Abstract
Functional and trophic perspectives on patterns of species occurrences have the potential to offer new and interesting insights into a range of spatially explicit problems in ecology and conservation. We present the function–area relationship (FAR) and explore linkages between functional and taxonomic species richness for South African birds. We first used beak morphology to classify a subset of 151 South African bird species into 18 functional groups and calculated both the species–area relationship and the FAR at quarter-degree resolution for South Africa. The relationship between functional and taxonomic richness by cell was quadratic rather than linear, with considerable scatter around the curve. We next looked at the spatial relationships between taxonomic diversity and response diversity (i.e. diversity within functional groups) using an a priori categorization of nearly all South African birds into nine functional groups. The spatial distribution of response richness also showed considerable variation in relation to taxonomic richness. Our results demonstrate a novel approach to linking taxonomic, functional and trophic patterns in space and suggest a way in which conservation planning, which has traditionally had a taxonomic focus, could formally incorporate a more functional and food-web-based approach.
Keywords: ecosystem function, resilience, conservation planning, food web, bird, South Africa
1. Introduction
The study of diversity has been the source of many insights in ecology and other disciplines. In ecology, diversity has been closely equated with the abundance and evenness of members of different species occurring within a single area. Diversity as a field of scientific research has focused on a few central themes that include: (i) an historical and evolutionary component (in particular, questions relating to how species evolved in or dispersed into an area), (ii) the pattern–process linkages that create spatial and temporal variation in community composition, ranging from abiotic variation through biotic interactions to anthropogenic effects, (iii) the importance of biodiversity, in terms of its contributions to ecosystem services and human wellbeing, and (iv) the conservation of biodiversity, with particular emphasis on rare and endangered species, highly diverse areas (‘hotspots’), umbrella and flagship species, and other heuristic approaches that are intended to guide conservation action.
Despite their common subject matter, these different themes have not always remained unified within the general area of biodiversity research. For example, the influence of competition on community structure and evolutionary processes remains an important area of research in theoretical ecology, but is seldom considered in conservation planning or site prioritization (with the possible exception of studies of alien invasive species). As a result, a certain disparity has arisen between some of the different threads of biodiversity research. This disparity is particularly apparent in the division between functional and taxonomic perspectives in both theoretical and applied ecology. Although many recent theoretical studies have shown that functional perspectives can offer valuable insights into ecosystem patterns and processes, and although there is a growing trend in ecosystem management to focus on processes, research tends to focus on either a functional or a taxonomic perspective and conservation biology remains firmly focused on taxonomic species for nearly all broad-scale planning and decision making (e.g. Pressey et al. 1996; Margules & Pressey 2000; Wilson et al. 2005). Similarly, food-web theory has only recently begun to explicitly incorporate aspects of spatial variation into its general paradigms (Polis et al. 2004).
Exercises such as the Millennium Ecosystem Assessment have further emphasized the importance of understanding the consequences of species loss for ecosystem function (Dobson et al. 2006; Cumming 2007). Quantification of species loss alone does not capture the likelihood of food web collapse, because similar functions may be performed by several different species that essentially provide the same ecosystem functions and services. While documentation of variation in functional groups does not fully capture food-web dynamics, it allows general consideration of changes by trophic level over broad scales and by extension offers a way of linking traditional taxonomic studies and studies of food webs. In this paper, we develop a novel approach to link functional, trophic and taxonomic perspectives through the consideration of similarities and differences in spatial patterns. We first discuss the functional equivalent of the species–area relationship (SAR) and then present two empirical case studies, one looking at taxonomic and functional richness and the other at taxonomic richness and response richness (where response richness is defined as the number of species within a given functional group). As our results show, quantifying the spatial differences between taxonomic and functional patterns offer a powerful way of reconciling different research streams in ecology, giving insights into fundamental changes in food webs and yielding novel and sometimes surprising insights.
2. The function–area relationship
While the SAR is one of the most widely accepted generalities in ecology (Fisher et al. 1943; Rosenzweig 1995), little attention has been paid to patterns of spatial variation in functional diversity. Functional classifications describe species according to what they do, providing an alternative taxonomy that relates closely to ecosystem function and trophic relationships (Simberloff & Dayan 1991; Kinzig et al. 2001; Lavorel & Garnier 2001; Naeem 2002). Functional perspectives on patterns of species occurrences have the potential to offer new and interesting insights into a range of spatially explicit problems in ecology and conservation, such as the quantification of spatial variation in the resilience of ecosystems to species loss and the integration of ecosystem processes into conservation planning exercises.
The SAR is commonly defined as
| (2.1) |
where S is the number of taxonomic species; c is a constant; A is the area; and z describes the slope of the curve. Although the values of z and c vary with scale and location (Rosenzweig 1995), and there has been considerable debate about both the mechanisms that produce the SAR and its biological significance (Hubbell 2001; Chave et al. 2002), the general form of the SAR has been shown to hold for a wide variety of different systems. As a consequence, the SAR has been used in numerous conservation and planning assessments as a method for quantifying the probable decline in local species richness that corresponds to a quantified decline in the total area of available habitat (Carpenter et al. 2005).
If individual species are each assigned to a single functional group based on a quantitative assessment of functional traits, we would expect most functional groups to contain more than one species. Consequently, there will always be fewer functional groups than taxonomic species. The character traits of species effectively define the dimensions along which functional traits can be distributed (Petchey & Gaston 2002). Given what is known about the distributions of characters within taxonomic groupings, representatives of some functional groups will be relatively common while others are relatively rare (Hubbell 2001). An empirically derived functional group–taxonomic species curve should therefore exhibit a logarithmic form, with early samples of individual organisms rapidly adding functional groups and subsequent sampling gradually contributing fewer and fewer novel functional groups. Based on this assumption, we propose that functional groups will be distributed among species in the same way that species are distributed in geographical space, according to a SAR-like equation of the form
| (2.2) |
where F is the number of functional groups; S is the number of species sampled; and b and y are constants.
By substituting the relationship in equation (2.2) into the SAR, we obtain the general expected form of a function–area relationship (FAR) as
| (2.3) |
where b, c, y and z are empirically derived constants from equations (2.1) and (2.2). Given that each of these constants is expected to be less than 1, the FAR will follow a similar form to the SAR but with an earlier asymptote and a shallower gradient. Note that permitting species to perform more than one function would alter the relative magnitudes of the SAR and the FAR, but the same equation should still hold. Although most species are clearly capable of performing more than one ecological function, limiting the problem initially to a single function per species offers a more approachable starting point for linking taxonomic and functional diversity.
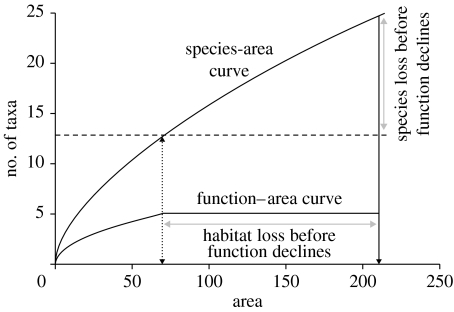
Comparison of the FAR and the SAR for the same data suggests that the FAR may offer a simple but effective way of quantifying two important variables (figure 1). The first of these is the total area of habitat that can be lost before ecological functions (as represented by entire functional groups) start to be lost. This quantity is only a rough approximation because it assumes random species loss by functional group, which is unlikely to be a tenable assumption in a real-world system; but it does provide a tentative first answer to the question of how large an area we can lose before the ecosystem functions derived from the taxonomic group under study are impacted. The second variable that the SAR–FAR contrast can help to quantify is the resilience of the system's functional components to species loss. If species are lost randomly from the system, the difference in areas under the SAR and the FAR respectively captures the degree to which redundancy in functions occurs and hence the depth of the pool of species that perform the same function. If the FAR is only slightly below the SAR, nearly every new species adds a new function and the system is less likely to be resilient to species loss. For example, suppose that out of ten vulture species, two have resistance to poisoning by a drug such as diclorofenac. In a system with eight species, it is four times more likely that the functions performed by vultures will be resilient to an episode of diclorofenac use than it is in a system with only one vulture species.
Figure 1.
Schematic diagram showing species–area and function–area curves for 25 species in a hypothetical 250 km2 landscape. Taxonomic richness is fully represented at 220 km2. In this hypothetical example, 65 km2 represents the area below which functional group loss would be expected to occur if local extinctions were random. The difference between the two curves indicates a ‘richness buffer’ for the maintenance of ecosystem function. Lower curve, function area; upper curve, species area.
Although the FAR curve defined here is new to ecology, it is not surprising. Its main value lies in the framework that it provides for thinking about the ways to contrast spatial patterns in taxonomic and functional richness. There should in nearly in all cases be discrepancies between species richness, functional group richness and response richness at any one point in space. Area curves average over spatial variation, but the most interesting ecological and conservation-oriented parts of the problem lie in spatially explicit details, to which we now turn.
3. Material and methods
(a) Defining functional groups by beak morphology
To provide an empirical test of the predicted form of the FAR and to explore spatial relationships between taxonomic and functional richness, we undertook an analysis using data for 151 randomly selected species of South African birds (constituting approximately a sixth of the known total fauna of approx. 950 bird species in southern Africa). For this test, we developed a quantitative classification based on beak morphology, which relates closely to what birds eat and consequently to their role in the ecosystem (Bock 1966; Ricklefs & Travis 1980; Simberloff & Dayan 1991; Hertel 1994). Specimens were sampled from collections held in the South African National Museum, Cape Town; and the Transvaal Museum, Pretoria. In total, 2532 specimens corresponding to 50 different families were sampled. Overall, 17±4 specimens were measured per species, the green barbet (Stactolaema olivacea) being the only species with less than 10 specimens (n=8). On each specimen gape width, bill depth, cere width, culmen length, culmen hypotenuse and head width were measured to the nearest 0.1 mm using a pair of Manostat dial callipers; lower beak length was measured to the nearest mm; and both degree of curvature (how far the upper beak tip protrudes past the lower mandible) and the slope of the upper mandible (tangential to upper beak tip) were measured to the nearest degree using a protractor (Fryer 1988; Simberloff & Dayan 1991; Hertel 1994; Winker 1998; Lavorel & Garnier 2001; Herrel et al. 2005a). Where applicable, measurements were taken from the anterior edge of the cere in order to make results comparable with other studies. Head width measurements were taken at the anterior edge of the eye socket. All measurements were recorded three times before the mean value was recorded. Only variables that were thought to directly relate to the functional efficacy of species were included in the final analysis (table 1). All metrics were directly measured from the specimens except the ‘active beak area’ that was calculated using the culmen length and culmen hypotenuse of specimens.
Table 1.
Justification for different morphological measurements used to define functional groups for 151 species of South African birds.
| functional variable | justification |
|---|---|
| gape width | limits the size of prey item a species is able to process per unit time (Herrera 1984; DeWitt et al. 2000; Nosil & Reimchen 2005) |
| bill depth/cere width | primary indicators of bite force (for example, Herrel et al. 2005a,b) |
| culmen length | functionally linked with cere width (Björklund 1992), thus auxiliary to bite force. Tool for probing specific substrates (e.g. Snow & Snow 1972) |
| head width | proxy of cranial kinesis (Bock 1964; Bout & Zweers 2001): lightly built kinetic skulls are characteristic of birds that feed on small plant material (including pecking birds) or insects, which do not require the resistance of large external forces on the jaws as in raptors eating larger prey (Bout & Zweers 2001). Determinant of bite force (Herrel et al. 2005a) |
| curvature | facilitates nectivore–host interactions (e.g. Temeles & Kress 2003). The extent of maxillary overhang is presumably important for raptors subduing prey |
| slope of beak | important component of compression stress during biting or pecking action (Bock 1966), such that a steeply sloping (strongly decurved) is most suitable for those birds capable of large bite forces |
| lower beak length | in some species (for example, within the Picidae family) there is a shock absorbing function (Bock 1966; see §3 for further discussion). This ability to directly alter hard substrates then opens up new resource pools for other species, such as bark probing gleaners (sensuOdling-Smee et al. 1996; Laland et al. 1999). This might be thought of as a latent functional tool for the indirect processing of such resources (‘processing chains’ sensuHeard 1994) |
| active beak area | delimits the surface over which it is posited that the beak interacts most with its environment, thereby potentially setting up a selective pressure for plasticity of the trait (Relyea & Auld 2005). The dorsal surface tracks the trajectories of greatest stress operating on the upper mandible (Bock 1966) |
These data were used to divide birds quantitatively into 18 functional groups (Milligan & Cooper 1987; Legendre & Legendre 1998; Jax 2006). There is no completely satisfactory method for determining the optimal number of clusters in a large multivariate dataset (McGarigal et al. 2000). We started by generating a plot of linkage distances across amalgamation steps and found that a clear plateau was reached at linkage distance Wkm=4. This corresponded to a cluster number of k=18 (figure 2). In order to assess this heuristically optimal cluster number, linkage distances corresponding to k=23 (Wkm=3) and k=13 (Wkm=7.8) were included for analysis. Although k=23 contains the maximum connectedness among species (average Co=0.57±0.35) and both the minimum sum of within-cluster distances and within-cluster standard error (average C-index of distance=1.17±0.74 and average standard error C-index=6.93±8.15), k=18 and 13 tend to maximize isolation (1.5±1.6 and 1.9±2, respectively for Euclidean distances; 20.7±21.5 and 31.5±24.1, respectively for ultrametric distances). This implies that k=18 offers an optimal combination of both cluster cohesion and isolation. The k=18 solution has 11 clusters in common with k=23 but only four in common with k=13, indicating only a small loss in information between k=18 and 23. Indeed, none of the parameters between k=18 and 23 are significantly different (−0.77<t44,42<0.43, all p>0.05, n=44).
Figure 2.
Plot of the first two morphological components with 18 clusters overlaid onto the reduced space domain. All group centroids are significantly different from one another (ANOVA: 67.9<F17,135<844.6, all p≪0.01, n=151). The first component (PC1) relates primarily to bill size, while the second component (PC2) captures bill shape. The appearance of cluster overlap is an artefact of projecting multidimensional data onto a two-dimensional space.
Comparison of results with a dendrogram produced from the component scores of the first two principal components suggested that the number of clusters remained most stable at linkage distance Wkm=4 and 7.8 (PCA groups=17 compared with 18; and PCA groups=11 compared with 11, respectively). For linkage distance Wkm=3 (k=26), however, there was a loss of five groups (PCA groups=21). At each linkage level, all morphometric variables were significant in distinguishing the functional group structure (MANOVA: all Wilk's lambda<0.01, all 2.5<F9,151<26.1, all p≪0.01). The group structure of k=13 (Wkm=7.8) provided the most satisfactory recovery with the discriminant model classifying 94.1 per cent of the species correctly. Both k=18 and 23 were on a par in classification recovery (92.1 and 92.6% correct, respectively).
Once we had developed the functional group classification, we used it together with data from the South African Bird Atlas (Harrison et al. 1997) to generate a FAR and to compare the relationship between taxonomic and functional diversity at a quarter-degree grid cell resolution across South Africa.
(b) Functional groups defined a priori
The massive, recent revision and expansion of ‘Roberts' Birds of Southern Africa’ (Hockey et al. 2005) was accompanied by the development of a database recording key attributes of different bird species. These data comprise the single most authoritative source presently available on the foraging ecology of Southern African birds. We used them to assign birds to nine different categories, based primarily on the breakdown provided by Sekercioglu (2006), although we added the category of granivores as an additional functional group. The classification included 950 species and differed from the beak morphological analysis in that birds could be assigned to multiple functional groups. The name and justification for each functional group are given in table 2.
Table 2.
Functional group categories derived from Sekercioglu (2006) and their definition. (The numbers in each cell are defined by the scores listed under ‘functional trait’. Note that raptors as used here includes animals not traditionally considered raptors, such as herons and egrets that feed on fishes and frogs; and that birds can belong to more than one functional group.)
| functional trait | categories | seed dispersers | pollinators | nutrient depositors | insectivores | granivores | grazers | raptors | scavengers | ecosystem engineers |
|---|---|---|---|---|---|---|---|---|---|---|
| habitat | includes wetland, estuarine, coastal, marine | 2,3 | ||||||||
| 3 main habitat | ||||||||||
| 2 secondary | ||||||||||
| habitat | ||||||||||
| 1 occasional habitat | ||||||||||
| 0 not used | ||||||||||
| food source | carrion | 1,2,3 | ||||||||
| mammal | 2,3 | |||||||||
| 3 main food | bird | 2,3 | ||||||||
| 2 secondary food | reptile | 2,3 | ||||||||
| 1 occasional food | fish | 2,3 | ||||||||
| 0 not eaten | other aquatic vertebrates | 2,3 | ||||||||
| terrestrial invertebrates | 3 | |||||||||
| freshwater invertebrates | 3 | |||||||||
| marine invertebrates | 3 | |||||||||
| fruits | 2,3 | |||||||||
| seeds | 3 | |||||||||
| nectar | 1,2,3 | |||||||||
| other plant parts | 2,3 | |||||||||
| engineering | makes cavities or burrows; or has large or highly colonial nests | 1 | ||||||||
| 1 yes | ||||||||||
| 0 no |
The occurrences of different functional groups were again mapped out using the South African Bird Atlas data (Harrison et al. 1997). The goal of this step of the analysis was to compare spatial patterns of within-functional group richness (‘response diversity’) to taxonomic richness; with 950 species being placed into only nine functional groups, it made little sense to compare the FAR directly to the SAR (or functional group numbers to species numbers by grid cell) because the data differ by an order of magnitude and most cells have six or more of the nine functional groups represented by at least one species. Response diversity was therefore plotted against taxonomic diversity for each grid cell and the results were graphed by functional group. The residuals of each of these relationships were summed and mapped to produce a picture of the degree of spatial correlation between species richness and response richness. This map captures the degree to which conventional conservation planning efforts based on taxonomic richness will provide a good surrogate for functional richness and hence of ecosystem resilience to loss of species (see Schweiger et al. (2007) for related caveats).
4. Results
(a) Functional groups defined by beak morphology
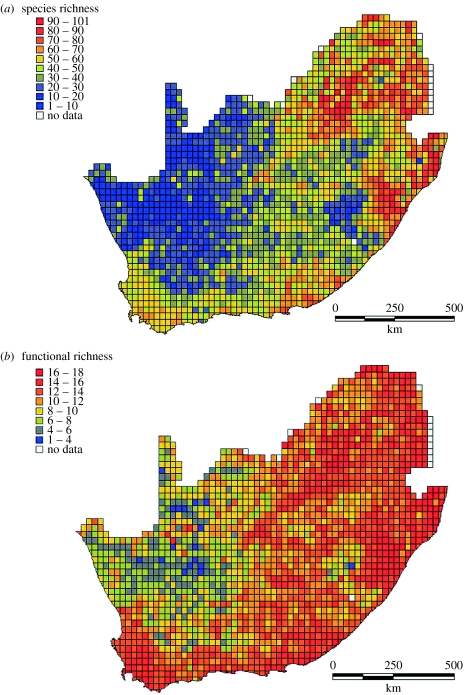
The results showed that a consistent FAR exists and that it matched our expectations. The inclusion of more or fewer functional groups had little effect on the overall relationship between functional and taxonomic accumulation curves, although this will not necessarily be the case in situations, where the ratio of functional richness to taxonomic richness is higher. Maps of functional and taxonomic richness demonstrate a high degree of similarity in the spatial clustering of centres of richness (figure 3). As expected from the difference in numbers of units in each group, functional richness showed less spatial variation than taxonomic richness. No single grid cell contained representatives of all functional groups (the maximum in any one cell was 17).
Figure 3.
Species richness (a) and functional richness (b) of a representative sample of 151 bird species in South Africa.
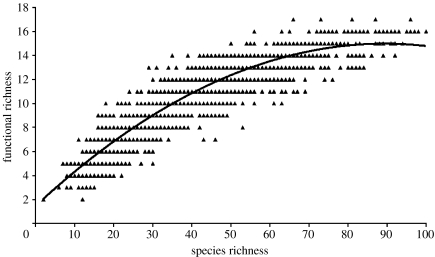
For our ‘optimal’ functional classification of 18 functional groups, plots of functional richness against taxonomic richness for each grid cell suggest that the form of the relationship is nonlinear, with functional richness apparently saturating in areas of higher species richness (figure 4). Interestingly, there is considerable variance around this relationship, with some grid cells exhibiting quite high residuals. This result indicates that some areas are species rich but are of low or average functional richness, while others may be less speciose but contain a greater variety of functional groups. In trophic terms, it implies that some areas may have food webs that are highly resilient to species loss, while in other cases the small number of members in key trophic levels (such as raptors or scavengers) may make these food webs more vulnerable.
Figure 4.
Plot of species richness against functional richness for each grid cell. The fitted curve (solid line) is quadratic (y=−0.0017x2+0.3025x+1.4651; r2=0.852; p<0.000). Note the wide scatter of points around the line. In areas that fall above the curve, functional richness is proportionally higher and consequently species loss is more likely to remove entire functional groups from the ecosystem.
(b) Functional groups defined a priori
South African birds were unevenly distributed between functional groups (figure 5). While the avifauna is rich in granivores and insectivores, there are relatively few birds that function as ecosystem engineers or nutrient dispersers. As predicted by food-web theory, the number of species in upper trophic levels (e.g. raptors and scavengers) is smaller than that in lower trophic levels (insectivores or granivores).
Figure 5.
Pie chart showing numbers of South African birds by functional group. The total number of species is 950; many birds fall into more than one functional group. Note that ‘raptors’ as used here include animals not traditionally considered raptors, such as herons and egrets that feed on fishes and frogs.
The relationship between taxonomic richness and response richness was linear in all cases (figure 6; table 3). The slopes of the lines for each functional group were markedly different; as one would expect, functional groups with fewer members also accumulated taxonomic species more slowly. More interestingly, there was a large degree of scatter around several of the regression lines. This level of variation was unexpected and suggests that even though the average relationship between taxonomic richness and response richness is linear, there are many individual instances in which taxonomic richness does not offer a good surrogate measure of response richness within a given functional group. The summed residuals by grid square indicate further that there is substantial variation between grid squares, with some grid squares having disproportionally high species redundancy and others having disproportionally low species redundancy.
Figure 6.
Examples of curves showing relationships between taxonomic richness and response richness for different functional groups. Each point represents data for a single SABAP grid cell. Lines are for insectivores (filled triangles); nutrient dispersers (open triangles); granivores (filled circles); and ecosystem engineers (open circles). Summary statistics for all functional groups are presented in table 3.
Table 3.
Summary of linear regressions between taxonomic richness and response richness, by SABAP grid cell, for different functional groups. (The sample size (i.e. total number of cells considered) is the same for all curves. Column headings: slope, intercept and r2-value of a fitted linear regression line; mean, mean number of species per cell per functional group; s.d., standard deviation in the number of species per cell; n members, number of species belonging to that functional group.)
| functional group | slope | intercept | r2 | mean | s.d. | n members |
|---|---|---|---|---|---|---|
| seed dispersers | 0.10 | 5.05 | 0.80 | 18.98 | 9.87 | 103 |
| pollinators | 0.15 | 3.36 | 0.86 | 24.55 | 14.5 | 110 |
| nutrient movers | 0.35 | −11.80 | 0.82 | 36.72 | 33.8 | 341 |
| insectivores | 0.67 | 1.80 | 0.99 | 93.01 | 58.1 | 560 |
| granivores | 0.13 | 7.68 | 0.83 | 25.46 | 12.4 | 131 |
| grazers | 0.08 | 0.40 | 0.72 | 11.38 | 8.2 | 54 |
| raptors | 0.20 | −4.20 | 0.90 | 23.16 | 18.3 | 192 |
| scavengers | 0.09 | 0.63 | 0.85 | 14.22 | 9.3 | 104 |
| ecological engineers | 0.07 | −1.19 | 0.83 | 8.27 | 6.6 | 41 |
5. Discussion
We started this paper by arguing that there is a need to link taxonomic, functional and trophic perspectives in biodiversity research. Our results demonstrate several ways in which such linkages can usefully be quantified in three different but closely related contexts: the FAR, the relationship between taxonomic and functional richness and the relationships between taxonomic and response richness.
We found that the FAR for South African birds was predictable and followed a similar form to the SAR. The FAR provides information that is complementary to the SAR and may also offer some additional or alternative answers to policy-oriented questions such as where critical thresholds for habitat loss occur, how habitat loss might influence the provision of ecosystem services, whether conservation plans that use taxonomic richness are in any way capturing ecosystem processes and the degree to which the ecosystem is likely to be resilient to species loss (Baskin 1994). Our examples from South African bird communities provide empirical examples of the kind of information that can be obtained from the analysis of functional patterns in space. The FAR in our first example also has some weaknesses; in particular, it does not presently take into account the potential for multifunctionality of single species, the fact that the pattern of species loss in a given system is likely to occur selectively in relation to different trophic levels (Dobson et al. 2006), or the potential importance of keystone species that influence ecosystem processes well beyond their numerical abundance or apparent functional group (Davic 2003). Inclusion of these additional facets of ecological change into a more general framework is clearly an important priority, both for our understanding of ecosystems and for our ability to apply ecological theory to spatially explicit conservation-related problems.
Deeper exploration of the details of the FAR—or more specifically, of the spatial variation that it glosses over at our scale of analysis—is provided by analyses of taxonomic patterns in regard to functional and response richness. These results demonstrate clearly the point that for a given location, taxonomic richness is not always a good surrogate for functional richness. In particular, we found that the relationship between taxonomic richness and functional richness (using groups defined by beak morphology) was nonlinear for South African birds; that there was considerable scatter around the line of best fit; and that this scatter remained prominent in the data even when an a priori classification of only nine classes was used. The nonlinear form of the relationship between taxonomic and functional diversity has been previously reported (Díaz & Cabido 2001) and is probably widespread. Both the form of the relationship and the degree of scatter around it imply that an individual ecosystem with a given number of species may be rich in functional groups or relatively depauperate; identifying areas and food webs that fall in either of these extremes may be important for conservation efforts (Walker 1992) and for understanding declines in the production of ecosystem services. For instance, it would hypothetically be possible in a typical conservation planning exercise to pick a grid cell that is species rich but function poor, ignoring an adjacent locality that has fewer species in total but more functional groups. Geographical ‘diversity hotspots’ in particular, are defined in taxonomic terms, but taxonomic hotspots will not always be functional hotspots and it makes sense that important but less speciose avian functional groups, such as ecosystem engineers (in our examples, particularly cavity-making birds), should be afforded special care because of their keystone role in many ecosystems and their provision of habitat for other species.
Since functional units relate more directly than taxonomic units to ecosystem processes, process-oriented conservation plans and assessments (Sala et al. 2000; Cowling et al. 2003; Oliver 2004; Carpenter et al. 2005) would benefit from considering local variations in the relationship between taxonomic and functional diversity when areas are prioritized for conservation. With the right kind of supporting information, these variations can offer considerable insight into food-web dynamics and may be useful indicators of looming trophic collapse. For example, areas in which within-group diversity is relatively low for birds in upper trophic levels may be on the verge of experiencing some form of trophic cascade with a resulting change in ecosystem services. In South Africa, for instance, declines in populations of predators may facilitate population explosions of small granivores, such as red-billed queleas Quelea quelea (‘the feathered locust’) that can be important agricultural pests.
One of the largest practical difficulties in applying functional classifications is that of defining functional groups in a rigorous and defensible way, and it is clear that the theoreticians must lead the way; further research is needed to develop objective ways of quantifying and mapping functional richness in different taxa (Walker & Langridge 2002) and to explore the similarities and differences between the spatial patterns of taxonomic and functional richness, both as drivers of ecosystem processes and as responses to system-wide changes such as nitrification or fragmentation (Lavorel et al. 1997). We anticipate that such research will yield many useful insights into further bridging the gap between taxonomic and functional perspectives while also clarifying the many linkages between individual species and the overall functioning of the ecosystem.
Acknowledgments
We are grateful to Rene Navarro of the ADU for providing the SABAP data and to Phil Hockey and Penn Lloyd for access to the Roberts' database. For access to museum collections, we are grateful to Denise Hamerton and Vincent Bartnik of the South African National Museum and Tamar Cassidy of the Transvaal Museum. Valuable comments on an earlier draft of this manuscript were provided by David Cumming and Jon Norberg. This research was supported by a URC grant from the University of Cape Town.
Footnotes
One contribution of 15 to a Theme Issue ‘Food-web assembly and collapse: mathematical models and implications for conservation’.
Supplementary Material
Functional group membership for 151 bird species based on beak morphology
References
- Baskin Y. Ecosystem function of biodiversity. Bioscience. 1994;44:657–660. doi:10.2307/1312507 [Google Scholar]
- Björklund M. Selection of bill proportions in the common rosefinch (Carpodacus erythrinus) Auk. 1992;109:637–642. [Google Scholar]
- Bock W.J. Kinetics of the avian skull. J. Morphol. 1964;114:1–42. doi:10.1002/jmor.1051140102 [Google Scholar]
- Bock W.J. An approach to the functional analysis of bill shape. Auk. 1966;83:10–51. [Google Scholar]
- Bout R.G., Zweers G.A. The role of cranial kinesis in birds. Comp. Biochem. Physiol. A. 2001;131:197–205. doi: 10.1016/s1095-6433(01)00470-6. doi:10.1016/S1095-6433(01)00470-6 [DOI] [PubMed] [Google Scholar]
- Carpenter S.R., Pingali P.L., Bennett E.M., Zurek M.B., editors. Ecosystems and human well-being: scenarios. The Millennium Ecosystem Assessment. Island Press; Washington, DC: 2005. [Google Scholar]
- Chave J., Muller-Landau H.C., Levin S.A. Comparing classical community models: theoretical consequences for patterns of diversity. Am. Nat. 2002;159:1–23. doi: 10.1086/324112. doi:10.1086/324112 [DOI] [PubMed] [Google Scholar]
- Cowling R.M., Pressey R.L., Rouget M., Lombard A.T. A conservation plan for a global biodiversity hotspot—the Cape floristic region, South Africa. Biol. Conserv. 2003;112:191–216. doi:10.1016/S0006-3207(02)00425-1 [Google Scholar]
- Cumming G.S. Global biodiversity scenarios and landscape ecology. Landsc. Ecol. 2007;22:671–685. doi:10.1007/s10980-006-9057-3 [Google Scholar]
- Davic R.D. Linking keystone species and functional groups: a new operational definition of the keystone species concept. Conserv. Ecol. 2003;7:1–11. [Google Scholar]
- DeWitt T.J., Robinson B.W., Sloan-Wilson D. Functional diversity among predators of a freshwater snail imposes an adaptive trade-off for shell morphology. Evol. Ecol. Res. 2000;2:129–148. [Google Scholar]
- Díaz S., Cabido M. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001;16:646–655. doi:10.1016/S0169-5347(01)02283-2 [Google Scholar]
- Dobson A., et al. Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology. 2006;87:1915–1924. doi: 10.1890/0012-9658(2006)87[1915:hltcat]2.0.co;2. doi:10.1890/0012-9658(2006)87[1915:HLTCAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fisher R.A., Corbet A.S., Williams C.B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943;12:42–58. doi:10.2307/1411 [Google Scholar]
- Fryer G. Functional morphology and functional ecology. Funct. Ecol. 1988;2:270–275. [Google Scholar]
- Harrison J.A., Allan D.G., Underhill L.G., Herremans M., Tree A.J., Parker V., Brown C.J., editors. The atlas of southern African birds. BirdLife South Africa; Johannesburg, Republic of South Africa: 1997. [Google Scholar]
- Heard S.B. Processing chain ecology: resource condition and interspecific interactions. J. Anim. Ecol. 1994;63:451–464. doi:10.2307/5562 [Google Scholar]
- Herrel A., Podos J., Huber S.K., Hendry A.P. Bite force and morphology in a population of Darwin's finches: implications for the evolution of beak shape. Funct. Ecol. 2005a;19:43–48. doi:10.1111/j.0269-8463.2005.00923.x [Google Scholar]
- Herrel A., Podos J., Huber S.K., Hendry A.P. Evolution of bite force in Darwin's finches: a key role for head width. J. Evol. Biol. 2005b;18:669–678. doi: 10.1111/j.1420-9101.2004.00857.x. doi:10.1111/j.1420-9101.2004.00857.x [DOI] [PubMed] [Google Scholar]
- Herrera C.M. Adaptation to frugivory of Mediterranean avian seed disperser. Ecology. 1984;65:609–617. doi:10.2307/1941423 [Google Scholar]
- Hertel F. Diversity in body size and feeding morphology within past and present vulture assemblages. Ecology. 1994;75:1074–1084. doi:10.2307/1939431 [Google Scholar]
- Hockey, P. A. R., Dean, W. R. J. & Ryan, P. G. 2005 Roberts' Birds of Southern Africa, 7th edn. Cape Town, South Africa: John Voelcker Bird Book Fund.
- Hubbell S.P. Princeton University Press; Princeton, NJ; Oxford, UK: 2001. The unified neutral theory of biodiversity and biogeography. [Google Scholar]
- Jax K. Ecological units: definitions and applications. Q. Rev. Biol. 2006;81:237–258. doi: 10.1086/506237. doi:10.1086/506237 [DOI] [PubMed] [Google Scholar]
- Kinzig, A. P., Pacala, S. W. & Tilman, D. 2001 The functional consequences of biodiversity: empirical progress and theoretical extensions Monographs in Population Biology. Princeton, NJ: Princeton University Press.
- Laland K.N., Odling-Smee F.J., Feldman M.W. Evolutionary consequences of niche construction and their implications for ecology. Proc. Natl Acad. Sci. USA. 1999;96:10 242–10 247. doi: 10.1073/pnas.96.18.10242. doi:10.1073/pnas.96.18.10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorel S., Garnier E. Aardvark to Zyzyxia—functional groups across kingdoms. New Phytol. 2001;149:360–363. doi: 10.1046/j.1469-8137.2001.00048.x. doi:10.1046/j.1469-8137.2001.00048.x [DOI] [PubMed] [Google Scholar]
- Lavorel S., Macintyre S., Landsberg J., Forbes T.D.A. Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends Ecol. Evol. 1997;12:474–478. doi: 10.1016/s0169-5347(97)01219-6. doi:10.1016/S0169-5347(97)01219-6 [DOI] [PubMed] [Google Scholar]
- Legendre P., Legendre L. Elsevier Science; Amsterdam, The Netherlands: 1998. Numerical ecology. [Google Scholar]
- Margules C.R., Pressey R.L. Systematic conservation planning. Nature. 2000;405:243–253. doi: 10.1038/35012251. doi:10.1038/35012251 [DOI] [PubMed] [Google Scholar]
- McGarigal K., Cushman S., Stafford S. Springer; New York, NY: 2000. Multivariate statistics for wildlife and ecology research. [Google Scholar]
- Milligan G.W., Cooper M.C. Methodology review: clustering methods. Appl. Psychol. Meas. 1987;11:329–354. doi:10.1177/014662168701100401 [Google Scholar]
- Naeem S. Disentangling the impacts of diversity on ecosystem functioning in combinatorial experiments. Ecology. 2002;83:2925–2935. doi:10.2307/3072027 [Google Scholar]
- Nosil P., Reimchen T.E. Ecological opportunity and levels of morphological variance within freshwater stickleback populations. Biol. J. Linn. Soc. 2005;86:297–305. doi:10.1111/j.1095-8312.2005.00517.x [Google Scholar]
- Odling-Smee F.J., Laland K.N., Feldman M.W. Niche construction. Am. Nat. 1996;147:641–648. doi:10.1086/285870 [Google Scholar]
- Oliver I. A framework and toolkit for scoring the biodiversity value of habitat, and the biodiversity benefits of land use change. Ecol. Manag. Restor. 2004;5:75–78. doi:10.1111/j.1442-8903.2004.180-5.x [Google Scholar]
- Petchey O.L., Gaston K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002;5:402–411. doi:10.1046/j.1461-0248.2002.00339.x [Google Scholar]
- Polis G.A., Power M.E., Huxel G.R., editors. Food webs at the landscape level. University of Chicago Press; Chicago, IL: 2004. [Google Scholar]
- Pressey R.L., Possingham H.P., Margules C.R. Optimality in reserve selection algorithms: when does it matter and how much? Biol. Conserv. 1996;76:259–267. doi:10.1016/0006-3207(95)00120-4 [Google Scholar]
- Relyea R.A., Auld J.R. Predator- and competitor- induced plasticity: how changes in foraging morphology affect phenotypic trade-offs. Ecology. 2005;86:1723–1729. doi:10.1890/04-1920 [Google Scholar]
- Ricklefs E., Travis J. A morphological approach to the study of avian community organisation. Auk. 1980;97:321–338. [Google Scholar]
- Rosenzweig M.L. Cambridge University Press; Cambridge, UK: 1995. Species diversity in space and time. [Google Scholar]
- Sala O.E., et al. Biodiversity—global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. doi:10.1126/science.287.5459.1770 [DOI] [PubMed] [Google Scholar]
- Schweiger O., et al. Functional richness of local hoverfly communities (Diptera, Syrphidae) in response to land use across temperate Europe. Oikos. 2007;116:461–472. doi: 10.1111/j.2007.0030-1299.15372.x. doi:10.1111/j.2007.0030-1299.15372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekercioglu C.H. Increasing awareness of avian ecological function. Trends Ecol. Evo. 2006;21:464–471. doi: 10.1016/j.tree.2006.05.007. doi:10.1016/j.tree.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Simberloff D., Dayan T. The guild concept and the structure of ecological communities. Annu. Rev. Ecol. Syst. 1991;22:115–143. doi:10.1146/annurev.es.22.110191.000555 [Google Scholar]
- Snow B.K., Snow D.W. Feeding niches of hummingbirds in a Trinidad valley. J. Anim. Ecol. 1972;41:471–485. doi:10.2307/3481 [Google Scholar]
- Temeles E.J., Kress W.J. Adaptation in a plant hummingbird association. Science. 2003;300:630–633. doi: 10.1126/science.1080003. doi:10.1126/science.1080003 [DOI] [PubMed] [Google Scholar]
- Walker B.H. Biodiversity and ecological redundancy. Conserv. Biol. 1992;6:18–23. doi:10.1046/j.1523-1739.1992.610018.x [Google Scholar]
- Walker B.H., Langridge J.L. Measuring functional diversity in plant communities with mixed life forms: a problem of hard and soft attributes. Ecosystems. 2002;5:529–538. doi:10.1007/s10021-002-0154-0 [Google Scholar]
- Wilson K.A., Westphal M.I., Possingham H.P., Elith J. Sensitivity of conservation planning to different approaches to using predicted species distribution data. Biol. Conserv. 2005;122:99–112. doi:10.1016/j.biocon.2004.07.004 [Google Scholar]
- Winker K. Suggestions for measuring the external characters of birds. Ornitol. Neotrop. 1998;9:23–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Functional group membership for 151 bird species based on beak morphology