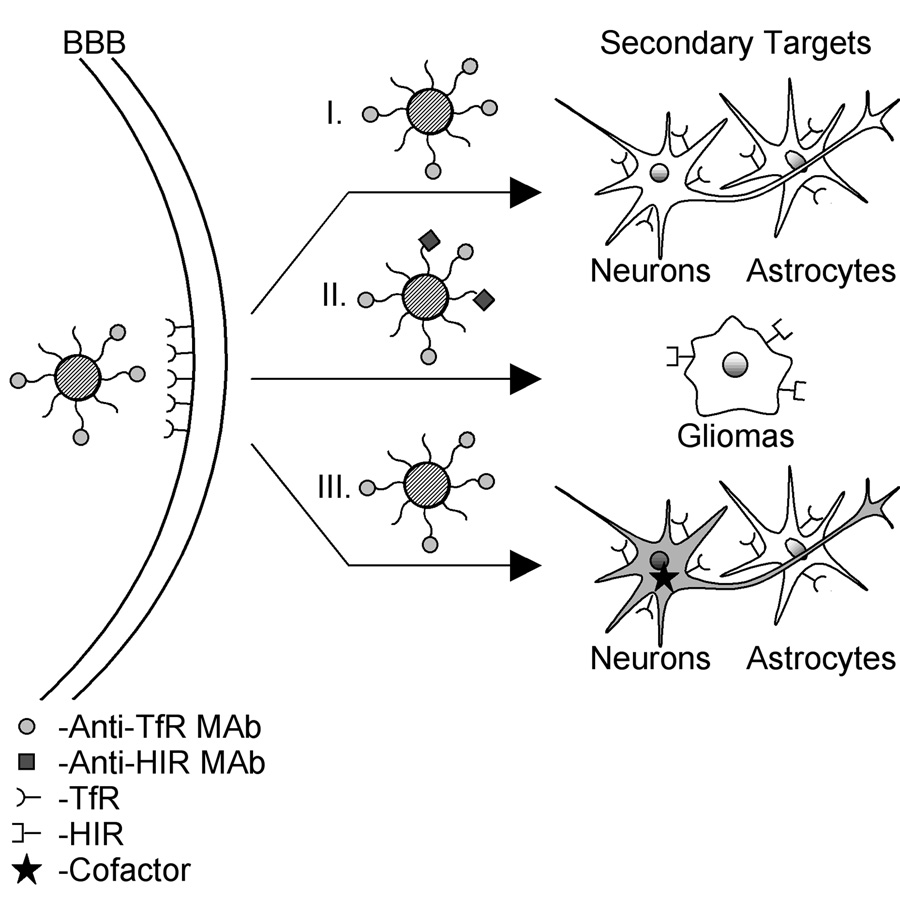
Schematic of secondary targeting strategies for therapeutic delivery to the brain. The figure depicts an anti-TfR antibody-targeted PEGylated liposome as the delivery system, but similar techniques can be used for other BBB targeting vectors and therapeutic payloads. For each depicted technique, the anti-TfR MAb binds to the TfR, and allows crossing of the BBB by transcytosis. I. Transporter present on both BBB and target cell population. For this situation, the anti-TfR MAb can also bind to the TfR of the neurons and the astrocytes, yielding intracellular delivery of the payload to both brain cell types. II. Sequential targeting. In addition to the anti-TfR MAb, a second targeting antibody, such as the anti-HIR MAb, can be conjugated to the surface of the PEGylated liposome. Thus, once the anti-TfR MAb allows delivery across the mouse BBB, the secondary targeting reagent can bind to the HIR on the surface of xenografted human glioma cells allowing targeted delivery of payload. With this proof-of-concept example, the strategy was designed based on the species difference of the xenograft. However, one could instead envision using tumor specific antigens to provide this secondary targeting. III. Selective action. The anti-TfR MAb binds to the TfR of the neurons and the astrocytes. However, specificity can be attained since neurons may produce the cofactor necessary for the activity of an enzyme payload. Similarly, delivering an expression plasmid with a cell-type specific promoter would allow for selective action by driving expression of the gene payload only in the target cell population.
II. Sequential targeting
In many cases, the targeting vector does not possess the fortuitous property of being able to traverse the BBB and reach the secondary target as was the case for the TfR examples above. However, it is still possible to accomplish secondary targeting by endowing the BBB vector-therapeutic conjugate with an additional targeting moiety. The first targeting agent would allow RMT across the BBB while the second targeting agent would discriminate the site of action within the central nervous system. Proof-of-principle experiments have supported the feasibility of this strategy. For instance, human U87 glial brain tumors were implanted in mouse brains, and mice were injected weekly with PEGylated liposomes containing either a nonviral expression plasmid encoding antisense mRNA against the human epidermal growth factor receptor (EGFR) gene (87) or an expression plasmid encoding a short interfering hairpin RNA (RNAi) directed against the human EGFR gene (88). The liposomes were decorated with both the anti-TfR 8D3 and the anti-HIR 83-14 antibodies. The 8D3 targeted the liposome through the mouse BBB while the anti-HIR 83-14 MAb targeted the human glioma cells and mediated endocytosis of the gene therapeutics (Figure 3). Continuing weekly injection of the antisense gene therapy increased the survival time by 100% (87), and the RNAi gene therapy increased survival time by 88% (88).
III. Selective action
It is also possible to gain a measure of secondary targeting by designing the therapeutic moiety to act selectively on a subset of cells. Gene therapeutics are particularly amenable to this approach as they can be customized using gene promoter elements that are cell-type specific thereby restricting gene expression to the cell type of interest. As an example, plasmid DNA encoding the tyrosine hydroxylase gene was loaded into PEGylated immunoliposomes decorated with the OX26 MAb for BBB transport and brain cell uptake by the TfR (85). The TH gene was designed to be under the control of the brain-restrictive GFAP promoter, so that liver expression of TH was eliminated. In addition, although the GFAP promoter drives expression in astrocytes and neurons, only the target population of nigral-striatal neurons produce the necessary cofactor for TH activity (Figure 3). In this way, introducing the gene-loaded immunoliposomes into a rat model of Parkinson’s disease had the effect of restoring TH activity solely in the target neuron population, and a reduction in disease symptoms was observed. Finally, a particularly elegant strategy that combines components of sequential targeting with selective action was recently demonstrated (89). First, a bifunctional fusion protein was engineered and consisted of an anti-beta amyloid scFv fused directly to the 83-14 MAb. The 83-14 portion mediated brain uptake, while the secondary targeting scFv portion was designed to bind and disaggregate amyloid plaques within the brain tissue. The selectivity arose from the constant regions (Fc) of the MAb. Since the neonatal Fc receptor is expressed at the BBB and operates in the efflux of MAb in the brain to blood direction, it was hypothesized that any amyloid bound by the scFv portion of the fusion protein could be actively carried out of the brain. In this way, it was shown that direct intracerebral injection of the fusion protein reduced the plaque burden, presumably by neonatal Fc receptor efflux of fusion protein with bound beta amyloid fragments (89).
CONCLUSIONS AND PROSPECTS
Throughout this review, we have defined the requirements of RMT-based delivery of brain therapeutics. These included choosing an appropriate RMT system and BBB-targeting vector, identifying a means of linking drug to the targeting vector, and taking into consideration requirements for secondary targeting within the CNS. Clearly the transferrin and insulin receptor approaches are currently the most well developed, but the research community continues to identify and develop alternative RMT-based transport systems. Ultimately, there is likely to be a host of options, each having its respective niche in terms of selectivity, capacity, and immunotolerance. As a measure of the promise of RMT-based CNS drug delivery methods, companies such as ArmaGen Technologies are in the process of further developing the anti-insulin receptor system for the treatment of stroke. In addition, the RAP-therapeutic protein fusion system is currently being developed by Raptor Pharmaceuticals, Inc. for the delivery of therapeutic proteins. Finally, the development of an RMT-based delivery system capable of overcoming the BBB requires expertise in BBB transport, pharmacokinetics, antibody engineering, and materials science. Thus, interdisciplinary efforts will likely be required to convert the “promise” of RMT approaches to “FDA-approved” therapeutics for noninvasive drug delivery to the brain.
Acknowledgments
This work was in part funded by National Institutes of Health Grant NS052649. A.R.J. is the recipient of a National Science Foundation Graduate Research Fellowship.
ABBREVIATIONS
- ADR
adriamycin
- AEM
analytical electron microscopy
- AUC
area under the curve
- Av
avidin
- AZT
azidothymidine
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- DSPE
distearoylphosphatidylethanolamine
- EGFR
human epidermal growth factor receptor
- FGF-2
fibroblast growth factor-2
- GFAP
glial fibrillary acidic protein
- GUS
β-glucuronidase
- HB-EGF
heparin binding epidermal growth factor-like growth factor
- HCEC
human cerebromicrovascular endothelial cells
- HD
Huntington’s disease
- HIR
human insulin receptor
- HRP
horseradish peroxidase
- IGF-II
insulin-like growth factor II
- LDL
low density lipoprotein
- LRP1/2
Low density lipoprotein receptor-related protein 1/2
- M6P
mannose 6-phosphate
- MAb
monoclonal antibody
- MCAO
middle cerebral artery occlusion
- NGF
nerve growth factor
- NHS
N-hydroxysuccinimide
- ODN
oligonucleotides
- PBCA
poly(butyl cyanoacrylate)
- PEG
poly(ethylene glycol)
- P-GUS
phosphorylated β-glucuronidase
- PNA
peptide nucleic acid
- RAP
receptor-associated protein
- RES
reticuloendothelial system
- RMT
receptor-mediated transcytosis
- rsCD4
recombinant human soluble CD4
- SA/B
streptavidin/ biotin
- SATA
N-Succinimidyl S-acetylthioacetate
- scFv
single-chain variable fragment
- sdAb
single-domain antibodies
- SMCC
Succinimidyl 4-[N-maleimidomethyl]-cyclohexane-1-carboxylate
- SV40
simian virus 40
- Tf
transferrin
- TfR
transferrin receptor
- TH
tyrosine hydroxylase
- tPA
tissue-type plasminogen activator
- VIP
vasoactive intestinal peptide
References
- 1.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6:494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. Brain Drug Targeting; the Future of Brain Drug Development. Cambridge UK: Cambridge University Press; 2001. [Google Scholar]
- 3.Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Delivery Rev. 2001;46:247–279. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 4.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 5.Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420:32–38. doi: 10.1016/0006-8993(87)90236-8. [DOI] [PubMed] [Google Scholar]
- 6.Golden PL, Maccagnan TJ, Pardridge WM. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Invest. 1997;99:14–18. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown VI, Greene MI. Molecular and Cellular Mechanisms of Receptor-Mediated Endocytosis. DNA Cell Biol. 1991;10:399–409. doi: 10.1089/dna.1991.10.399. [DOI] [PubMed] [Google Scholar]
- 8.Pardridge WM. Peptide Drug Delivery to the Brain. New York: Raven Press; 1991. [Google Scholar]
- 9.Lee HJ, Pardridge WM. Pharmacokinetics and delivery of tat and tat-protein conjugates to tissues in vivo. Bioconjug Chem. 2001;12:995–999. doi: 10.1021/bc0155061. [DOI] [PubMed] [Google Scholar]
- 10.Pardridge WM. Vector-mediated drug delivery to the brain. Advanced Drug Delivery Reviews. 1999;36:299–321. doi: 10.1016/s0169-409x(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 11.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Controlled Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 12.Koziara JM, Lockman PR, Allen DD, Mumper RJ. The blood-brain barrier and brain drug delivery. J Nanosci Nanotechnol. 2006;6:2712–2735. doi: 10.1166/jnn.2006.441. [DOI] [PubMed] [Google Scholar]
- 13.Wu DF, Pardridge WM. Central nervous system pharmacologic effect in conscious rats after intravenous injection of a biotinylated vasoactive intestinal peptide analog coupled to a blood-brain barrier drug delivery system. J Pharmacol Exp Ther. 1996;279:77–83. [PubMed] [Google Scholar]
- 14.Pardridge WM, Wu DF, Sakane T. Combined use of carboxyl-directed protein pegylation and vector-mediated blood-brain barrier drug delivery system optimizes brain uptake of brain-derived neurotrophic factor following intravenous administration. Pharm Res. 1998;15:576–582. doi: 10.1023/a:1011981927620. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Schlachetzki F, Pardridge WM. Global non-viral gene transfer to the primate brain following intravenous administration. Mol Ther. 2003;7:11–18. doi: 10.1016/s1525-0016(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 16.Cerletti A, Drewe J, Fricker G, Eberle AN, Huwyler J. Endocytosis and transcytosis of an immunoliposome-based brain drug delivery system. J Drug Targeting. 2000;8:435–436. doi: 10.3109/10611860008997919. [DOI] [PubMed] [Google Scholar]
- 17.Shi N, Boado RJ, Pardridge WM. Receptor-mediated gene targeting to tissues in vivo following intravenous administration of pegylated immunoliposomes. Pharm Res. 2001;18:1091–1095. doi: 10.1023/a:1010910523202. [DOI] [PubMed] [Google Scholar]
- 18.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, Martin FJ. Sterically stabilized liposomes improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroder U, Sabel BA. Nanoparticles, a drug carrier system to pass the blood-brain barrier, permit central analgesic effects of i.v. dalargin injections. Brain Res. 1996;710:121–124. doi: 10.1016/0006-8993(95)01375-x. [DOI] [PubMed] [Google Scholar]
- 20.Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16:1564–1569. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 21.Steiniger SCJ, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, Severin SE, Uhi R, Kock M, Geiger KD, Gelperina SE. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109:759–767. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 22.Ambruosi A, Yamamoto H, Kreuter J. Body distribution of polysorbate-80 and doxorubicin-loaded [C-14]poly(butyl cyanoacrylate) nanoparticles after i.v. administration in rats. J Drug Targeting. 2005;13:535–542. doi: 10.1080/10611860500411043. [DOI] [PubMed] [Google Scholar]
- 23.Ambruosi A, Khalansky AS, Yamamoto H, Gelperina SE, Begley DJ, Kreuter J. Biodistribution of polysorbate 80-coated doxorubicin-loaded [C-14]-poly(butyl cyanoacrylate) nanoparticles after intravenous administration to glioblastoma-bearing rats. J Drug Targeting. 2006;14:97–105. doi: 10.1080/10611860600636135. [DOI] [PubMed] [Google Scholar]
- 24.Alyautdin RN, Petrov VE, Langer K, Berthold A, Kharkevich DA, Kreuter J. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm Res. 1997;14:325–328. doi: 10.1023/a:1012098005098. [DOI] [PubMed] [Google Scholar]
- 25.Gao K, Jiang X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int J Pharm. 2006;310:213–219. doi: 10.1016/j.ijpharm.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Targeting. 2002;10:317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier transferrin receptor. Metabolism. 1987;36:892–895. doi: 10.1016/0026-0495(87)90099-0. [DOI] [PubMed] [Google Scholar]
- 28.Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2000;20:77–95. doi: 10.1023/a:1006948027674. [DOI] [PubMed] [Google Scholar]
- 29.Qian ZM, Li HY, Sun HZ, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 30.Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res Dev Brain Res. 1990;55:35–42. doi: 10.1016/0165-3806(90)90103-6. [DOI] [PubMed] [Google Scholar]
- 31.Crowe A, Morgan EH. Iron and transferrin uptake by brain and cerebrospinal fluid in the rat. Brain Res. 1992;592:8–16. doi: 10.1016/0006-8993(92)91652-u. [DOI] [PubMed] [Google Scholar]
- 32.Morgan EH, Moos T. Mechanism and developmental changes in iron transport across the blood-brain barrier. Dev Neurosci. 2002;24:106–113. doi: 10.1159/000065699. [DOI] [PubMed] [Google Scholar]
- 33.Shin SU, Friden P, Moran M, Olson T, Kang YS, Pardridge WM, Morrison SL. Transferrin-antibody fusion proteins are effective in brain targeting. Proc Natl Acad Sci USA. 1995;92:2820–2924. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra V, Mahor S, Rawat A, Gupta PN, Dubey P, Khatri K, Vyas SP. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J Drug Targeting. 2006;14:45–53. doi: 10.1080/10611860600612953. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther. 2000;292:1048–1052. [PubMed] [Google Scholar]
- 36.Zhang Y, Pardridge WM. Blood-brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion. Brain Res. 2006;1111:227–229. doi: 10.1016/j.brainres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Lyons MK, Anderson RE, Meyer FB. Basic fibroblast growth factor promotes in vivo cerebral angiogenesis in chronic forebrain ischemia. Brain Res. 1991;558:315–320. doi: 10.1016/0006-8993(91)90784-s. [DOI] [PubMed] [Google Scholar]
- 38.Song BW, Vinters HV, Wu DF, Pardridge WM. Enhanced neuroprotective effects of basic fibroblast growth factor in regional brain ischemia after conjugation to a blood-brain barrier delivery vector. J Pharmacol Exp Ther. 2002;301:605–610. doi: 10.1124/jpet.301.2.605. [DOI] [PubMed] [Google Scholar]
- 39.Kordower JH, Charles V, Bayer R, Bartus RT, Putney S, Walus LR, Friden PM. Intravenous administration of a transferrin receptor antibody nerve growth-factor conjugate prevents the degeneration of cholinergic striatal neurons in a model of Huntington disease. Proc Natl Acad Sci USA. 1994;91:9077–9080. doi: 10.1073/pnas.91.19.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huwyler J, Wu DF, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA. 1996;93:14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huwyler J, Yang J, Pardridge WM. Receptor mediated delivery of daunomycin using immunoliposomes: Pharmacokinetics and tissue distribution in the rat. J Pharmacol Exp Ther. 1997;282:1541–1546. [PubMed] [Google Scholar]
- 42.Boado RJ, Tsukamoto H, Pardridge WM. Drug delivery of antisense molecules to the brain for treatment of Alzheimer’s disease and cerebral AIDS. J Pharm Sci. 1998;87:1308–1315. doi: 10.1021/js9800836. [DOI] [PubMed] [Google Scholar]
- 43.Shi N, Boado RJ, Pardridge WM. Antisense imaging of gene expression in the brain in vivo. Proc Natl Acad Sci USA. 2000;97:14709–14714. doi: 10.1073/pnas.250332397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki T, Wu DF, Schlachetzki F, Li JY, Boado RJ, Pardridge WM. Imaging endogenous gene expression in brain cancer in vivo with In-111-peptide nucleic acid antisense radiopharmaceuticals and brain drug-targeting technology. J Nucl Med. 2004;45:1766–1775. [PubMed] [Google Scholar]
- 45.Penichet ML, Kang YS, Pardridge WM, Morrison SL, Shin SU. An antibody-avidin fusion protein specific for the transferrin receptor serves as a delivery vehicle for effective brain targeting: Initial applications in anti-HIV antisense drug delivery to the brain. J Immunol. 1999;163:4421–4426. [PubMed] [Google Scholar]
- 46.Shi NY, Zhang Y, Zhu CN, Boado RJ, Pardridge WM. Brain-specific expression of an exogenous gene after i.v. administration. Proc Natl Acad Sci USA. 2001;98:12754–12759. doi: 10.1073/pnas.221450098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Pardridge WM. Delivery of beta-galactosidase to mouse brain via the blood-brain barrier transferrin receptor. J Pharmacol Exp Ther. 2005;313:1075–1081. doi: 10.1124/jpet.104.082974. [DOI] [PubMed] [Google Scholar]
- 48.Lee HJ, Boado RJ, Braasch DA, Corey DR, Pardridge WM. Imaging gene expression in the brain in vivo in a transgenic mouse model of Huntington’s disease with an antisense radiopharmaceutical and drug-targeting technology. J Nucl Med. 2002;43:948–956. [PubMed] [Google Scholar]
- 49.Lee HJ, Zhang Y, Zhu CN, Duff K, Pardridge WM. Imaging brain amyloid of Alzheimer disease in vivo in Transgenic mice with an A beta peptide radiopharmaceutical. J Cereb Blood Flow Metab. 2002;22:223–231. doi: 10.1097/00004647-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Friden PM, Walus LR, Musso GF, Taylor MA, Malfroy B, Starzyk RM. Antitransferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc Natl Acad Sci USA. 1991;88:4771–4775. doi: 10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friden PM, Olson TS, Obar R, Walus LR, Putney SD. Characterization, receptor mapping and blood-brain barrier transcytosis of antibodies to the human transferrin receptor. J Pharmacol Exp Ther. 1996;278:1491–1498. [PubMed] [Google Scholar]
- 52.Havrankova J, Brownstein M, Roth J. Insulin and insulin receptors in rodent brain. Diabetologia. 1981;20:268–273. [PubMed] [Google Scholar]
- 53.Smith MW, Gumbleton M. Endocytosis at the blood-brain barrier: From basic understanding to drug delivery strategies. J Drug Targeting. 2006;14:191–214. doi: 10.1080/10611860600650086. [DOI] [PubMed] [Google Scholar]
- 54.Gaillard PJ, Visser CC, de Boer AG. Targeted delivery across the blood-brain barrier. Expert Opin Drug Deliv. 2005;2:299–309. doi: 10.1517/17425247.2.2.299. [DOI] [PubMed] [Google Scholar]
- 55.Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, Gray A, Coussens L, Liao YC, Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- 56.Pardridge WM, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transctosis through the blood-brain barrier. Pharm Res. 1995;12:807–816. doi: 10.1023/a:1016244500596. [DOI] [PubMed] [Google Scholar]
- 57.Coloma MJ, Lee HJ, Kurihara A, Landaw EM, Boado RJ, Morrison SL, Pardridge WM. Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res. 2000;17:266–274. doi: 10.1023/a:1007592720793. [DOI] [PubMed] [Google Scholar]
- 58.Boado RJ, Zhang YF, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood-brain barrier. Biotechnol Bioeng. 2007;96:381–391. doi: 10.1002/bit.21120. [DOI] [PubMed] [Google Scholar]
- 59.Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA. 1984;81:6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36:3–10. doi: 10.1016/j.ymeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Wu D, Yang J, Pardridge WM. Drug targeting of a peptide radiopharmaceutical through the primate blood-brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J Clin Invest. 1997;100:1804–1812. doi: 10.1172/JCI119708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Schlachetzki F, Li JY, Boado RJ, Pardridge WM. Organ-specific gene expression in the rhesus monkey eye following intravenous non-viral gene transfer. Mol Vis. 2003;9:465–472. [PubMed] [Google Scholar]
- 63.Boado RJ, Zhang Y, Pardridge WM. Genetic engineering, expression, and activity of a fusion protein of a human neurotrophin and a molecular Trojan horse for delivery across the human blood-brain barrier. Biotechnol Bioeng. 2007 doi: 10.1002/bit.21369. [DOI] [PubMed] [Google Scholar]
- 64.Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogler C, Levy B, Galvin NJ, Thorpe C, Sands MS, Barker JE, Baty J, Birkenmeier EH, Sly WS. Enzyme replacement in murine mucopolysaccharidosis type VII: Neuronal and glial response to beta-glucuronidase requires early initiation of enzyme replacement therapy. Pediatr Res. 1999;45:838–844. doi: 10.1203/00006450-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Demeule M, Poirier J, Jodoin J, Bertrand Y, Desrosiers RR, Dagenais C, Nguyen T, Lanthier J, Gabathuler R, Kennard M, Jefferies WA, Karkan D, Tsai S, Fenart L, Cecchelli R, Beliveau R. High transcytosis of melanotransferrin (P97) across the blood brain barrier. J Neurochem. 2002;83:924–933. doi: 10.1046/j.1471-4159.2002.01201.x. [DOI] [PubMed] [Google Scholar]
- 67.Gabathuler R, Arthur G, Kennard M, Chen Q, Tsai S, Yang J, Schoorl W, Vitalis TZ, Jeffereies WA. Development of a potential protein vector (NeuroTrans) to deliver drugs across to the blood-brain barrier. Int Congres Series. 2005;1277:171–184. [Google Scholar]
- 68.Pan WH, Kastin AJ, Zankel TC, van Kerkhof P, Terasaki T, Bu GJ. Efficient transfer of receptor-associated protein (RAP) across the blood-brain barrier. J Cell Sci. 2004;117:5071–5078. doi: 10.1242/jcs.01381. [DOI] [PubMed] [Google Scholar]
- 69.Sun W, Wang H, Xie C, Hu Y, Yang X, Xu H. An attempt to directly trace polymeric nanoparticles in vivo with electron microscopy. J Controlled Release. 2006;115:259–265. doi: 10.1016/j.jconrel.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Olivier JC. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2:108–119. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaillard P, Brink A, de Boer AG. Diphtheria toxin receptor-targeted brain drug delivery. Int Congres Series. 2005;1277:185–198. [Google Scholar]
- 72.Anderson P, Pichichero ME, Insel RA. Immunogens consisting of oligosaccharides from the capsule of Haemophilusinfluenzae type-b coupled to diphtheria toxoid or the toxin protein CRM197. J Clin Invest. 1985;76:52–59. doi: 10.1172/JCI111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buzzi S, Rubboli D, Buzzi G, Buzzi AM, Morisi C, Pironi F. CRM197 (nontoxic diphtheria toxin): effects on advanced cancer patients. Cancer Iimmunol Immunother. 2004;53:1041–1048. doi: 10.1007/s00262-004-0546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang YS, Bickel U, Pardridge WM. Pharmacokinetics and saturable blood-brain barrier transport of biotin bound to a conjugate of avidin and a monoclonal antibody to the transferrin receptor. Drug Metab Dispos. 1994;22:99–105. [PubMed] [Google Scholar]
- 75.Pissarra N. Changes in the business of culture. Nat Biotechnol. 2004;22:1355–1356. doi: 10.1038/nbt1104-1355. [DOI] [PubMed] [Google Scholar]
- 76.Muruganandam A, Tanha J, Narang S, Stanimirovic D. Selection of phage-displayed llama single-domain antibodies that transmigrate across human blood-brain barrier endothelium. FASEB J. 2002;16:240–242. doi: 10.1096/fj.01-0343fje. [DOI] [PubMed] [Google Scholar]
- 77.Abulrob A, Sprong H, Henegouwen P, Stanimirovic D. The blood-brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem. 2005;95:1201–1214. doi: 10.1111/j.1471-4159.2005.03463.x. [DOI] [PubMed] [Google Scholar]
- 78.Cortez-Retamozo V, Backmann N, Senter PD, Wernery U, De Baetselier P, Muyldermans S, Revets H. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 2004;64:2853–2857. doi: 10.1158/0008-5472.can-03-3935. [DOI] [PubMed] [Google Scholar]
- 79.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 80.Wang XX, Cho YK, Shusta EV. Mining a yeast library for brain endothelial cell-binding antibodies. Nat Methods. 2007;4:143–145. doi: 10.1038/nmeth993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giometto B, Bozza F, Argentiero V, Gallo P, Pagni S, Piccinno MG, Tavolato B. Transferrin receptors in rat central nervous system. An immunocytochemical study. J Neurol Sci. 1990;98:81–90. doi: 10.1016/0022-510x(90)90183-n. [DOI] [PubMed] [Google Scholar]
- 82.Mash DC, Pablo J, Buck BE, Sanchezramos J, Weiner WJ. Distribution and number of transferrin receptors in Parkinson’s disease and in MPTP-treated mice. Exp Neurol. 1991;114:73–81. doi: 10.1016/0014-4886(91)90086-r. [DOI] [PubMed] [Google Scholar]
- 83.Kratz F, Beyer U. Serum proteins as drug carriers of anticancer agents: A review. Drug Delivery. 1998;5:281–299. doi: 10.3109/10717549809065759. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Calon F, Zhu CN, Boado RJ, Pardridge WM. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum Gene Ther. 2003;14:1–12. doi: 10.1089/10430340360464660. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Schlachetzki F, Zhang YF, Boado RJ, Pardridge WM. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum Gene Ther. 2004;15:339–350. doi: 10.1089/104303404322959498. [DOI] [PubMed] [Google Scholar]
- 86.Haavik J, Toska K. Tyrosine hydroxylase and Parkinson’s disease. Mol Neurobiol. 1998;16:285–309. doi: 10.1007/BF02741387. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Zhu CN, Pardridge WM. Antisense gene therapy of brain cancer with an artificial virus gene delivery system. Mol Ther. 2002;6:67–72. doi: 10.1006/mthe.2002.0633. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Zhang YF, Bryant J, Charles A, Boado RJ, Pardridge WM. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res. 2004;10:3667–3677. doi: 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- 89.Boado RJ, Zhang Y, Xia CF, Pardridge WM. Fusion antibody for Alzheimer’s disease with bidirectional transport across the blood-brain barrier and abeta fibril disaggregation. Bioconjug Chem. 2007;18:447–455. doi: 10.1021/bc060349x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyajima Y, Nakamura H, Kuwata Y, Lee JD, Masunaga S, Ono K, Maruyama K. Transferrin-loaded nido-carborane liposomes: Tumor-targeting boron delivery system for neutron capture therapy. Bioconjug Chem. 2006;17:1314–1320. doi: 10.1021/bc060064k. [DOI] [PubMed] [Google Scholar]
- 91.Walus LR, Pardridge WM, Starzyk RM, Friden PM. Enhanced uptake of rsCD4 across the rodent and primate blood-brain barrier after conjugation to anti-transferrin receptor antibodies. J Pharmacol Exp Ther. 1996;277:1067–1075. [PubMed] [Google Scholar]
- 92.Bickel U, Yoshikawa T, Landaw EM, Faull KF, Pardridge WM. Pharmacological effects in vivo in brain by vector-mediated peptide drug delivery. Proc Natl Acad Sci USA. 1993;90:2618–2622. doi: 10.1073/pnas.90.7.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saito Y, Buciak J, Yang J, Pardridge WM. Vector-mediated delivery of I-125 labeled beta-amyloid peptide A-beta(1–40) through the blood-brain barrier and binding to Alzheimer Disease amyloid of the A-beta(1–40)/vector complex. Proc Natl Acad Sci USA. 1995;92:10227–10231. doi: 10.1073/pnas.92.22.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Pardridge WM. Neuroprotection in transient focal brain ischemia after delayed intravenous administration of brain-derived neurotrophic factor conjugated to a blood-brain barrier drug targeting system. Stroke. 2001;32:1378–1383. doi: 10.1161/01.str.32.6.1378. [DOI] [PubMed] [Google Scholar]
- 95.Kurihara A, Deguchi Y, Pardridge WM. Epidermal growth factor radiopharmaceuticals: In-111 chelation, conjugation to a blood-brain barrier delivery vector via a biotin-polyethylene linker, pharacokinetics, and in vivo imaging of experimental brain tumors. Bioconjug Chem. 1999;10:502–511. doi: 10.1021/bc980123x. [DOI] [PubMed] [Google Scholar]
- 96.Wu DF, Song BW, Vinters HV, Pardridge WM. Pharmacokinetics and brain uptake of biotinylated basic fibroblast growth factor conjugated to a blood-brain barrier drug delivery system. J Drug Targeting. 2002;10:239–245. doi: 10.1080/10611860290022679. [DOI] [PubMed] [Google Scholar]
- 97.Kreuter J, Ramge P, Petrov V, Hamm S, Gelperina SE, Engelhardt B, Alyautdin R, von Briesen H, Begley DJ. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res. 2003;20:409–416. doi: 10.1023/a:1022604120952. [DOI] [PubMed] [Google Scholar]
- 98.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674:171–174. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 99.Soni S, Babbar AK, Sharma RK, Maitra A. Delivery of hydrophobised 5-fluorouracil derivative to brain tissue through intravenous route using surface modified nanogels. J Drug Targeting. 2006;14:87–95. doi: 10.1080/10611860600635608. [DOI] [PubMed] [Google Scholar]
- 100.Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, Kreuter J, Langer K. Covalent linkage of apolipoprotein E to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–1253. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- 101.Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu GJ, Cecchelli R, Touzani O, Vivien D. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]