Abstract
Neuroglobin (Ngb) is a recently discovered protein that shows only minor sequence similarity with myoglobin and hemoglobin but conforms to the typical 3-over-3 α-helical fold characteristic of vertebrate globins. An intriguing feature of Ngb is its heme hexacoordination in the absence of external ligands, observed both in the ferrous and ferric (met) forms. In Ngb, the imidazole of a histidine residue (His-64) in the distal position, above the heme plane, provides the sixth coordination bond. In this work, a valine residue was introduced at position 64 (H64V variant) to clarify the possible role(s) of the distal residue in protecting the heme iron of Ngb from attack by strong oxidants. SDS-PAGE analyses revealed that the oxidation of the H64V variant of metNgb by H2O2 resulted in the formation of dimeric and trimeric products in contrast to the native protein. Dityrosine cross-links were shown by their fluorescence to be present in the oligomeric products. When the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was included in the reaction mixture, nitrone adducts were detected by immuno-spin trapping. The specific location of the DMPO adducts on the H64V variant protein was determined by a mass spectrometry method that combines off-line immuno-spin trapping and chromatographic procedures. This method revealed Tyr-88 to be the site of modification by DMPO. The presence of His-64 in the wild-type protein results in the nearly complete loss of detectable radical adducts. Together, the data support the argument that wild-type Ngb is protected from attack by H2O2 by the coordinated distal His.
Neuroglobin (Ngb) is a recently discovered protein that belongs to the globin family of proteins (1). Like other globins, Ngb reversibly binds small ligands such as dioxygen (O2), nitric oxide (NO), and carbon monoxide (CO) via an iron (Fe2+) ion of the heme group (2, 3). Comparison of Ngb amino acid sequences with other vertebrate globins such as myoglobins (Mb) and hemoglobins (Hb) shows only minor similarity (<21 % and <25 % identity, respectively) (1, 4). Nevertheless, X-ray crystallographic studies performed on both human and murine Ngbs clearly indicate that the two proteins conform to the canonical 3-over-3 α-helical fold characteristic of vertebrate globins (5, 6). Ngb was initially discovered in the human and murine brains, and, since then, it has been identified in a wide range of vertebrate species (1, 7-11). In the rat, Ngb is expressed in the cerebral cortex, hippocampus, thalamus, hypothalamus, and cerebellum of the brain (9, 12). Ngb is also found in nonneural cells of the endocrine system such as the pituitary gland, the adrenal gland and the testis (1, 10).
A distinguishing characteristic of Ngb is its heme hexacoordination in the absence of external ligands, observed both in the ferrous and ferric (Fe3+, met) forms (2, 5, 6). This contrasts with the heme environment of Hb and Mb showing pentacoordination in the ferrous form and distal water coordination for the ferric form of the protein. In Ngb, the imidazole of a histidine residue (His-64) provides the sixth coordination bond, which needs to thermally dissociate from the heme iron before an external ligand can bind. Rapid mixing and flash photolysis experiments have demonstrated that the overall affinity constant for O2 or other exogenous ligands results from a competition with the internal ligand, the His-64 residue (2, 3, 13). Therefore, ligand affinity is not modulated exclusively by the on and off rate constants to the pentacoordinate state.
An additional unusual feature of Ngb that distinguishes this protein from Mb and Hb is the presence of a wide internal cavity (about 120 Å3 for human Ngb (5)) enclosing part of the heme distal site. This peculiarity was first observed in 3D structures of human and murine Ngb in their ferric form (5, 6). Later, the determination of the murine Ngb structure in the CO-ligated state (14) revealed an unprecedented heme-sliding mechanism to bind the CO ligand. In this mechanism, His-64 essentially retains its position, but the docking of the heme in a new position, deeper inside the internal cavity, creates enough space at the sixth heme coordination site to accommodate the external ligand.
In addition to this heme-sliding mechanism, another component of Ngb that may also affect its ability to bind exogenous ligands is the oxidation state of two reactive thiols (Cys-46 and Cys-55, in human Ngb). Formation of an intramolecular disulfide bond between these two cysteine residues increases O2 affinity up to 10-fold, presumably by making the heme pocket more accessible to the solvent (15). However, the general physiological relevance of this observation is questionable because Cys-46 is absent in other mammalian Ngbs (16).
The general physiological functions of pentacoordinated vertebrate globins are well defined. In man and other vertebrates, Hb is localized to erythrocytes and transports O2 between the lungs and other tissues (17). Mb, typically found in skeletal and cardiac myocytes, facilitates O2 diffusion or acts as an NO oxygenase, converting toxic NO to the innocuous nitrate (18). However, the precise physiological functions of Ngb and most other newly discovered hexacoordinated globins are, as yet, unclear. It seems unlikely that Ngb functions as an oxygen storage or delivery protein in the brain because of its fairly low concentration in the neurons (∼ 1 μM) (1) and its weak O2-binding affinity (P50 ∼ 7.5 Torr) (19). However, this function is not yet excluded in the mammalian retina, where particularly high Ngb concentrations (∼ 100 μM) have been reported (20). Other potential functions for Ngb include a role as a sensor for O2/NO levels (21), as a scavenger of reactive oxygen species, nitric oxide and other reactive nitrogen species (19, 22, 23) and/or a guanine nucleotide dissociation inhibitor (24). Each hypothesis is supported by some evidence, but no clear-cut picture has yet emerged. It has been reported that expression of Ngb is upregulated under hypoxic and ischemic stress and has neuroprotective functions both in vitro and in vivo (25-29). However, the precise mechanism by which Ngb protects neurons against hypoxic or ischemic injury remains unclear.
In the presence of oxygen, the ferrous form of the pentacoordinated vertebrate globins, hemoglobin and myoglobin, is unstable and continuously undergoes a spontaneous oxidation (autoxidation), producing the ferric form and a superoxide radical (30, 31). Dismutation of the superoxide radical generates O2 and H2O2. The latter is a potent two-electron oxidizing agent that can react further with both the ferrous and ferric forms of those globins to give a spectroscopically detectable ferryl (FeIV=O) species and, in the case of the ferric form, a transient protein radical (32, 33). The globin radical can be directly observed by EPR (32, 33), can be spin-trapped (34, 35), and, in the case of some hemoglobins and the myoglobin of sperm whales (36, 37), gives rise to dityrosine cross-linked oligomers. These studies have identified protein radicals on a variety of amino acid residues, primarily tyrosine (34, 38, 39), tryptophan (40), histidine (38, 39) and cysteine (38, 39, 41).
Spectroscopic studies with human and mouse recombinant Ngb show that the autoxidation of this type of protein is high as compared with hemoglobin and myoglobin (2, 19, 42). However, the ferric form of Ngb does not react with the oxidizing agents H2O2 and peroxynitrite and, hence, does not produce the highly reactive ferryl species and a transient protein radical (22). The purpose of the present investigation is to clarify the possible role(s) of the distal histidine residue which provides the sixth coordination bond, in inhibiting these reactions.
EXPERIMENTAL PROCEDURES
Materials
Horse heart metmyoglobin (HoMb) acquired from USB (Cleveland, OH) was purified before use by passage through a Sephadex G-25 gel filtration column (GE Healthcare Bio-Sciences, Piscataway, NJ) and elution with 50 mM potassium phosphate buffer. HoMb concentrations were determined by measuring the absorbance of the heme Soret band using εMetMb,408 = 188 mM-1cm-1 (43). Trypsin (from bovine pancreas, modified, sequencing grade) and catalase from beef liver (suspension, 64000 units mg) were obtained from Roche Molecular Biochemicals (Indianapolis, IN). Gelatin from cold water fish skin was obtained from Sigma (St Louis, MO). DMPO (high purity, ≥ 99%) from Alexis Biochemicals (San Diego, CA) was sublimed twice under vacuum at room temperature and stored under an argon atmosphere at -70 °C before use. The DMPO concentration was measured at 228 nm, assuming a molar extinction coefficient of 7800 M-1cm-1 (44). All aqueous solutions were prepared using water passed through a Picopure 2UV Plus system (Hydro Services and Supplies, Inc, RTP, NC) equipped with a 0.2 μm pore size filter. Diluted H2O2 solutions, obtained from a 30% solution (Fisher Scientific Co., Fairlawn, NJ), were used within 1 h of preparation. The H2O2 concentration was confirmed by absorbance measurements at 240 nm (εH2O2, 240 = 39.4 M-1 cm-1) (45). All other chemicals were of analytical grade and were purchased from Sigma (St Louis, MO) or Roche Molecular Biochemicals (Indianapolis, IN). Absorption spectra were recorded on a Cary 100 UV-visible spectrometer (Varian, Victoria, Australia).
Preparation of Recombinant Proteins
The original construct of Ngb (cDNA of human Ngb in pET3a) was a generous gift from Dr. Tilmann Laufs and Prof. Thorsten Burmester. The full-length cDNA sequence of Ngb was subcloned into the Nde I and Hind III sites of vector pUC18 for prokaryotic expression. The two primers flanking the human neuroglobin gene were 5′-GGAATTCCATATGGAGCGCCCGGAGCCCGAGCTGATCCG-3′ (forward primer) and 5′-CCCAAGCTTTTACTCGCCATCCCAGC-3′ (reverse primer) with NdeI and Hind III restriction sites underlined. PCR was carried out with a GeneAmp 9700 DNA thermal cycler (Applied Biosystems, Foster City, CA) using PfuTurbo DNA polymerase (Stratagene, La Jolla, CA). The PCR reaction was checked by agarose electrophoresis, and the amplified DNA fragment was purified with a QIAquick PCR purification kit (Qiagen Inc., Valencia, CA). The purified DNA fragment and pUC18 were digested separately with Nde I and Hind III and were ligated. The final plasmid was verified by DNA sequencing using BigDye terminator cycle sequencing and an ABI Model 3100 Sequencer (Applied Biosystems). The ligated plasmid was then used to transform Escherichia coli DH5α.
Cells were grown at 37 °C in Luria-Bertani medium containing 100 μg/mL ampicillin. After 18 hours, the cells were harvested by centrifugation. All purification steps described below were performed at 4 °C. The cell pellets were resuspended in a 4-fold excess (with respect to the initial weight of cells) of lysis buffer (50 mM Tris-HCl buffer, pH 8.0, containing 1 mM EDTA, 1 mM dithiothreitol, 0.1 mM phenylmethanesulfonyl fluoride, 4.5 μM leupeptin, 2.9 μM pepstatin, 3.3 μM antipain, 50 units of Dnase I/mL, 5 units of Rnase/mL and 5% lysozyme). The solution was stirred for 60 min, after which the cells were sonicated using a Branson sonifer (Danbury, CT). Cell debris was removed by centrifugation and fractionated by ammonium sulfate precipitation (first 30 % then 45 % saturation). The 45 % ammonium sulfate pellet containing the crude Ngb was resuspended in a minimal volume of 10 mM Tris-HCl buffer, pH 7.5 (typically 1-2 pellet volumes) and dialyzed overnight against the same buffer. The crude dialyzed protein solution was then loaded onto a DEAE D52 anion exchange column (Whatman, Maidstone, UK), washed and equilibrated in 10 mM Tris-HCl buffer, pH 7.5. After washing off unbound material, Ngb was eluted with 10 mM Tris-HCl buffer, pH 7.5, supplemented with 100 mM NaCl. The fractions containing the Ngb (as determined by absorbance at 400 nm) were pooled and concentrated by ultrafiltration using centricon YM-10 cartridges (Millipore Corporation, Bedford, USA; molecular weight cut off = 10,000). The protein was then further purified on a Sephacryl S-200 HR column (2.5 × 67 cm) (GE Healthcare Bio-Sciences, Piscataway, NJ). The column was equilibrated and separations were performed at a flow rate of 0.34 mL/min using 50 mM potassium phosphate buffer, pH 6.8. The fractions having the highest Ngb content (A412/A275 ≥ 2.9) were pooled and concentrated by ultrafiltration using centricon YM-10 cartridges. The purified protein was then stored at 4 °C in 50 mM potassium phosphate buffer, pH 6.8.
The expression of Ngb yields the protein in the Fe2+-O2 form. The met (Fe3+) form (metNgb) was obtained by treating the protein with a 20% excess of K3Fe(CN)6 and quickly passing the resulting solution over a Sephadex G-25 gel filtration column equilibrated with 50 mM potassium phosphate buffer, pH 6.8. Samples were stored at -70 °C. Once thawed, the protein was kept on ice until use. Except when noted, in all the experiments described below, the proteins (wild-type Ngb and H64V variant protein) were utilized in their met form. MetNgb concentrations were estimated by measuring the absorbance of the heme Soret band using ε 414 nm = 129 mM-1cm-1 (46).
Site-directed Mutagenesis
A QuickChange site-directed mutagenesis system (Stratagene, La Jolla, CA) was used to replace the distal His64 with Val64 in human Ngb. The primers were 5′-CGCCTGAGTTTCTGGACGTGATCAGGAAGGTGATGC-3′ (forward primer) and 5′-GCATCACCTTCCTGATCACGTCCAGAAACTCAGGCG-3′ (reverse primer) with the mutations underlined. The point mutation was confirmed by DNA sequencing. The recombinant mutant Ngb was subsequently expressed and purified as described above.
Oligomerization and SDS-PAGE of Protein Samples
Cross-linking experiments were performed by incubating solutions of either wild-type or H64V variant Ngb (protein concentration 100 μM, each in 50 mM potassium phosphate, pH 6.8) with 1 eq H2O2 at 25 °C. After 30 min, the reactions were terminated by addition of the SDS-PAGE sample buffer supplemented with 190 mM DTT (protein solution:sample buffer = ∼ 4 v/v, final concentration 38 mM for DTT and 80 μM for Ngb). Samples were allowed to stand in reducing sample buffer for 10 min and heated at 85 °C for 2 min before loading and separation onto 4-12% Bis-Tris NuPAGE (Invitrogen, Carlsbad, CA, USA) gels, which were stained with Coomassie Blue.
Fluorescence detection of dityrosine cross-links
Ngb solutions were prepared in potassium buffer and reacted with 1 eq H2O2 at 25 °C, as described above. After a 30 min incubation, the reaction mixtures were cooled to 4° C and acidified to pH 2.5 by the addition of concentrated HCl. The heme was then extracted with 1 volume of cold 2-butanone. The organic layer containing the extracted heme was discarded. The aqueous solution containing the apoprotein was filtered using a Sephadex G-25 gel filtration column previously equilibrated and eluted with 200 mM sodium bicarbonate buffer, pH 9.4. The resulting mixtures were then diluted with the same buffer to a concentration of 2.1 μM. After 315-nm excitation, the fluorescence intensities of the resulting mixtures were measured over a range of wavelengths that bracket 410 nm, which is the emission maximum of the Tyr-Tyr bond, using an FluoroLog 3 spectrofluorimeter (Horiba Jobin Yvon, Edison, NY). All measurements were performed at 25 °C in a 5 × 5 mm supracil fluorescence cuvette. Dityrosine standard was synthesized and purified as described previously (37). The purity of the compound was checked by reverse phase HPLC and mass spectrometry.
Sample Preparation for Proteolytic Digestion
A 150 μM Ngb solution was prepared in 50 mM potassium phosphate buffer, pH 6.8, containing 50 μM DTPA. The reaction was conducted at 25 °C using 100 mM DMPO and was initiated by the addition of 3 equivalents of H2O2. After a 30 sec incubation, the excess H2O2 was removed by adding catalase (250 units/ml). The resulting solution was cooled to 4° C and the heme was then extracted with 1 volume of cold 2-butanone, as described above. The aqueous solution containing the apoprotein was filtered using a Sephadex G-25 gel filtration column previously equilibrated and eluted with 100 mM Tris-HCl, pH 8.5. The fraction having the highest Ngb content (∼ 50 μM) was digested using a 20:1 substrate-to-trypsin ratio for 16 h at 37 °C. Reactions were stopped by injecting the final mixture directly onto a reverse phase high pressure liquid chromatography (RP-HPLC) column.
Fractionation of Protein Digest by Reverse Phase HPLC
All RP-HPLC experiments were performed on an Chemstation 1100 liquid chromatography system (Agilent Technologies, Palo Alto, CA), equipped with a control module, vacuum degasser, binary pump, manual injector, and diode-array UV-visible and fluorescence detectors. HPLC fractions were collected using a fraction collector (Model 2110, Bio-Rad, Richmond, CA). Digested samples (50 μL) were injected onto a Vydac 218TP54, 4.6 mm × 250 mm, C18 RP-HPLC column (Vydac, Hesperia, CA). Peptides were eluted using a flow rate of 0.8 mL/min and a linear gradient rising from 100% solvent A to 50% solvent B in 80 min: solvent A, 0.1% trifluoroacetic acid in water; solvent B, 0.085% trifluoroacetic acid in acetonitrile. A rapid gradient to 90% solvent B and then back to 100% solvent A was used to regenerate the column. The eluent was monitored with a UV-visible detector set at 216 and 280 nm. 0.4 min fractions were collected in Eppendorf tubes containing 200 μL of 100 mM NH4HCO3, pH 7.8. 50 μL of each fraction was analyzed by ELISA; the rest of the fraction was lyophilized and stored at -70 °C for subsequent analyses.
ELISA
The rabbit anti-DMPO nitrone adduct polyclonal antiserum developed in this laboratory has already been described (47). Protein-derived nitrone adducts were determined using standard ELISAs in 96-well plates (Greiner Labortechnik, Frickenhausen, Germany). Reaction mixtures were first diluted with coating buffer (100 mM sodium bicarbonate, pH 9.6) to a concentration of 0.2 μM, and 300 μL of this dilution was applied to each well (1.0 μg protein per well). ELISA analyses were then performed as previously described (39). ELISA analyses of HPLC fractions were also performed as described (39).
Electrospray Mass Spectrometry
A Micromass Q-TOF Micro (Waters Micromass Corporation, Manchester, UK) hybrid tandem mass spectrometer was used for the acquisition of the electrospray ionization (ESI) mass spectra and tandem mass spectra. All experiments were performed in the positive ion mode. HPLC fractions showing positive ELISA signals were resuspended in a minimal volume (∼50 μL) of 50:50 water:acetonitrile containing 0.1% formic acid and infused into the mass spectrometer at ∼300 nL/min using a pressure injection vessel. The needle voltage was ∼3000 V and the collision energy was 10 eV for the MS analyses. Collision-induced dissociation experiments employed argon as the collision gas with a collision energy between 30 and 50 eV. Data analysis was accomplished with a MassLynx data system and MaxEnt deconvolution software supplied by the manufacturer.
RESULTS
A key feature of Ngb is its six-coordinate heme structure in both the ferrous and ferric states, with the distal histidine (His 64) forming an endogenous ligand (2, 5, 6). To clarify the possible role(s) of the distal histidine residue in protecting the Fe(III) center of Ngb from attack by strong oxidants, a mutant of Ngb where the distal histidine is replaced by valine, an oxidation-inert amino acid, was constructed and expressed. The mutant protein was purified by the same protocol as, and in comparable yields to, the wild-type protein. Purity was checked by SDS-PAGE and shows a single band at approximately 17 kDa (> 95 % pure, not shown).
Direct observation of a peroxidase-like ferryl (FeIV=O) species
Absorption spectra recorded directly after the purification of the wild-type Ngb and the H64V variant proteins were similar (data not shown) and showed the typical features of oxyferrous globins (43). The oxyferrous Ngbs were converted to their ferric forms (metNgb) by addition of ferricyanide. The spectrum obtained for wild-type Ngb after treatment by the oxidizing reagent (Fig. 1A, solid line) is typical for hexacoordinated ferric globins (λmax at 412, 534 nm and a shoulder around 554 nm) (2). When the H64V variant protein was treated in the same way (Fig. 1A, dotted line), the spectrum became similar to the ferric form of pentacoordinated globins (λmax at 405, 502 and 630 nm), but differed markedly from that of the ferric form of wild-type Ngb; this is in accord with previous observations (48).
Figure 1.
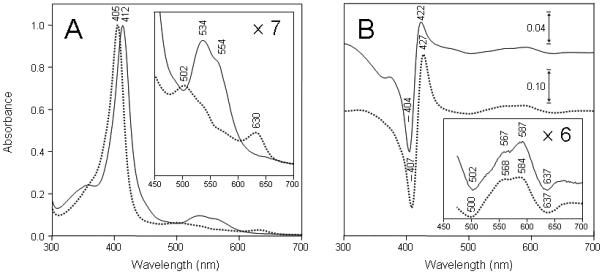
Absorption spectra of wild-type and H64V variant of human neuroglobin. A, wild-type human metNgb (solid lines) and metH64V variant (dotted lines). The spectra were recorded directly after oxidation by ferricyanide. B, difference between spectra of metH64V variant (solid lines) and of commercial horse heart metmyoglobin (dotted lines) recorded before and after the addition of 1 eq H2O2. Spectra in B were taken before and within 6 s after addition of H2O2. The visible region of each spectrum (450-700 nm) has been enlarged at the magnification indicated on the right side of each figure. Experimental conditions: metNgb (7.8 μM) or metMb (5.3 μM) in 50 mM potassium phosphate buffer, pH 6.8, at 25 °C.
The formation of ferryl (FeIV=O) species in the reaction of wild-type human Ngb and H64V variant proteins with H2O2 was then investigated. In agreement with previous investigations (22), no changes were observed in the spectra of oxyferrous forms of either wild-type or H64V variant proteins or the wild-type metNgb, even after addition of a large excess of the oxidant (up to 2 mM H2O2) (data not shown). However, small but significant changes can be observed in the spectrum of the metH64V mutant after the addition of 1 eq H2O2 (Fig. 1B, solid line). The data are presented as the difference between spectra to better visualize the changes because changes were too weak to provide clear information in direct mode. The difference between spectra of horse metmyoglobin recorded before and after the addition of 1 eq H2O2 is shown in same figure for comparison (Fig. 1B, dotted line).
Addition of H2O2 to the metH64V mutant resulted in a red shift in the Soret absorbance maximum (maximum variations in difference spectra at 404 and 422 nm) coupled with an increase in the visible range (maximum variations in difference spectra at 502, 567, 587 and 637 nm) (Fig. 1B, solid line). These maxima correspond closely to those for horse metMb after the addition of H2O2 (maximum variations in difference spectra at 407, 427, 500, 568, 584 and 637 nm) (Fig. 1B, dotted line) and are typical for the conversion of a ferric heme to a ferryl species. The metH64V mutant ferryl species was stable for a few seconds up to 2 minutes depending on the excess of H2O2 (data not shown). The addition of catalase within 30 seconds following the addition of H2O2 resulted in a quick reversion of a fraction of the ferryl to the native ferric form. However, differences between the original and final heights at 404 nm suggest a partial degradation of the prosthetic heme group during the process (not shown).
H2O2-mediated oligomerization of H64V mutant
In addition to its notable impact upon absorption spectra, the addition of H2O2 led to metNgb oligomerization. SDS-PAGE analysis of nontreated metNgb showed a single band at approximately 17 kDa corresponding to the neuroglobin monomer (Fig. 2A, lane 1). However, analysis of metNgb after incubation with H2O2 showed that the monomeric H64V mutant was partially converted to dimeric and trimeric products (Fig. 2A, lane 4, arrows) while the wild-type human metNgb was not (Fig. 2A, lane 2). Formation of trimeric products suggests that at least two residues in separate locations on the Ngb surface can participate in oligomerization reactions. These cross-linking reactions cannot be attributed to S-S bond formation, as the protein samples were treated with DTT before electrophoresis.
Figure 2.
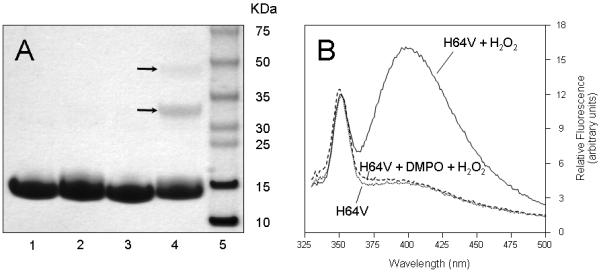
A, SDS-PAGE analysis of human neuroglobin following H2O2 treatment. Lane 1, wild-type metNgb; lane 2, wild-type metNgb + H2O2; lane 3, metH64V mutant; lane 4, metH64V mutant + H2O2; lane 5, molecular weight standards. Arrows indicate positions for dimeric and trimeric products. The samples were reduced with DTT and were boiled before electrophoresis. B, Fluorescence emission spectra of H64V mutant before and after addition of 1 eq H2O2. Doted line, metH64V mutant; solid line, metH64V mutant + H2O2; dashed line, metH64V mutant + DMPO + H2O2. The excitation wavelength was set at 315 nm. The emission spectra were measured over a range of wavelengths that bracket 410 nm, the emission maximum of the Tyr-Tyr bond. Incubations and heme extraction procedures were carried out as described under ‘experimental procedures’. The following concentrations were used: [Ngb] = [H2O2] = 100 μM (panels A and B) and [DMPO] = 100 mM (panel B).
Previous investigations have established that the reaction of some hemoproteins with H2O2 results in oligomerization of the protein due to the formation of tyrosine-tyrosine cross-links (37). The dityrosine cross-links can be detected by their characteristic ultraviolet fluorescence (49). This led us to compare the fluorescence spectrum of H64V variant metNgb protein before and after incubation with H2O2. As can be seen in Fig. 2B (solid line), the H2O2-treated mutant protein exhibits an additional emission maximum around 410 nm when exposed to 315-nm light, consistent with formation of dityrosine cross-links (49). Dityrosine is a covalently bound diphenol, produced by the reaction of two tyrosyl radicals. Thus, the formation of a dityrosine crosslink between Ngb molecules provides the first solid evidence that a protein radical is indeed formed in the reaction of metNgb with H2O2. The low-intensity peak which occurs at 355 nm, the Raman band for water, is also observed in the two spectra. Interestingly, dityrosine fluorescence was not detected when the H64V variant metNgb protein was treated with H2O2 in the presence of the spin trap DMPO (Fig. 2B, dashed line).
Immunochemical detection of globin-centered radicals
Immuno-spin trapping (47) was used to confirm protein-derived radical formations in the reaction of metNgb with H2O2. Anti-DMPO antibody binding to the globin-DMPO adduct was measured using ELISA (Fig. 3). When the H64V variant metNgb protein was treated with H2O2 in the presence of DMPO, nitrone adducts were detected (Fig. 3B). In contrast, no adducts were detected when the wild-type metNgb protein was treated with H2O2 (Fig. 3A) or in any of the control samples lacking DMPO or H2O2.
Figure 3.
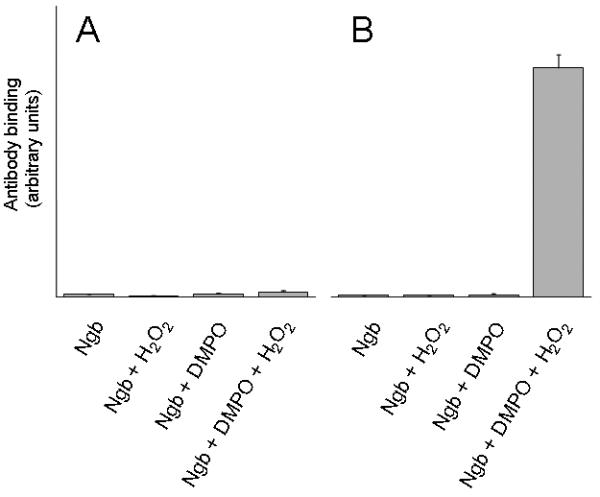
Immunochemical detection of wild-type and H64V recombinant human metNgb radical-derived nitrone adducts. A, wild-type metNgb; B, H64V metNgb. H2O2-dependent formation of globin radical-derived DMPO nitrone adduct was examined by ELISA analyses. Reaction mixtures containing globin in met form (1 μM), DMPO (100 mM), H2O2 (10 μM), or subsets of these as indicated, were incubated in 50 mM potassium phosphate buffer, pH 6.8. The reactions were initiated by adding H2O2 to the mixture of globin and the spin trap. After 30 min incubation at 37 °C, reactions were stopped by the addition of 250 units/mL catalase and were diluted with coating buffer for ELISA analysis. Values are mean ± S.D (n = 3).
Mass spectrometry assessment of disulfide bonds and of DMPO trapping yields
Analysis of wild type Ngb and H64V variant protein by flow injection electrospray ionization mass spectrometry, indicated the presence of one free cysteine and one disulfide bond (data not shown); this result is in accord with previous observations obtained for the wild-type protein (15). When the H64V variant metNgb protein was analyzed by ESI, no ions corresponding to protein-DMPO adducts were observed after reaction with H2O2 in the presence of DMPO (data not shown). This result indicates that under the conditions used in this study the trapping yields are low.
MS Identification of the amino acid residue labeled by the spin trap
Identification of the specific location of the DMPO adduct on the H64V variant Ngb protein was determined by a mass spectrometry method that combines off-line immuno-spin trapping and chromatographic procedures (39). In this technique, the protein-DMPO nitrone adducts are proteolytically digested, peptides are separated by HPLC, and the collected fractions are analyzed by ELISA using polyclonal anti-DMPO nitrone antiserum. Fractions showing positive ELISA signals are then concentrated and characterized by tandem mass spectrometry (MS/MS). This method adds a dimension of selectivity prior to the MS analysis, thereby facilitating the identification of the actual amino acid(s) labeled by the spin trap (39).
The HPLC chromatograms for the tryptic digest of the H64V variant metNgb protein treated with H2O2 in the presence of DMPO are shown in Fig. 4 (top and middle panels). The bottom panel shows the results of ELISA analyses. Only one HPLC fraction showed a strong positive ELISA signal (peak 1), so this fraction was further characterized using flow injection analysis coupled to electrospray ionization mass spectrometry (ESI-MS) and tandem mass spectrometry (MS/MS). The ESI mass spectrum of the ELISA-positive fraction is shown in Fig. 5. The ions of m/z 969.79 (+3) and 1454.13 (+2) correspond in mass to tryptic peptide T8 (residue 70-96), which has a theoretical mass of 2906.5 Da (calculated mass-to-charge (m/z) values: 1454.26 (+2) and 969.84 (+3)). The ions of m/z 1006.80 (+3) and 1509.63 (+2) correspond in mass to the addition of one molecule of DMPO to tryptic peptide T8. The insert in Fig. 5 depicts one pair of triply charged ions, which shows a mass-to-charge difference of 37.0. This mass difference corresponds to the loss of one molecule of DMPO (111.07, theoretical mass shift) and depends on the charge state, i.e., Δm = 111.07 (+1), 55.54 (+2) or 37.02 (+3). As demonstrated in our previous investigation (39), these pairs of ions can greatly facilitate the selection of DMPO-containing ions of interest and are characteristic of Tyr-DMPO modifications.
Figure 4.
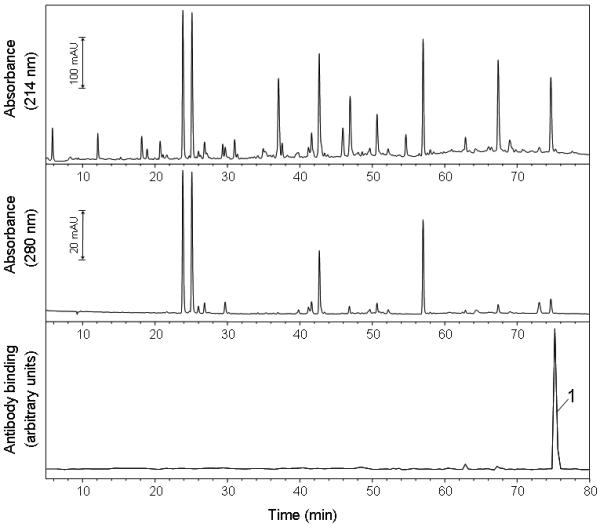
Reversed-phase HPLC of the tryptic digest of metNgb H64V mutant reacted with H2O2 in the presence of DMPO. Absorbances were monitored at 214 nm (peptide backbone) and 280 nm (aromatic amino acid). Fractions of 320-μl were collected every 0.4 min and a 25-μl aliquot of each fraction was analyzed by ELISA (bottom trace). Only one fraction showed strong anti-DMPO binding (peak 1) and was further characterized by tandem mass spectrometry.
Figure 5.
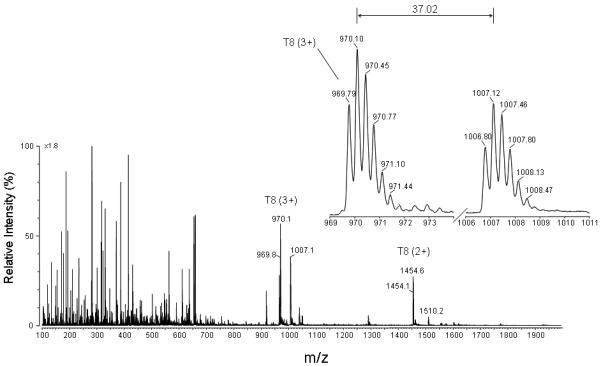
Full scan mass spectrum of the fraction that showed anti-DMPO binding. The fraction corresponding to peak 1 in Fig. 4 and eluting at approximately 75.4 min was lyophilized, resuspended in 50:50 acetonitrile/H2O + 0.1 % formic acid, and directly infused into the electrospray source of a Micromass Q-TOF hybrid tandem mass spectrometer. An expanded view of a pair of ions having the same charge state (+ 3 ions) and a mass-to-charge (m/z) difference of 37.0 is shown. This mass difference corresponds to the loss of one molecule of DMPO.
To determine the position of DMPO within this peptide, each pair of ions observed in Fig. 5 was subjected to MS/MS analysis. An example of an MS/MS spectrum which allows for the sequence determination for the DMPO-containing triply charged pair of ions is presented in Fig. 6. These data show a series of carboxy-terminal y ions (y4, y6, y8-y25) and amino-terminal b ions (b2-b5, b7-b8), accounting for most of the amino acids in the Val68-Arg94 sequence. The series y8-y25 corresponds to a shift in mass of an additional 111 Da when compared to the same series in the MS/MS spectrum of the unmodified peptide. These data, together with normal y-ions y6 and y4, allow the assignment of a DMPO molecule either to Glu-87 or to Tyr-88 of the tryptic peptide T8 of the H64V variant of Ngb. Given that Glu-87 is an oxidation-inert amino acid, the data are most consistent with the formation of the DMPO nitrone adduct at Tyr-88.
Figure 6.
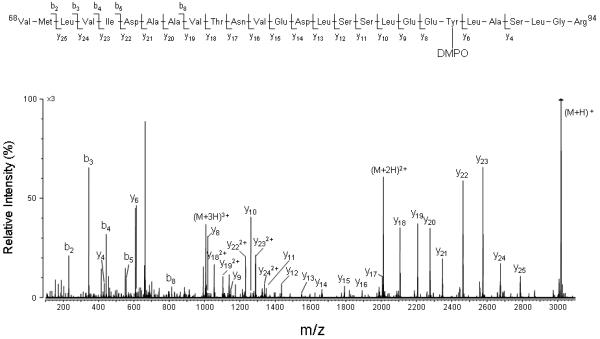
Deconvoluted MS/MS spectrum acquired from parent of m/z 1006.8 (+3 ion), which corresponds in mass to tryptic peptide T8 plus DMPO. For clarity reasons, not all identified fragment ions are labeled on the spectrum.
DISCUSSION
In contrast to the ferric forms of myoglobin (metMb) and hemoglobin (metHb), the ferric form of Ngb (metNgb) does not produce a peroxidase-like ferryl (FeIV=O) species and a transient protein radical when treated with H2O2 (22). Also, in contrast to metMb and metHb, metNgb does not generate an FeIV=O species on reaction with ONOO- (peroxynitrite) (22). To clarify the possible role(s) of the distal histidine residue in inhibiting these reactions, a mutant of Ngb in which the distal histidine is replaced by valine, an oxidation-inert amino acid, was constructed and expressed. It is shown here that, in the presence of H2O2, the metH64V mutant produced DMPO-trappable protein radicals, as detected by immuno-spin trapping (Fig. 3). This finding provides the first solid evidence that a protein radical is formed in the reaction of H2O2 with a variant of metNgb lacking a distal coordination bond with His-64. In contrast, no adducts were detected when the hexacoordinated wild-type metNgb was treated with H2O2, which confirms the critical role of the distal histidine residue in inhibiting this reaction.
The specific location(s) of the DMPO adduct(s) on H64V variant protein was determined by a mass spectrometry method that combines off-line immuno-spin trapping and chromatographic procedures. Only one HPLC fraction showed a strong positive ELISA signal (Fig. 4); therefore, this fraction was further characterized by electrospray ionization mass spectrometry (ESI-MS) and MS/MS. An abundant ion that corresponds in mass to tryptic peptide T8 (amino acids residues 68-94) plus a DMPO molecule was observed (Fig. 5). MS/MS analysis by collision-induced dissociation revealed Tyr-88 to be the sole site of modification by DMPO on this peptide (Fig. 6). No other DMPO modified tryptic peptide was detected.
There are four tyrosine residues in human Ngb (i.e., Tyr-44, Tyr-88, Tyr-115 and Tyr-137). From the crystal structure of human Ngb in its hexacoordinated ferric form, determined at 1.95 Å resolution (5), three of the tyrosyl residues exhibit at least some degree of solvent accessibility (i.e., Tyr-44, Tyr-88 and Tyr-115) while Tyr-137 is an internal residue. Tyr-88, the only tyrosine residue detectably modified by DMPO, is the tyrosine residue nearest to the heme group, and the shortest distance separating these two groups is 3.85 Å (Fig. 7). This distance is similar to the corresponding distance in both the unliganded ferric (6) and the CO-bound ferrous (14) forms of murine Ngb. In the human variant protein, the phenol oxygen of Tyr-88 actually points toward a large cavity (about 120 Å3 for human Ngb, (5)) which stretches from the heme distal site toward the protein surface. Tyr-88 is thus optimally placed for oxidation by reactive species generated on the porphyrin ring structure and for the production of DMPO-trappable tyrosyl radicals.
Figure 7.
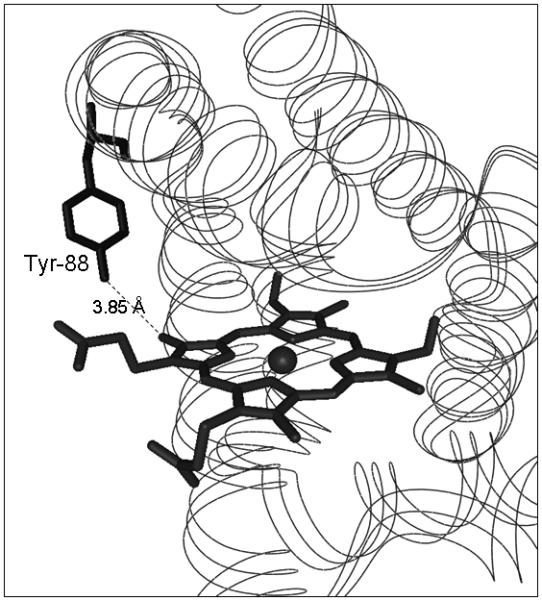
Location of the radical-forming tyrosyl residue identified in the current study and its position relative to the heme prosthetic group. The atomic co-ordinates were obtained from (5) and the Protein Data Bank (accession number 1OJ6). Tyr-88 is the tyrosine residue nearest to the prosthetic heme group. The point of closest approach is the phenol oxygen, which is located at a distance of 3.85 Å from the carbon atom of one of the heme methyl groups.
The finding that the H64V Ngb mutant undergoes a dimerization reaction in which a tyrosine residue in each of two Ngb molecules couples to give a dityrosine cross-link provides additional evidence for a protein radical and confirms that the radical generated on the globin is partly localized on a tyrosine residue. The nature of the cross-link was definitively established by fluorescence spectrometry (Fig. 2B, solid line), but the identity of the two cross-linked residues remains to be determined. One possibility is that the reaction of the H64V mutant with H2O2 yields dimers linked by Tyr88-Tyr88 cross-links. The finding that preincubation of Ngb with DMPO prevents dityrosine formation (Fig. 2B, dashed line) may indicate that formation of a nitrone adduct at position Tyr-88 suppresses subsequent cross-linking of this residue. It is also possible that the protection by DMPO against dimerization is due to reducing impurities in the spin trap, which is present in very high concentration. However, the observation that in absence of DMPO the monomeric H64V variant protein was partially converted, not only to dimeric but also trimeric products, implicates at least one residue in addition to Tyr-88 as a locus of unpaired electron density. Note that the dimeric and trimeric products were not observed when the hexacoordinated wild-type metNgb was treated with H2O2, again showing the critical role of the distal histidine ligand in inhibiting this reaction.
The present study indicates that a ferryl species is formed in parallel with formation of the protein radicals in the reaction of the metH64V variant protein with H2O2 (Fig. 1B). Mb and Hb have long been known to form ferryl species upon treatment of the ferric state with peroxide (32, 33). In the reaction of the ferric form of Ngb with H2O2, however, a ferryl species has never been reported. Previous stopped-flow UV-vis rapid-scan experiments (50) suggest that the reaction of metMb may first produce a transient Compound I-like intermediate in which the ferryl species is coupled to a porphyrin rather than a protein radical. Electron transfer from the protein to the porphyrin radical cation would then produce the observed protein radical.
Although the reaction of metNgb with H2O2 has not yet been investigated by stopped-flow, it is likely that the H64V variant metNgb, lacking a distal coordination bond with His-64, acts as a pentacoordinated metMb and, thus, that a Compound I-like intermediate with a porphyrin-centered radical is also formed in this reaction. Compound I may subsequently channel the oxidizing equivalent located on the porphyrin ring to Tyr-88 and also, possibly via additional intra- and/or intermolecular electron transfer reactions, toward additional tyrosine residues. Some of the resulting Tyr-phenoxyl radicals may further react in a bimolecular reaction to generate the dityrosine crosslinks detected in this study while the Tyr88-phenoxyl radical can be spin trapped by DMPO.
In summary, the replacement of distal histidine (His-64) by an non-oxidizable amino acid enabled us to observe a ferryl species and a transient protein radical in the reaction of metNgb with H2O2. More specifically, Tyr-88 was identified as a site of tyrosyl radical formation in the peroxide-treated H64V variant metNgb, as detected with the spin-trap agent DMPO. The presence of His-64 in the wild-type protein results in a complete loss of any detectable radical adduct and of the ferryl species. Together, the data reinforce the contention that wild-type Ngb is protected from attack by H2O2 and other strong oxidants by the coordinated distal His. In addition, this work further illustrates the power of the newly developed immuno-spin-trapping approach in conjunction with chromatographic and mass spectrometric procedures to select DMPO-labeled peptides and identify sites of DMPO covalent attachments, an attachment which occurs only subsequent to DMPO trapping of free radicals.
ACKNOWLEDGEMENTS
The authors thank Dr. Arno Siraki and Dr. Marilyn Ehrenshaft for their careful review of the manuscript and Ms. Jean Corbett, Ms. Anne Froment, Mrs. Mary J. Mason and Dr. Ann Motten for their valuable assistance in the preparation of this manuscript. We thank Dr. Robert Petrovich and all the members of the Protein Expression Core Facility for their kind access to PCR machines and shakers and for their comments and suggestions. We are also grateful to Piotr Bilski for performing the fluorescence measurements. The original construct of Ngb in pET3a was a generous gift from Dr. Tilmann Laufs and Prof. Thorsten Burmester from the Institute of Zoology of the University of Mainz, Germany.
Abbreviations
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- ESI
electrospray ionization
- Ngb
neuroglobin
- metNgb
metneuroglobin
Footnotes
This work has been supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences.
REFERENCES
- (1).Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- (2).Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden MC, Caubergs R, Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- (3).Van Doorslaer S, Dewilde S, Kiger L, Nistor SV, Goovaerts E, Marden MC, Moens L. Nitric oxide binding properties of neuroglobin. A characterization by EPR and flash photolysis. J Biol Chem. 2003;278:4919–4925. doi: 10.1074/jbc.M210617200. [DOI] [PubMed] [Google Scholar]
- (4).Pesce A, Bolognesi M, Bocedi A, Ascenzi P, Dewilde S, Moens L, Hankeln T, Burmester T. Neuroglobin and cytoglobin. Fresh blood for the vertebrate globin family. EMBO Rep. 2002;3:1146–1151. doi: 10.1093/embo-reports/kvf248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pesce A, Dewilde S, Nardini M, Moens L, Ascenzi P, Hankeln T, Burmester T, Bolognesi M. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. Structure. 2003;11:1087–1095. doi: 10.1016/s0969-2126(03)00166-7. [DOI] [PubMed] [Google Scholar]
- (6).Vallone B, Nienhaus K, Brunori M, Nienhaus GU. The structure of murine neuroglobin: Novel pathways for ligand migration and binding. Proteins. 2004;56:85–92. doi: 10.1002/prot.20113. [DOI] [PubMed] [Google Scholar]
- (7).Mammen PP, Shelton JM, Goetsch SC, Williams SC, Richardson JA, Garry MG, Garry DJ. Neuroglobin, a novel member of the globin family, is expressed in focal regions of the brain. J Histochem Cytochem. 2002;50:1591–1598. doi: 10.1177/002215540205001203. [DOI] [PubMed] [Google Scholar]
- (8).Awenius C, Hankeln T, Burmester T. Neuroglobins from the zebrafish Danio rerio and the pufferfish Tetraodon nigroviridis. Biochem Biophys Res Commun. 2001;287:418–421. doi: 10.1006/bbrc.2001.5614. [DOI] [PubMed] [Google Scholar]
- (9).Zhang C, Deng M, Li L, Wang H, Fan M, Xu W, Meng F, Qian L, He F. Full-length cDNA cloning of human neuroglobin and tissue expression of rat neuroglobin. Biochem Biophys Res Commun. 2002;290:1411–1419. doi: 10.1006/bbrc.2002.6360. W. C. [DOI] [PubMed] [Google Scholar]
- (10).Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T, Hankeln T. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience. 2002;115:645–656. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- (11).Kugelstadt D, Haberkamp M, Hankeln T, Burmester T. Neuroglobin, cytoglobin, and a novel, eye-specific globin from chicken. Biochem Biophys Res Commun. 2004;325:719–725. doi: 10.1016/j.bbrc.2004.10.080. [DOI] [PubMed] [Google Scholar]
- (12).Hankeln T, Wystub S, Laufs T, Schmidt M, Gerlach F, Saaler-Reinhardt S, Reuss S, Burmester T. The cellular and subcellular localization of neuroglobin and cytoglobin -- a clue to their function? IUBMB Life. 2004;56:671–679. doi: 10.1080/15216540500037794. [DOI] [PubMed] [Google Scholar]
- (13).Kriegl JM, Bhattacharyya AJ, Nienhaus K, Deng P, Minkow O, Nienhaus GU. Ligand binding and protein dynamics in neuroglobin. Proc Natl Acad Sci U S A. 2002;99:7992–7997. doi: 10.1073/pnas.082244399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Vallone B, Nienhaus K, Matthes A, Brunori M, Nienhaus GU. The structure of carbonmonoxy neuroglobin reveals a heme-sliding mechanism for control of ligand affinity. Proc Natl Acad Sci U S A. 2004;101:17351–17356. doi: 10.1073/pnas.0407633101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hamdane D, Kiger L, Dewilde S, Green BN, Pesce A, Uzan J, Burmester T, Hankeln T, Bolognesi M, Moens L, Marden MC. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J Biol Chem. 2003;278:51713–51721. doi: 10.1074/jbc.M309396200. [DOI] [PubMed] [Google Scholar]
- (16).Vinck E, Van Doorslaer S, Dewilde S, Moens L. Structural change of the heme pocket due to disulfide bridge formation is significantly larger for neuroglobin than for cytoglobin. J Am Chem Soc. 2004;126:4516–4517. doi: 10.1021/ja0383322. [DOI] [PubMed] [Google Scholar]
- (17).Brunori M. Hemoglobin is an honorary enzyme. Trends Biochem Sci. 1999;24:158–161. doi: 10.1016/s0968-0004(99)01380-8. [DOI] [PubMed] [Google Scholar]
- (18).Brunori M. Nitric oxide moves myoglobin centre stage. Trends Biochem Sci. 2001;26:209–210. doi: 10.1016/s0968-0004(01)01824-2. [DOI] [PubMed] [Google Scholar]
- (19).Fago A, Hundahl C, Dewilde S, Gilany K, Moens L, Weber RE. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin. Molecular mechanisms and physiological significance. J Biol Chem. 2004;279:44417–44426. doi: 10.1074/jbc.M407126200. [DOI] [PubMed] [Google Scholar]
- (20).Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- (21).Brunori M, Giuffre A, Nienhaus K, Nienhaus GU, Scandurra FM, Vallone B. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc Natl Acad Sci U S A. 2005;102:8483–8488. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Herold S, Fago A, Weber RE, Dewilde S, Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- (23).Fordel E, Thijs L, Moens L, Dewilde S. Neuroglobin and cytoglobin expression in mice. Evidence for a correlation with reactive oxygen species scavenging. Febs J. 2007;274:1312–1317. doi: 10.1111/j.1742-4658.2007.05679.x. [DOI] [PubMed] [Google Scholar]
- (24).Wakasugi K, Nakano T, Morishima I. Oxidized human neuroglobin acts as a heterotrimeric Gα protein guanine nucleotide dissociation inhibitor. J Biol Chem. 2003;278:36505–36512. doi: 10.1074/jbc.M305519200. [DOI] [PubMed] [Google Scholar]
- (25).Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Li RC, Lee SK, Pouranfar F, Brittian KR, Clair HB, Row BW, Wang Y, Gozal D. Hypoxia differentially regulates the expression of neuroglobin and cytoglobin in rat brain. Brain Res. 2006;1096:173–179. doi: 10.1016/j.brainres.2006.04.063. [DOI] [PubMed] [Google Scholar]
- (28).Shang A, Zhou D, Wang L, Gao Y, Fan M, Wang X, Zhou R, Zhang C. Increased neuroglobin levels in the cerebral cortex and serum after ischemia-reperfusion insults. Brain Res. 2006;1078:219–226. doi: 10.1016/j.brainres.2006.01.064. [DOI] [PubMed] [Google Scholar]
- (29).Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rifkind JM, Ramasamy S, Manoharan PT, Nagababu E, Mohanty JG. Redox reactions of hemoglobin. Antioxid Redox Signal. 2004;6:657–666. doi: 10.1089/152308604773934422. [DOI] [PubMed] [Google Scholar]
- (31).Tajima G, Shikama K. Autoxidation of oxymyoglobin. An overall stoichiometry including subsequent side reactions. J Biol Chem. 1987;262:12603–12606. [PubMed] [Google Scholar]
- (32).Keilin D, Hartree EF. Reaction of methaemoglobin with hydrogen peroxide. Nature. 1950;166:513–514. doi: 10.1038/166513a0. [DOI] [PubMed] [Google Scholar]
- (33).King NK, Winfield ME. The mechanism of metmyoglobin oxidation. J Biol Chem. 1963;238:1520–1528. [PubMed] [Google Scholar]
- (34).Gunther MR, Tschirret-Guth RA, Witkowska HE, Fann YC, Barr DP, Ortiz De Montellano PR, Mason RP. Site-specific spin trapping of tyrosine radicals in the oxidation of metmyoglobin by hydrogen peroxide. Biochem J. 1998;330(Pt 3):1293–1299. doi: 10.1042/bj3301293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Davies MJ. Detection of myoglobin-derived radicals on reaction of metmyoglobin with hydrogen peroxide and other peroxidic compounds. Free Radic Res Commun. 1990;10:361–370. doi: 10.3109/10715769009149905. [DOI] [PubMed] [Google Scholar]
- (36).Giulivi C, Davies KJ. Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19 S) proteasome. J Biol Chem. 1993;268:8752–8759. [PubMed] [Google Scholar]
- (37).Lardinois OM, Ortiz de Montellano PR. Intra- and intermolecular transfers of protein radicals in the reactions of sperm whale myoglobin with hydrogen peroxide. J Biol Chem. 2003;278:36214–36226. doi: 10.1074/jbc.M304726200. [DOI] [PubMed] [Google Scholar]
- (38).Deterding LJ, Ramirez DC, Dubin JR, Mason RP, Tomer KB. Identification of free radicals on hemoglobin from its self-peroxidation using mass spectrometry and immuno-spin trapping: observation of a histidinyl radical. J Biol Chem. 2004;279:11600–11607. doi: 10.1074/jbc.M310704200. [DOI] [PubMed] [Google Scholar]
- (39).Lardinois OM, Detweiler CD, Tomer KB, Mason RP, Deterding LJ. Identifying the site of spin trapping in proteins by a combination of liquid chromatography, ELISA, and off-line tandem mass spectrometry. Free Radic Biol Med. 2007;44:893–906. doi: 10.1016/j.freeradbiomed.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).DeGray JA, Gunther MR, Tschirret-Guth R, Ortiz de Montellano PR, Mason RP. Peroxidation of a specific tryptophan of metmyoglobin by hydrogen peroxide. J Biol Chem. 1997;272:2359–2362. doi: 10.1074/jbc.272.4.2359. [DOI] [PubMed] [Google Scholar]
- (41).Witting PK, Douglas DJ, Mauk AG. Reaction of human myoglobin and H2O2. Involvement of a thiyl radical produced at cysteine 110. J Biol Chem. 2000;275:20391–20398. doi: 10.1074/jbc.M000373200. [DOI] [PubMed] [Google Scholar]
- (42).Trent JT, 3rd, Watts RA, Hargrove MS. Human neuroglobin, a hexacoordinate hemoglobin that reversibly binds oxygen. J Biol Chem. 2001;276:30106–30110. doi: 10.1074/jbc.C100300200. [DOI] [PubMed] [Google Scholar]
- (43).Antonini E, Brunori M. Hemoglobin and Myoglobin in the Reactions with Ligands. Elsevier; Amsterdam, The Netherlands, North-Holland: 1971. [Google Scholar]
- (44).Buettner GR. On the reaction of superoxide with DMPO/.OOH. Free Radic Res Commun. 1990;10:11–25. doi: 10.3109/10715769009145928. [DOI] [PubMed] [Google Scholar]
- (45).Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H2O2 solutions in the UV) Anal Biochem. 1972;49:474–478. doi: 10.1016/0003-2697(72)90451-4. [DOI] [PubMed] [Google Scholar]
- (46).Nicolis S, Monzani E, Ciaccio C, Ascenzi P, Moens L, Casella L. Reactivity and endogenous modification by nitrite and hydrogen peroxide: does human neuroglobin act only as a scavenger? Biochem J. 2007;407:89–99. doi: 10.1042/BJ20070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med. 36:1214–1223. doi: 10.1016/j.freeradbiomed.2004.02.077. 200. [DOI] [PubMed] [Google Scholar]
- (48).Uno T, Ryu D, Tsutsumi H, Tomisugi Y, Ishikawa Y, Wilkinson AJ, Sato H, Hayashi T. Residues in the distal heme pocket of neuroglobin. Implications for the multiple ligand binding steps. J Biol Chem. 2004;279:5886–5893. doi: 10.1074/jbc.M311748200. [DOI] [PubMed] [Google Scholar]
- (49).Malencik DA, Anderson SR. Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids. 2003;25:233–247. doi: 10.1007/s00726-003-0014-z. [DOI] [PubMed] [Google Scholar]
- (50).Egawa T, Shimada H, Ishimura Y. Formation of compound I in the reaction of native myoglobins with hydrogen peroxide. J Biol Chem. 2000;275:34858–34866. doi: 10.1074/jbc.M004026200. [DOI] [PubMed] [Google Scholar]


