Abstract
Inbred mouse strains C57BL/6J (B6) and C3H/HeJ (C3H) exhibit a marked difference in atherosclerotic lesion formation when deficient in apolipoprotein E (apoE−/−), and the arterial wall has been identified as a source of the difference in atherosclerosis susceptibility. In the present study, differences in gene expression in aortic walls of the two strains were analyzed by microarrays. Total RNA was extracted from the aorta of 6-wk-old female B6 and C3H apoE−/− mice fed a chow or Western diet. There were 1,514 genes in chow fed mice and 590 genes in Western fed mice that were found to be differentially expressed between the two strains. Pathway analysis of differentially expressed genes suggested a role for the calcium signaling pathway in regulating atherosclerosis susceptibility. Oxidized LDL (oxLDL) induced a dose-dependent rise in cytosolic calcium levels in B6 endothelial cells. oxLDL-induced monocyte chemoattractant protein-1 production was inhibited by pretreatment with calcium chelator EGTA or intracellular calcium trapping compound BAPTA, indicating that calcium ions mediate the effect of oxLDL on monocyte chemoattractant protein-1 induction. The present findings demonstrate involvement of the calcium signaling pathway in the inflammatory process of atherogenesis.
Keywords: gene profiling, genetic susceptibility, monocyte chemoattractant protein-1
atherosclerosis is a chronic inflammatory disease of the large- and medium-sized arteries involving numerous genes, as well as their interactions with the environment. The mouse has been a powerful force in elucidating the genetic basis of atherogenesis. More than 80 genes have been confirmed to play a role in atherosclerosis by using gene-targeted or transgenic mice (21). Inbred mouse strains that display quantitative differences in susceptibility to atherosclerosis or associated traits have also been used to search for genes and pathways that give rise to the traits. C57BL/6 (B6) and C3H/HeJ (C3H) mice are two inbred strains that exhibit marked differences in atherosclerosis susceptibility when fed an atherogenic diet or when deficient in apolipoprotein E (apoE−/−) (8, 14). Strain B6 readily develops atherosclerosis, while strain C3H is highly resistant to it. We have observed various differences between the two strains in atherogenic processes involving the arterial wall, including differences in the retention of apoB-containing lipoproteins in the arterial wall (2), in the capacity to oxidize low-density lipoprotein (LDL) (2), and in the expression of proinflammatory genes upon stimulation with oxidized LDL (13). Through aorta transplantation, whereby aortic segments from B6.apoE−/− and C3H.apoE−/− mice were transplanted into the infrarenal aorta of their F1 strains, we have demonstrated that the arterial wall is a source of the difference in atherosclerosis susceptibility (9). However, atherosclerosis-susceptible genes acting at the level of the arterial wall remain to be identified.
Microarrays, which are capable of quantifying thousands of genes for expression differences between samples simultaneously, have been used to define genetic variation associated with disease risk (3, 11). They have an even greater utility in identifying regulatory and functional pathways in which up- or downregulated genes are functionally clustered into specific biological processes from the classification systems of the gene ontology annotation (1). In the present study, we performed microarray analysis to compare gene expression in the arterial wall of B6.apoE−/− and C3H.apoE−/− mice when fed a chow or Western diet. Further analysis of differentially expressed genes suggested the involvement of the calcium signaling pathway in control of atherosclerosis susceptibility. The role of calcium signaling in oxidized LDL-induced monocyte chemoattractant protein-1 (MCP-1) production was further evaluated with cultured endothelial cells in vitro.
MATERIALS AND METHODS
Mice.
Female B6.apoE−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Female C3H.apoE−/− mice at the N12 generation were generated in our laboratory. The animals were weaned at 3 wk of age onto a rodent chow diet. At 4 wk of age, mice either continued with the chow diet or were switched onto a Western diet containing 21% fat, 0.2% cholesterol, and 19.5% casein without sodium cholate (TD 88137, Harlan Teklad, Madison, WI) for a period of 2 wk. All procedures were in accordance with current National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee.
RNA preparation and microarray assays.
The descending aorta of each mouse was prepared separately, as our laboratory previously described (15, 16). Briefly, the vessel was flushed thoroughly with phosphate-buffered saline (PBS), containing 5 U/ml of heparin, through the left ventricle of the heart, cleaned of periadventitial fat and connective tissues, and snap-frozen in liquid nitrogen. The frozen aorta was mechanically broken up, followed by extraction of total RNA from the tissue with Qiagen RNeasy Mini Kit. Total RNA was pooled in an equal amount from four individual mice of each group and treated with DNase I to remove residual genomic DNA. cDNA synthesis and labeling and array hybridization were performed at our GeneChip/Microarray Bioinformatics Facility, according to Affymetrix's instructions (Affymetrix, Santa Clara, CA). Briefly, the above prepared RNA was reverse transcribed into cDNA, which was then used to generate biotin-labeled cRNA by in vitro transcription with an oligo(T7) primer and biotin-labeled UTP and CTP. cRNA was fragmented and hybridized to a mouse genome MOE430A array (Affymetrix). Four separate RNA samples prepared from B6.apoE−/− and C3H.apoE−/− mice fed a chow or Western diet were each hybridized to one array. The hybridized arrays were stained in the Fluidics Station 450 and scanned on the Affymetrix Scanner 3000, and fluorescent signal intensities for each spot on the arrays were analyzed using the Gene Chip Operating System (Affymetrix).
Microarray data were analyzed using the standard analysis procedures established at the UVA GeneChip/Microarray Bioinformatics Core. [The microarray data have been deposited in the NCBI Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/), and the GEO series accession number is GSE14854.] The procedures included assessment of the overall quality of array data, statistical evaluation of differentially expressed genes, and advanced pathway analysis. Six parameters were examined to assess overall assay performance, including background, noise, scale factor, 3′/5′ ratios for the housekeeping genes GAPDH and β-actin, and signal intensity for spiked cRNA BioB. Once the quality of array data was confirmed, the Gene Chip Operating System was used to calculate signal intensities, detection calls, and their associated P values for each transcript on the array. Signal intensity denoted the difference in intensity values between perfect match (PM) and mismatch (MM) probes in a probe set. A probe set was composed of a series of probe pairs, each containing one PM probe and one MM probe. Wilcoxon one-sided rank test was used to calculate P value for each transcript based on measured values of PM probes and MM probes after corrections for background and noise of hybridization. A probe set with a detection P value < 0.04 was considered present, a P value between 0.06 and 0.04 was considered marginal, and a P value > 0.06 was considered absent. When a comparison expression analysis was conducted, the algorithm computed a change call, signal difference, fold change, and fold change P value for each transcript represented on the arrays. A probe set with a fold change P value < 0.05 was considered present.
Pathway analysis.
Differentially expressed genes (>1.5-fold) that had a comparison P value < 0.05 but were not labeled as “absent” in microarray analysis for both strains were subject to pathway analysis. Data sets, each consisting of gene identifier, fold change in expression levels, and a comparison P value for each of the genes, were imported into Ingenuity Pathway Analysis software (Ingenuity Systems, Mountain View, CA) to detect biological pathways. The significance of the association of uploaded microarray data with the canonical pathway was measured in two ways: 1) a ratio of the number of genes from the microarray data that map to the pathway over the total number of genes that map to the canonical pathway was calculated; and 2) Fisher's exact test was used to assess the significance of the association between the observed data and the data in the canonical pathway. Each generated pathway was assigned a significance score, according to the number of differentially regulated focus genes in the data set. This score was the negative logarithm of the P value, indicative of the likelihood that focus genes were found together in a pathway randomly.
Real-time PCR analysis.
A set of genes identified by the pathway analysis to be involved in calcium signaling were further examined for their expression in the aortic wall of B6 and C3H mice using real-time PCR. Total RNA was prepared from female B6.apoE−/− and C3H.apoE−/− mice fed the Western diet for 2 wk, as described above, treated with DNase I, and reverse transcribed to cDNA using ThermoScript RT-PCR system (Invitrogen). cDNA was mixed with SYBR Green supermix reagent (Bio-Rad) and gene-specific primers (see supplementary data; the online version of this article contains supplementary data). Real-time PCR on each sample was run in triplicate on an iCycler iQ5 machine (Bio-Rad) under the condition of 15 s at 95°C, 30 s at 55°C, and 30 s at 72°C for 40 cycles. Expression levels of each gene were determined in three to six biologically independent samples for each strain and were normalized to GAPDH.
Culture and treatment of endothelial cells.
Endothelial cells were isolated from the aorta of B6 mice by an established explant technique (13). Briefly, under sterile conditions, the thoracic aorta was harvested from the animals, cleaned of periadventitial fat and connective tissue, and cut into rings ∼3 mm long. The aortic segments were placed on Matrigel in a 35-mm tissue culture plate and incubated in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, 90 μg/ml heparin, 60 μg/ml endothelial cell growth supplements, and 100 U/ml fungizone at 37°C in a 95% air/5% CO2 incubator. The vessel rings were removed once cell outgrowth was observed. Approximately 4 days later, the cells were passaged with Dispase and plated onto 0.1% gelatin-coated 60-mm culture dishes. The subsequent passages were performed with 0.25% trypsin-EDTA, and cells were split in a 1:4 ratio. Multiple independent isolates with this method have given reproducible results with regards to the expression of endothelium-specific markers.
Confluent cells at passages 4–6 were incubated overnight in DMEM containing 1% FBS and 1% penicillin-streptomycin. The cells of each mouse were then treated in triplicate with various reagents for 4 h in DMEM supplemented with 1% FBS and 1% penicillin-streptomycin.
Lipoprotein isolation and modification.
LDL (d = 1.019–1.069 g/ml) was isolated from the plasma of healthy human donors by density-gradient ultracentrifugation, as described (4), dialyzed in PBS containing 0.3 mM EDTA, filtered through 0.22-μm filters, and stored at 4°C. Lipoprotein concentrations were expressed as protein content. Oxidized LDL was prepared by incubating LDL in a dialysis tube at a concentration of 5 mg/ml with 7 μM FeSO4 in 0.9% NaCl solution at room temperature (13). Iron oxidation of LDL produced ∼1.8 nM thiobarbituric acid-reactive substances per milligram protein after dialysis.
Measurement of cytosolic calcium.
Cytosolic Ca2+ concentrations ([Ca2+]i) in endothelial cells were measured using fluorescent dye fura 2, as described (12). Briefly, cells cultured in 35-mm dishes were made quiescent by incubating overnight in serum-free medium. Cells were loaded with 16 μM fura 2-AM and 0.083% pluronic F-127 (Molecular Probes) for 30 min and then washed with Krebs-Ringer buffer (125 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 11 mM glucose, 2.5 mM CaCl2, and 25 mM HEPES, pH 7.4). Fluorescence of a cluster of six to eight cells located inside the light path was continuously monitored at 340- and 380-nm excitation and 510-nm emission with a Nikon Eclipse TE2000-U (Nikon Instruments, Melville, NY) equipped with a CoolSnap ES Camera (Photometrics, Tucson, AZ) and a Prior ProScan II (Prior Scientific, Rockland, MD). Fluorescent images were analyzed using MetaFlour software (Universal Imaging, Downingtown, PA). The ratio of fura 2 fluorescence intensity at the two excitation wavelengths (340/380 ratio) was used to determine the relative change in cytosolic Ca2+ levels. In certain experiments, cells were pretreated for 30 min with 30 μM BAPTA-AM or 2 mM EGTA (Calbiochem) before addition of oxidized LDL.
Quantification of MCP-1 protein.
MCP-1 in culture media that had been incubated with endothelial cells for 4 h was quantified with a sandwich ELISA technique, according to the manufacturer's instructions (R&D Systems).
Statistical analysis.
Student's t-test was used to determine the statistical significance of differences in measurements between B6 and C3H with the same treatment or between two different treatments within the same strain. Differences were considered statistically significant at P < 0.05.
RESULTS
Global gene expression analysis.
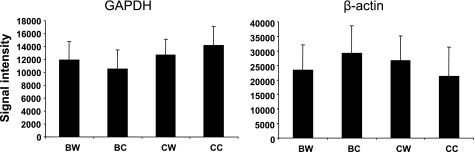
The overall quality of each expression chip was examined using six parameters, including background, noise, scale factor, 5′/3′ end ratios of GAPDH and β-actin transcripts, and signal intensity for low-abundance control cRNA BioB. As shown in Table 1, all quality assessment variables are within the acceptance limits suggested by Affymetrix.
Table 1.
Overall statistics on the quality of each chip used for assessing gene expression in the aorta of B6.apoE−/− and C3H.apoE−/− mice fed a chow or Western diet
| Acceptance Limits | BW | CW | BC | CC | |
|---|---|---|---|---|---|
| Background | <100 | 50.29 | 48.26 | 50.09 | 51.87 |
| Noise | <4 | 1.77 | 1.68 | 1.72 | 1.73 |
| Scale factor | 19.578 | 20.586 | 22.77 | 27.189 | |
| GAPDH 3′/5′ ratio | <4 | 1.28 | 1.15 | 1.03 | 1.19 |
| b-actin 3′/5′ ratio | <4 | 1.83 | 1.63 | 1.64 | 2.28 |
| BioB 3′ detection call | M or P | P | P | P | P |
| BioB 5′ detection call | M or P | P | P | P | P |
apoE−/−, apolipoprotein E deficient; BW, B6 mice fed with Western diet; CW, C3H mice on Western diet; BC, B6 mice on chow diet; CC, C3H mice on chow diet; M, mismatch; P, perfect match.
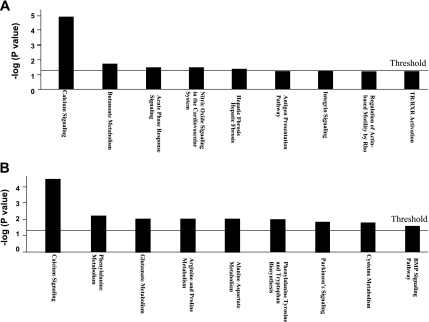
Gene expression in the aortic wall was compared between B6.apoE−/− and C3H.apoE−/− mice when fed a chow or Western diet. On the chow diet, 1,514 genes, which represented 14.7% of the 10,328 genes spotted on the array, were significantly differentially expressed between the two strains (P < 0.05; Supplemental Table 1). On the Western diet, 590 genes, which accounted for 5.7% of the genes on the array, were significantly differentially expressed (P < 0.05; Supplemental Table 2). In contrast, the signal intensities of two abundantly expressed housekeeping genes, GAPDH and β-actin, on the arrays were comparable among the four groups of mice (Fig. 1). The differentially expressed genes were then analyzed using Ingenuity Pathway Analysis software to identify underlying biological pathways. The most significantly enriched pathway was the calcium signaling pathway (Fig. 2; P = 1.48 × 10−5 for chow and P = 3.1 × 10−5 for Western). On the chow diet, 11 focus genes in the pathway were highly expressed in B6 relative to C3H, which included Actc1, Itpr2, Mef2c, Myh6, MyL3, MyL4, MyL7, Ryr2, Tnnc1, Tnni3, and Tnnt2 (Table 2). On the Western diet, 12 genes in the pathway were differentially expressed between the two strains, including Actc1, Atp2a2, Casq2, Chrna3, Myh6, MyL4, MyL7, Nfatc2, Ryr2, Tnnc1, Tnni3, and Tnnt2. In addition, on the chow diet, the acute phase response signaling pathway was significantly enriched (P = 0.03). Five focus genes in the pathway, including Hamp, Il6st, Rbp7, Socs3, and Trf, were differentially expressed between the two strains (Table 2). On the Western diet, the phenylalanine metabolism pathway was significantly enriched (P = 0.006). Got1, Got2, and Prdx2 in the pathway were highly expressed in B6 relative to C3H.
Table 2.
Pathways detected by Ingenuity pathway analysis of genes significantly differentially expressed in the aortic wall of B6.apoE−/− and C3H.apoE−/− mice fed a chow or Western diet
| Pathway Name | Gene Symbol | Gene Name | Affymetrix ID | Fold Change In | Fold Change P | Gene Product Location | Gene Ontology Type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chow diet | ||||||||||||||
| Calcium signaling | ACTC1 | Actin, α, cardiac muscle 1 | 1415927_at | 25.992 | 0.000020 | Cytoplasm | Other | |||||||
| MEF2C | Myocyte enhancer factor 2C | 1421028_a_at | 9.19 | 0.000101 | Nucleus | Transcription regulator | ||||||||
| MYH6 | Myosin, heavy chain 6, cardiac muscle, α (cardiomyopathy, hypertrophic 1) | 1448554_s_at | 157.586 | 0.000020 | Cytoplasm | Enzyme | ||||||||
| MYL3 | Myosin, light chain 3, alkali; ventricular, skeletal, slow | 1427768_s_at | 6.063 | 0.000618 | Cytoplasm | Other | ||||||||
| MYL4 | Myosin, light chain 4, alkali; atrial, embryonic | 1422580_at | 59.714 | 0.000052 | Cytoplasm | Other | ||||||||
| MYL7 | Myosin, light chain 7, regulatory | 1449071_at | 194.012 | 0.000020 | Cytoplasm | Enzyme | ||||||||
| RCAN2 | Regulator of calcineurin 2 | 1450243_a_at | 12.126 | 0.000046 | Unknown | Other | ||||||||
| RYR2 | Ryanodine receptor 2 (cardiac) | 1450123_at | 9.849 | 0.000552 | Plasma membrane | Ion channel | ||||||||
| TNNC1 | Troponin C type 1 (slow) | 1418370_at | 8.574 | 0.000068 | Cytoplasm | Other | ||||||||
| TNNI3 | Troponin I type 3 (cardiac) | 1422536_at | 24.251 | 0.000020 | Cytoplasm | Transporter | ||||||||
| TNNT2 | Troponin T type 2 (cardiac) | 1424967_x_at | 181.019 | 0.000020 | Cytoplasm | Other | ||||||||
| Butanoate metabolism | ALDH1A1 | Aldehyde dehydrogenase 1 family, member A1 | 1416468_at | 9.19 | 0.002250 | Cytoplasm | Enzyme | |||||||
| HMGCS2 | 3-Hydroxy-3-methylglutaryl-Coenzyme A synthase 2 (mitochondrial) | 1423858_a_at | 45.255 | 0.000030 | Cytoplasm | Enzyme | ||||||||
| MYO5B | Myosin VB | 1452298_a_at | −7.464 | 0.001486 | Cytoplasm | Enzyme | ||||||||
| Acute phase response signaling | HAMP | Hepcidin antimicrobial peptide | 1419197_x_at | 19.698 | 0.000078 | Extracellular space | Other | |||||||
| IL6ST | Interleukin 6 signal transducer (gp130, oncostatin M receptor) | 1421239_at | 12.996 | 0.000189 | Plasma membrane | Transmembrane receptor | ||||||||
| RBP7 | Retinol binding protein 7, cellular | 1449461_at | −36.758 | 0.000020 | Cytoplasm | Other | ||||||||
| SOCS3 | Suppressor of cytokine signaling 3 | 1455899_x_at | 32 | 0.000618 | Cytoplasm | Other | ||||||||
| TRF | Transferrin | 1425546_a_at | −12.996 | 0.000030 | Extracellular space | Transporter | ||||||||
| Nitric oxide signaling in the cardiovascular system | GUCY1A3 | Guanylate cyclase 1, soluble, α3 | 1420534_at | 12.996 | 0.000020 | Cytoplasm | Enzyme | |||||||
| PLN | Phospholamban | 1460332_at | 11.314 | 0.000130 | Cytoplasm | Other | ||||||||
| RYR2 | Ryanodine receptor 2 (cardiac) | 1450123_at | 9.849 | 0.000552 | Plasma membrane | Ion channel | ||||||||
| Hepatic fibrosis | COL1A2 | Collagen, type I, α2 | 1423110_at | 6.063 | 0.000020 | Extracellular space | Other | |||||||
| MYH6 | Myosin, heavy chain 6, cardiac muscle, α (cardiomyopathy, hypertrophic 1) | 1448554_s_at | 157.586 | 0.000020 | Cytoplasm | Enzyme | ||||||||
| MYL3 | Myosin, light chain 3, alkali; ventricular, skeletal, slow | 1427768_s_at | 6.063 | 0.000618 | Cytoplasm | Other | ||||||||
| MYL4 | Myosin, light chain 4, alkali; atrial, embryonic | 1422580_at | 59.714 | 0.000052 | Cytoplasm | Other | ||||||||
| MYL7 | Myosin, light chain 7, regulatory | 1449071_at | 194.012 | 0.000020 | Cytoplasm | Enzyme | ||||||||
| Western diet | ||||||||||||||
| Calcium signaling | ACTC1 | Actin, α, cardiac muscle 1 | 1415927_at | 68.594 | 0.00002 | Cytoplasm | Other | |||||||
| ATP2A2 | ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2 | 1452363_a_at | 3.732 | 0.00003 | Cytoplasm | Transporter | ||||||||
| CASQ2 | Calsequestrin 2 (cardiac muscle) | 1422529_s_at | 3.482 | 0.001486 | Cytoplasm | Other | ||||||||
| CHRNA3 | Cholinergic receptor, nicotinic, α3 | 1452010_at | 16 | 0.000618 | Plasma membrane | Transmembrane receptor | ||||||||
| MYH6 | Myosin, heavy chain 6, cardiac muscle, α (cardiomyopathy, hypertrophic 1) | 1448554_s_at | 119.428 | 0.000023 | Cytoplasm | Enzyme | ||||||||
| MYL4 | Myosin, light chain 4, alkali; atrial, embryonic | 1422580_at | 16 | 0.00002 | Cytoplasm | Other | ||||||||
| MYL7 | Myosin, light chain 7, regulatory | 1449071_at | 128 | 0.00002 | Cytoplasm | Enzyme | ||||||||
| NFATC2 | Nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 2 | 1425901_at | −8.574 | 0.00107700 | Nucleus | Transcription regulator | ||||||||
| RYR2 | Ryanodine receptor 2 (cardiac) | 1450123_at | 2.83 | 0.003355 | Plasma membrane | Ion channel | ||||||||
| TNNC1 | Troponin C type 1 (slow) | 1418370_at | 10.556 | 0.00013 | Cytoplasm | Other | ||||||||
| TNNI3 | Troponin I type 3 (cardiac) | 1422536_at | 137.187 | 0.00002 | Cytoplasm | Transporter | ||||||||
| TNNT2 | Troponin T type 2 (cardiac) | 1424967_x_at | 147.033 | 0.00002 | Cytoplasm | Other | ||||||||
| Phenylalanine metabolism | GOT1 | Glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1) | 1450970_at | 3.732 | 0.003355 | Cytoplasm | Enzyme | |||||||
| GOT2 | Glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) | 1417716_at | 4 | 0.001077 | Cytoplasm | Enzyme | ||||||||
| PRDX2 | Peroxiredoxin 2 | 1430979_a_at | 11.314 | 0.00225 | Cytoplasm | Enzyme | ||||||||
| Glutamate metabolism | GOT1 | Glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1) | 1450970_at | 3.732 | 0.003355 | Cytoplasm | Enzyme | |||||||
| GOT2 | Glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) | 1417716_at | 4 | 0.001077 | Cytoplasm | Enzyme | ||||||||
| GPT2 | Glutamic pyruvate transaminase (alanine aminotransferase) 2 | 1455007_s_at | −45.255 | 0.00003000 | Unknown | Enzyme | ||||||||
| Arginine and proline metabolism | CKMT2 | Creatine kinase, mitochondrial 2 (sarcomeric) | 1428722_at | 13.929 | 0.000046 | Cytoplasm | Kinase | |||||||
| GAMT | Guanidinoacetate N-methyltransferase | 1422558_at | −12.126 | 0.00249000 | Cytoplasm | Enzyme | ||||||||
| GOT1 | Glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1) | 1450970_at | 3.732 | 0.003355 | Cytoplasm | Enzyme | ||||||||
| GOT2 | Glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) | 1417716_at | 4 | 0.001077 | Cytoplasm | Enzyme | ||||||||
| Alanine and aspartate metabolism | GOT1 | Glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1) | 1450970_at | 3.732 | 0.003355 | Cytoplasm | Enzyme | |||||||
| GOT2 | Glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) | 1417716_at | 4 | 0.001077 | Cytoplasm | Enzyme | ||||||||
| GPT2 | Glutamic pyruvate transaminase (alanine aminotransferase) 2 | 1455007_s_at | −45.255 | 0.00003000 | Unknown | Enzyme | ||||||||
| GOT1 | Glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1) | 1450970_at | 3.732 | 0.003355 | Cytoplasm | Enzyme | ||||||||
| GOT2 | Glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) | 1417716_at | 4 | 0.001077 | Cytoplasm | Enzyme | ||||||||
| Parkinson's signaling | MAPK14 | Mitogen-activated protein kinase 14 | 1416704_at | −6.063 | 0.00304100 | Cytoplasm | Kinase | |||||||
| SNCA | Synuclein, α (non-A4 component of amyloid precursor) | 1436853_a_at | −6.964 | 0.00011400 | Cytoplasm | Other | ||||||||
| Cysteine metabolism | GOT1 | Glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1) | 1450970_at | 3.732 | 0.003355 | Cytoplasm | Enzyme | |||||||
| GOT2 | Glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) | 1417716_at | 4 | 0.001077 | Cytoplasm | Enzyme | ||||||||
| HS3ST1 | Heparan sulfate (glucosamine) 3-O-sulfotransferase 1 | 1423450_a_at | −3.482 | 0.00016700 | Cytoplasm | Enzyme | ||||||||
| BMP signaling pathway | BMPR2 | Bone morphogenetic protein receptor, type II (serine/threonine kinase) | 1419616_at | −17.148 | 0.00034600 | Plasma membrane | Kinase | |||||||
| EGFR | Epidermal growth factor receptor [erythroblastic leukemia viral (v-erb-b) oncogene homolog, avian] | 1424932_at | 7.464 | 0.003355 | Plasma membrane | Kinase | ||||||||
| MAPK14 | Mitogen-activated protein kinase 14 | 1416704_at | −6.063 | 0.00304100 | Cytoplasm | Kinase | ||||||||
| SMAD4 | SMAD family member 4 | 1422485_at | 3.249 | 0.00013 | Nucleus | Transcription regulator | ||||||||
Fig. 1.
Expression levels of the housekeeping genes GAPDH and β-actin in the aorta of B6.apoE−/− (apolipoprotein E deficient) and C3H.apoE−/− mice fed a chow or Western diet, as determined in microarray experiment. Results are means ± SD of 3 separate probe sets for each gene. Expression levels are depicted as relative signal intensities of specific probes on the expression arrays. BW and BC, B6 mice on Western and chow diet, respectively; CW and CC, C3H mice on Western and chow diet, respectively.
Fig. 2.
Canonical pathway analysis of genes differentially expressed in the aortic wall of B6.apoE−/− and C3H.apoE−/− mice fed a chow (A) or Western diet (B). The x-axis depicts the pathways detected, and the y-axis depicts the negative log of the P values calculated using the right-tailed Fisher's exact test. The horizontal line on the plot represents significance threshold for all pathways, as calculated by Ingenuity Pathway Analysis software. Only significantly differentially expressed genes that were not labeled as “absent” for both B6 and C3H mice in the microarray analysis were subject to pathway analysis. TR, thyroid hormone receptor; RXR, retinoid X receptor; BMP, bone morphogenetic protein.
Confirmation of microarray result by real-time PCR.
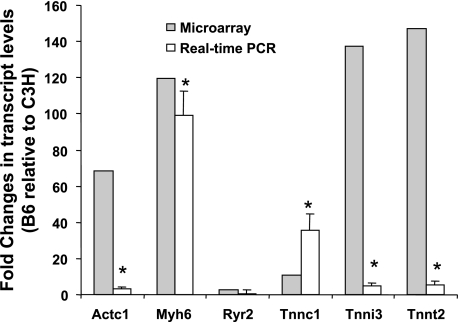
Six genes in the calcium signaling pathway, including Actc1, Myh6, Ryr2, Tnnc1, Tnni3, and Tnnt2, which were found to be highly expressed in B6 mice on both chow and Western diets in the microarray analysis, were further analyzed by real-time PCR using RNA prepared from the mice fed the Western diet. Five of six genes tested were confirmed to be highly expressed in the aorta of B6 relative to C3H (Fig. 3).
Fig. 3.
Fold changes in expression levels of 6 genes (Actc1, Myh6, Ryr2, Tnnc1, Tnni3, Tnnt2) in the aortic walls of B6.apoE−/− mice relative to C3H.apoE−/− mice when fed a Western diet. The shaded bars show fold changes in gene expression assessed by microarray analysis. The open bars show fold changes in gene expression measured by real-time PCR. Microarray analysis was performed on RNA pooled from four individual mice for each strain. Real-time PCR was performed on 3–6 biologically independent RNA samples for each strain. *P < 0.05 vs. C3H.
Calcium signaling pathway mediates oxidized LDL-induced MCP-1 production.
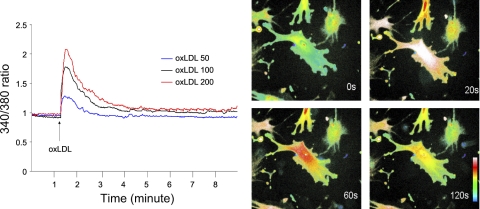
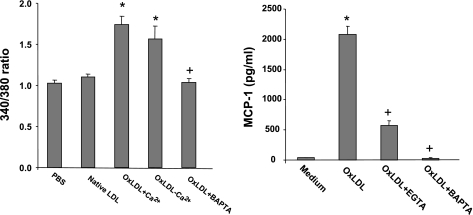
We previously demonstrated a marked difference between B6 and C3H mice in the response of endothelial cells to oxidized LDL with regard to the induction of inflammatory genes (13). Thus the role of calcium signaling in oxidized LDL-induced gene induction was tested in endothelial cells from B6 mice. Oxidized LDL induced a prompt but transient increase in [Ca2+]i levels in a dose-dependent manner (Fig. 4). The dose-dependent effect was observed with 50, 100, and 200 μg/ml oxidized LDL. [Ca2+]i reached a peak ∼20 s after addition of oxidized LDL and then decreased gradually. In contrast, neither PBS nor native LDL had any influence on [Ca2+]i levels (Fig. 5). The contribution of extracellular Ca2+ to the rise in [Ca2+]i was evaluated by incubating the cells with Ca2+-free Krebs-Ringer buffer. Absence of extracellular Ca2+ had little influence on the rise, indicating that Ca2+ was primarily released from intracellular stores (Fig. 5). Pretreatment with the intracellular calcium-trapping compound BAPTA completely abolished the Ca2+ increase induced by oxidized LDL.
Fig. 4.
Oxidized low-density lipoprotein (oxLDL)-induced Ca2+ release in endothelial cells from B6 mice. Left: changes over time in cytosolic Ca2+ levels after stimulation with various doses of oxLDL. The increase in the ratio of fluorescence at wave length 340 nm to 380 nm indicates a rise in intracellular free Ca2+. oxLDL induced a dose-dependent increase in Ca2+ levels. Right: confocal microscopy images showing changes in intracellular distribution of Ca2+ labeled with fluorescent dye fura 2 at 0, 20, 60, and 120 s in endothelial cells from B6 mice after addition of oxLDL.
Fig. 5.
Left: cytosolic Ca2+ levels in endothelial cells of B6 mice after stimulation with PBS, native LDL, oxLDL in buffer with Ca2+, oxLDL in buffer without Ca2+, or oxLDL + BAPTA. *P < 0.05 compared with PBS; +P < 0.05 compared with oxLDL. Values are means ± SD of 3–5 assays. Right: effects of calcium chelator EGTA or intracellular calcium trapping compound BAPTA on oxLDL-induced monocyte chemoattractant protein-1 (MCP-1) production in endothelial cells from B6 mice. Endothelial cells were treated with medium only, 100 μg/ml oxLDL, oxLDL + EGTA, or oxLDL + BAPTA for 4 h. Results are means ± SD of 4 experiments. *P < 0.05 vs. medium; +P < 0.05 compared with oxLDL.
We then examined the role of calcium signaling in oxidized LDL-induced MCP-1 production in endothelial cells from B6 mice. Treatment with oxidized LDL (100 μg/ml) resulted in a 48-fold increase over control in MCP-1 concentration in the medium incubated with endothelial cells (2,080 ± 138 vs. 44 ± 2 pg/ml; P = 0.00004; Fig. 5). Pretreatment with the calcium chelator EGTA resulted in a significant reduction in MCP-1 levels (566 ± 92 pg/ml; P = 0.000002), and pretreatment with BAPTA abolished MCP-1 production induced by oxidized LDL (33.2 ± 3.5 pg/ml; P = 0.00004).
DISCUSSION
In this study, we used a microarray expression profiling approach to identify genes and pathways that might contribute to the marked difference between B6.apoE−/− and C3H.apoE−/− mice in atherosclerosis susceptibility. We examined gene expression in the aortic walls when the two strains were fed a chow or Western diet and identified numerous genes that were significantly differentially expressed in the two strains fed either diet. Pathway analysis of differentially expressed genes identified the calcium signaling pathway, as well as several other pathways that might be implicated in control of atherosclerosis susceptibility. In vitro studies with endothelial cells from B6 mice demonstrated that calcium signaling mediated the effect of oxidized LDL on inflammatory gene induction.
Our laboratory previously demonstrated that the arterial wall is a source of the marked difference between B6 and C3H mice in atherosclerosis susceptibility (9). Early studies of the arterial wall of the two strains focused on one or a few genes of interest (13, 14), thus severely narrowing the scope of the findings. Due to the polygenic nature of atherosclerosis, recent studies have taken advantage of the microarray technology to examine genome-wide gene expression differences between the strains (18, 20). The genome-wide transcriptional profiling of aortas from wild-type B6 and C3H mice identified a list of candidate genes relevant to growth, differentiation, inflammation, cathecholamine synthesis, phosphatase activity, peroxisome function, insulin-like growth factor activity, and antigen presentation (18). However, in the diet-induced mouse model, atherosclerotic lesions are small and consist almost entirely of macrophage foam cells, and they are largely limited to the aortic root (8). Thus the findings from this model may not adequately reflect the situation of human atherosclerosis, which typically occurs at vessel branch points and progresses from fatty streaks to fibrous plaques to complicated lesions. In contrast, apoE−/− mice develop lesions that are similar in many aspects to human lesions (7). In an intercross derived from B6.apoE−/− and C3H.apoE−/− parental strains, Wang et al. (20) performed expression array analysis of the liver and adipose tissues and identified > 10,000 expression quantitative trait loci. However, the vessel wall, a tissue most relevant to atherosclerosis, had not been examined. In the present study, we evaluated transcript levels in the aortic wall of 6-wk-old apoE−/− mice that had been fed a chow or a Western diet for 2 wk. Under these conditions, the descending aorta, whereby RNA was extracted, had no detectable atherosclerotic lesions for both apoE−/− strains (data not shown). ApoE−/− mice develop spontaneous hyperlipidemia and atherosclerosis in a time-dependent manner. In previous studies, Nakashima et al. (7) examined atherosclerotic lesion formation in apoE−/− mice fed chow and Western diets and at ages ranging from 6 to 40 wk and observed no foam cell formation at 6 wk. At 8 wk of age, when fed the Western diet, apoE−/− mice developed foam cell lesions. Gene expression profiles would be difficult to interpret if atherosclerotic lesions had developed, because B6.apoE−/− mice develop larger atherosclerotic lesions in the aorta than C3H.apoE−/− mice (14) and because different cellular components have different gene expression patterns. It is worth noting that female mice become sexually mature at 6 wk after birth (http://ko.cwru.edu/docs/breeding_strategies_manual.pdf).
There were much more differentially expressed genes in the two strains when fed the chow diet than the Western diet. The two strains also exhibited much larger differences in atherosclerotic lesion size on the chow diet (>100-fold) than on the Western diet (∼10-fold) (14). The reasons for the discrepancy in gene expression or lesion size on the chow vs. the Western diet are unknown. One likely explanation is that, on the Western diet, both strains develop severe hyperlipidemia, which might overwhelm the influence of genetic factors on gene expression or lesion formation, whereas, on the chow diet, the two strains have mild hyperlipidemia, which might have little impact on genetic influence to gene expression or lesion formation.
Pathway analysis of differentially expressed genes in the aortic walls of the two strains pointed out a role for the calcium signaling pathway in regulation of atherosclerosis susceptibility. Ca2+ is a key intracellular second messenger in almost all eukaryotic cells, including endothelial cells and vascular smooth muscle cells. In endothelial cells, it serves as a positive or negative regulatory signal for a variety of cell functions, such as secretion of vasoactive substances, regulation of blood-tissue permeability, and modulation of vascular tone (19). There is also evidence supporting that calcium signaling in endothelial cells is involved in the pathogenesis of atherosclerosis. It is well established that the regions of disturbed blood flow and low wall shear stress at arterial bifurcations are predisposing to atherosclerosis. Endothelial calcium levels in these regions are found to be altered, and this alteration has been suggested to be a contributing factor to the onset of atherosclerosis (10). Oxidized LDL is also known to play a key role in the initiation and progression of atherosclerosis. It is a potent inducer of a variety of inflammatory genes involved in atherogenesis (17). Our present study clearly showed that oxidized LDL induced a [Ca2+]i rise in a dose-dependent manner. The oxidized LDL-induced intracellular Ca2+ rise has been found in macrophages (5), smooth muscle cells (22), and T lymphocyte cells (6). Moreover, we found that the Ca2+ rise after stimulation with oxidized LDL was primarily due to release of Ca2+ from intracellular stores, as absence of extracellular Ca2+ had little influence on the rise.
In the present study, we found that the calcium signaling pathway mediated oxidized LDL-induced MCP-1 production in endothelial cells. Indeed, the Ca2+ chelator EGTA and the Ca2+ trapping compound BAPTA nearly completely abolished the induction. Our laboratory previously observed that oxidized LDL induces dramatic induction of MCP-1, macrophage-colony-stimulating factor, and heme oxygenase-1 in endothelial cells from B6 mice, whereas the induction in C3H is limited (13). Our present study shows that calcium signaling mediates the activation process by which oxidized LDL results in increased inflammatory molecule production in endothelial cells, leading to vascular wall inflammation. Thus our findings suggest that genetic variations (polymorphisms/mutations) that affect the expression of genes involved in calcium signaling contribute to variation in atherosclerosis susceptibility. Female mice were used for the present study because they are more susceptible to atherosclerosis than their male counterparts. However, these findings may not be extrapolated to male mice since they were not studied.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL75433.
Supplementary Material
Acknowledgments
This study has been partly reported in Arteriosclerosis, Thrombosis and Vascular Biology Annual Conference 2008 as an oral presentation.
REFERENCES
- 1.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 1: 25–29, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MD, Jin L, Jien ML, Matsumoto AH, Helm GA, Lusis AJ, Frank JS, Shi W. Lipid retention in the arterial wall of two mouse strains with different atherosclerosis susceptibility. J Lipid Res 45: 1155–1161, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bystrykh L, Weersing E, Dontje B, Sutton S, Pletcher MT, Wiltshire T, Su AI, Vellenga E, Wang J, Manly KF, Lu L, Chesler EJ, Alberts R, Jansen RC, Williams RW, Cooke MP, de Haan G. Uncovering regulatory pathways that affect hematopoietic stem cell function using “genetical genomics”. Nat Genet 37: 225–232, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Havel RJ, Eder EA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins of human serum. J Clin Invest 43: 1345–1353, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumura T, Sakai M, Kobori S, Biwa T, Takemura T, Matsuda H, Hakamata H, Horiuchi S, Shichiri M. Two intracellular signaling pathways for activation of protein kinase C are involved in oxidized low-density lipoprotein-induced macrophage growth. Arterioscler Thromb Vasc Biol 17: 3013–3020, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Maziere C, Morliere P, Massy Z, Kamel S, Louandre C, Conte MA, Maziere JC. Oxidized low-density lipoprotein elicits an intracellular calcium rise and increases the binding activity of the transcription factor NFAT. Free Radic Biol Med 38: 472–480, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 14: 133–140, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Paigen B, Holmes PA, Mitchell D, Albee D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis 64: 215–221, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Pei H, Wang Y, Miyoshi T, Zhang Z, Matsumoto AH, Helm GA, Tellides G, Shi W. Direct evidence for a crucial role of the arterial wall in control of atherosclerosis susceptibility. Circulation 114: 2382–2389, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Plank MJ, Wall DJ, David T. Atherosclerosis and calcium signalling in endothelial cells. Prog Biophys Mol Biol 91: 287–313, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, Colinayo V, Ruff TG, Milligan SB, Lamb JR, Cavet G, Linsley PS, Mao M, Stoughton RB, Friend SH. Genetics of gene expression surveyed in maize, mouse and man. Nature 422: 297–302, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, Tuttle JB. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer's disease. J Neurosci 17: 4612–4622, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation 102: 75–81, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Shi W, Wang NJ, Shih DM, Sun VZ, Wang X, Lusis AJ. Determinants of atherosclerosis susceptibility in the C3H and C57BL/6 mouse model: evidence for involvement of endothelial cells but not blood cells or cholesterol metabolism. Circ Res 86: 1078–1084, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Shi W, Wang X, Wang NJ, McBride WH, Lusis AJ. Effect of macrophage-derived apolipoprotein E on established atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 20: 2261–2266, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Shi W, Wang X, Wong J, Hedrick CC, Wong H, Castellani LW, Lusis AJ. Effect of macrophage-derived apolipoprotein E on hyperlipidemia and atherosclerosis of LDLR-deficient mice. Biochem Biophys Res Commun 317: 223–229, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg D Thematic review series: the pathogenesis of atherosclerosis: an interpretive history of the cholesterol controversy. III. Mechanistically defining the role of hyperlipidemia. J Lipid Res 46: 2037–2051, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Tabibiazar R, Wagner RA, Spin JM, Ashley EA, Narasimhan B, Rubin EM, Efron B, Tsao PS, Tibshirani R, Quertermous T. Mouse strain-specific differences in vascular wall gene expression and their relationship to vascular disease. Arterioscler Thromb Vasc Biol 25: 302–308, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Tran QK, Watanabe H. Calcium signalling in the endothelium. Handb Exp Pharmacol 176: 145–187, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Wang SS, Schadt EE, Wang H, Wang X, Ingram-Drake L, Shi W, Drake TA, Lusis AJ. Identification of pathways for atherosclerosis in mice: integration of quantitative trait locus analysis and global gene expression data. Circ Res 101: e11–e30, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Ishimori N, Korstanje R, Rollins J, Paigen B. Identifying novel genes for atherosclerosis through mouse-human comparative genetics. Am J Hum Genet 77: 1–15, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisser B, Locher R, Mengden T, Vetter W. Oxidation of low density lipoprotein enhances its potential to increase intracellular free calcium concentration in vascular smooth muscle cells. Arterioscler Thromb 12: 231–236, 1992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.