Abstract
The mesophilic methanogenic archaeon Methanosarcina mazei strain Gö1 is able to utilize molecular nitrogen (N2) as its sole nitrogen source. We have identified and characterized a single nitrogen fixation (nif) gene cluster in M. mazei Gö1 with an approximate length of 9 kbp. Sequence analysis revealed seven genes with sequence similarities to nifH, nifI1, nifI2, nifD, nifK, nifE and nifN, similar to other diazotrophic methanogens and certain bacteria such as Clostridium acetobutylicum, with the two glnB-like genes (nifI1 and nifI2) located between nifH and nifD. Phylogenetic analysis of deduced amino acid sequences for the nitrogenase structural genes of M. mazei Gö1 showed that they are most closely related to Methanosarcina barkeri nif2 genes, and also closely resemble those for the corresponding nif products of the gram-positive bacterium C. acetobutylicum. Northern blot analysis and reverse transcription PCR analysis demonstrated that the M. mazei nif genes constitute an operon transcribed only under nitrogen starvation as a single 8 kb transcript. Sequence analysis revealed a palindromic sequence at the transcriptional start site in front of the M. mazei nifH gene, which may have a function in transcriptional regulation of the nif operon.
Keywords: GlnB-like proteins, nif genes, nitrogen fixation, nitrogen regulation
Introduction
Biological nitrogen fixation, the enzymatic reduction of atmospheric nitrogen (N2) to ammonia, is not limited to the bacterial domain, but is also observed in several methanogenic members of the archaeal domain. Nitrogenase, the enzyme complex of dinitrogenase and dinitrogenase reductase, is responsible for the reduction of molecular nitrogen; it is highly conserved in structure, function, and amino acid sequence across both domains (Lobo and Zinder 1992, Young 1992, Fischer 1994, Rees and Howard 1999). The dinitrogenase, which is an α2β2 heterotetramer containing the P-cluster and the FeMo-cofactor, is encoded by nifD and nifK; the nitrogenase reductase is a homodimer with a single [4Fe-4S]-cluster linking the subunits and is encoded by nifH (Georgiadis et al. 1992, Rees and Howard 1999 and papers cited therein). In bacteria, the genes nifH, nifD and nifK, which encode the molybdenum-containing nitrogenase, are typically found together in a single operon and are physically adjacent to other nif genes as part of a larger nif regulon. Downstream of nifK, the nifE and nifN genes, which are essential for FeMo-cofactor assembly (Dean et al. 1993), are found in a separate operon. In Archaea, genes homologous to the bacterial nif genes have been identified, and nitrogen fixation has been observed in several methanogenic species (Lobo and Zinder 1992, Young 1992, Bult et al. 1996, Chien and Zinder 1996, Haselkorn and Buikema 1996, Kessler et al. 1997, Smith et al. 1997). The discovery of genes homologous to nifH, nifD and nifK suggests that the basic mechanism of nitrogen fixation is similar in Bacteria and Archaea and predicts that most methanogenic nitrogenases contain a molybdenum-cofactor (Chien and Zinder 1996, Kessler et al. 1997). It was recently shown that, unique among the archaea, Methanosarcina acetivorans appears to contain all three types of nitrogenases: the molybdenum nitrogenase and two alternative nitrogenases (Galagan et al. 2002). In methanogenic archaea, the nitrogen fixation genes nifH, nifD, nifK, nifE and nifN are present in the same order as in bacteria (Dean and Jacobson 1992). However, in contrast, (i) methanogenic nif gene promoters are typical archaeal promoters, and the transcriptional apparatus is similar to that of Eucarya (Langer and Zillig 1993, Marsh et al. 1994, Langer et al. 1995, Qureshi et al. 1995, Hausner et al. 1996, Thomm 2000, Bell and Jackson 2001), (ii) the archaeal nif genes are present in a single operon, and (iii) all diazotrophic methanogens contain two open reading frames (ORFs) inserted between nifH and nifD that show a strong similarity to glnB (Sibold et al. 1991, Merrick and Edwards 1995, Arcondeguy et al. 2001, Kessler et al. 2001). Recently, this nif gene organization with the two glnB-like genes, which have been renamed nifI1 and nifI2 (Arcondeguy et al. 2001), has also been found in Clostridium acetobutylicum (Nölling et al. 2001) and Clostridium beijerinckii (Chen et al. 2001). The presence of the nifI ORFs within the bacterial nif operon may be the result of a horizontal interdomain gene transfer, or this respective arrangement is ancestral and other bacteria have lost it.
As with bacterial nitrogen fixation, nif gene transcription in methanogenic archaea is regulated by the availability of nitrogen (Souillard and Sibold 1989, Chien and Zinder 1994, Kessler et al. 1998). However, because of the major differences between archaeal and bacterial transcription, it is likely that the mechanisms of methanogenic nif gene regulation differ significantly from bacterial nif regulation. Transcriptional regulation of nif genes in response to the nitrogen status of the cells has been studied in detail for Methanococcus maripaludis and Methanosarcina barkeri 227; experimental evidence obtained by Leigh and coworkers showed that nitrogen metabolism genes in Methanococcus and Methanobacterium species are coordinately regulated at the transcriptional level by a common repressor binding site (Cohen-Kupiec et al. 1997, Kessler and Leigh 1999). In M. barkeri 227, nif regulation is also mediated by a negative mechanism; however, in contrast to Methanococcus, this mechanism does not appear to be based on an operator site (Chien et al. 1998).
Our goal was to analyze the regulatory network of nitrogen metabolism and nitrogen fixation in M. mazei strain Gö1. This mesophilic archaeon belongs to the methylotrophic methanogens of the order Methanosarcinales and is able to grow on H2 plus CO2, methanol, methylamines and acetate. The pathways of methanogenesis from these substrates have been analyzed in detail in recent years (Deppenmeier et al. 1990, Thauer 1998, Ferry 1999) and the genome of M. mazei strain Gö1 has been sequenced by the Göttingen Genomics Laboratory (Deppenmeier et al. 2002). Here we report on the arrangement and expression of nif genes in M. mazei.
Materials and methods
Bacterial strains and plasmids
Methanosarcina mazei strain Gö1 (DSM3647) was obtained from the DSM. The pTZ19R sequencing vector (Pharmacia) and pSK+ Bluescript (Stratagene, La Jolla, CA) were used for subcloning and DNA sequencing.
Growth
Methanosarcina mazei Gö1 was grown without shaking at 37 °C, in 5- or 25-ml closed growth tubes on 150 mM methanol in a minimal medium described previously (Deppenmeier et al. 1990). Growth generally took place in a nitrogen atmosphere containing 20% CO2. For nitrogen-limiting growth conditions, ammonium was omitted from the medium. Control growth experiments for nitrogen fixation were performed in either an argon atmosphere or a hydrogen atmosphere containing 20% CO2. In general, growth was monitored by determining the optical density of the cultures at 600 nm (OD600).
Cloning and nucleotide sequencing
The complete genomic sequence of M. mazei strain Gö1 was determined by a whole-genome-shotgun approach (Deppenmeier et al. 2002). The generated sequence readings were assembled into contigs with P. Greens PHRAP assembling tools and were edited with GAP, which is part of the STADEN software package (Staden et al. 2000). Sequence analysis was performed with the Genetics Computer Group (GCG) software package (Devereux et al. 1984). The nucleotide sequences for nifH, nifI1, nifI2, nifD, nifK, nifE and nifN have been submitted to GenBank (Accession Number AY029234).
RNA isolation and Northern blot analyses
Methanosarcina mazei Gö1 cells from exponentially growing cultures (5 ml or 50 ml, OD600 = 0.3–0.4), grown with N2 or 10 mM as the nitrogen source, were anaerobically centrifuged in the growth tubes at 3000 g for 10 min and resuspended in 30 mM sodium acetate, pH 5.2. After incubation with 1.5% sodium dodecyl sulfate (SDS), RNA was extracted using the RNeasy Kit (Qiagen, Santa Clarita, CA) according to the manufacturer’s protocol and treated with DNAseI. Following ethanol precipitation in the presence of 4 M LiCl, the RNA pellet was resuspended in 30 or 60 µl of RNAse-free water and stored at –70 °C. The RNA (9–12 µg) was separated by electrophoresis in a 1% denaturing agarose gel containing 6% formaldehyde, and then transferred to nylon membranes (Hybond-N, Amersham/Pharmacia, Piscataway, NJ) by vacuum blotting according to the manufacturer’s directions. After 3 min of UV cross-linking, Northern hybridization was performed to locate the mRNA of interest (Sambrook et al. 1989). Filters were hybridized overnight at 55 °C with [α-32P] ATP-labeled nifH, nifK and nifN probes in the presence of 5× SSC (1× SSC is 0.15 M NaCl plus 0.15 M sodium citrate), 0.02% SDS and 0.1% laurylsarcosine. After hybridization, the membranes were washed twice with 2× SSC containing 0.1% SDS at room temperature for 5 min, twice with 2× SSC containing 0.1% SDS at 55 °C for 30 min, and twice with 0.1× SSC containing 0.1% SDS at room temperature for 30 min. The hybridized mRNA was detected with a PhosphorImager and analyzed with ImageQuant 1.2 software (Molecular Dynamics). The RNA marker standard used was obtained from New England Biolabs (U.K.).
Generating DNA probes
The DNA probes for nifH, nifK and nifN and for 16S rRNA were amplified by PCR using genomic DNA from M. mazei Gö1 as template. The oligonucleotides used were: nifH sense primer (5′-GCAATCTACGGAAAGGGCGG-3′) and antiprimer (5′-CATCAGTTCCCCACTGGCAAC-3′); nifK sense primer (5′-CCCATGTGAAGAGATAACCAGAG-3′) and antiprimer (5′-CGTCTTCAATGATAACTCCGACG- 3′); nifN sense primer (5′-GAAGTGCCCTTGCCTTTAAA GG-3′) and antiprimer (5′-CCGGACTTTCAGGTTTTTCCG -3′); glnK1 sense primer (5′-TAGGATAGAGAATTCCTAC TGGTGGTC-3′) and antiprimer (5′-CCATACAGTGTAAG CTTCGTTTATAGCC-3′); and 16S rDNA sense primer (5′- GCAGCAGGCGCGAAAAC-3′) and antiprimer (5′-CGTTT ACGGCTGGGACTA-3′). Reactions were carried out in 100 µl volumes with Taq polymerase (New England Biolabs, U.K.) and primers at a concentration of 0.3 µM. The annealing temperature was 58 °C (49 °C for 16S rDNA) and synthesis was carried out over 25 cycles of 30 s each. The PCR products, which were 450 bp (nifH), 417 bp (nifK), 438 bp (nifN), 415 bp (glnK1) and 420 bp (16S rDNA), respectively, were purified by gel electrophoresis and extracted with the QIAQuick extraction kit (Qiagen). The purified PCR products (1–1.5 µg) were labeled with α-32P-ATP using the random labeling system from Gibco BRL (Random Primers Labeling System) according to the manufacturer’s protocol.
Reverse transcription PCR
Transcriptional analysis by reverse transcription PCR (RT-PCR) was carried out on DNA-free RNA extracted with phenol/chloroform and treated with DNAseI (Sambrook et al. 1989) from cells grown with N2 or 10 mM as the nitrogen source. Control PCR reactions using RNA in the absence of reverse transcriptase showed that the isolated RNA preparations were free of genomic DNA. The RT-PCR reactions were carried out using the OneStep RT-PCR Kit (Qiagen) as recommended by the supplier, using 0.1 µg RNA and 0.6 µM sense primer and antiprimer for nifH, nifK, nifN, glnK1 and 16S rDNA (see above). The control RT-PCR of 16S rDNA was carried out with 10 ng RNA and the respective primers. The annealing temperatures were 58 °C (nifH, nifK, nifN, glnK1) and 49 °C (16S rDNA). Products for each primer pair and growth condition were separated on 1.5% agarose gels and quantified using the GelDoc2000 Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Determination of the transcriptional start site
The nif transcriptional start site was determined with the 5′-RACE system, as recommended by the supplier (Gibco BRL), using 1 µg of total RNA (DNA-free) from cells grown under nitrogen starvation and the primers nifH-GSP1 (5′-TGC CCCTGCCTGCACATCC-3′) and nifH-GSP2 (5′-CTCGGG CCCTCCTGATTCC-3′), which hybridize to bases +297 to +278 and bases +269 to +250 of nifH, respectively. The resulting PCR product (310 bp) was cloned into pSK+ Bluescript (Stratagene) and sequenced in both directions with an ABI PRISM 377 DNA sequencer.
Results and discussion
Our goal was to study nitrogen regulation in the methanogenic archaeon M. mazei strain Gö1. By analyzing the sequences obtained from the genome sequencing project of M. mazei Gö1, we identified a single nitrogen fixation (nif) gene cluster. We characterized growth of M. mazei during nitrogen starvation and analyzed the structure and transcriptional regulation of this nif cluster.
Diazotrophic growth of M. mazei Gö1
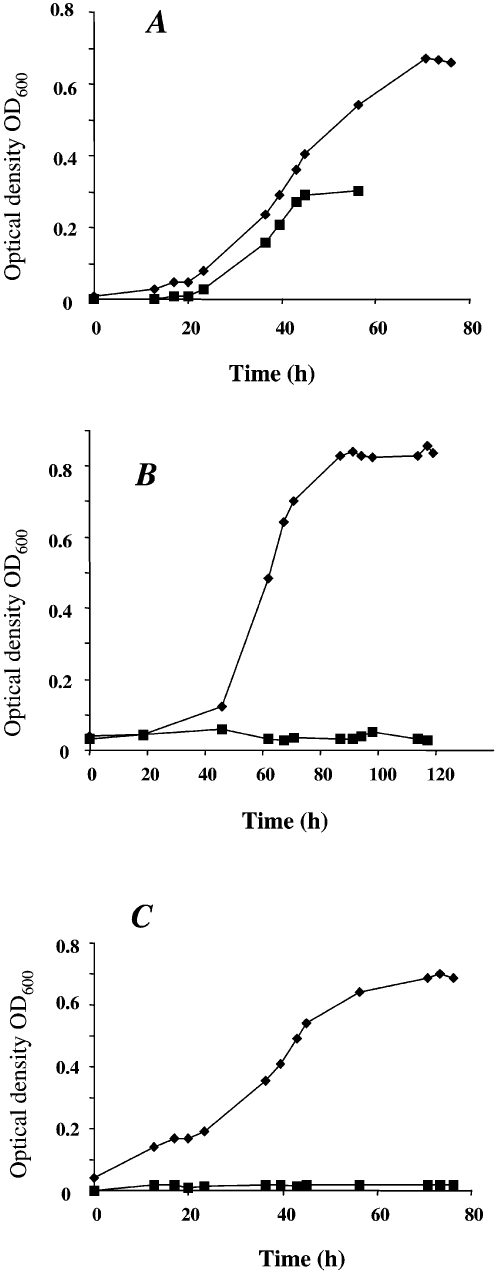
To determine if M. mazei is able to fix molecular nitrogen and use it as the sole nitrogen source, growth under nitrogen-limiting conditions was analyzed. Growth experiments were performed under anaerobic conditions in defined nitrogen-depleted mineral media in an N2/CO2 (80%/20%) gas atmosphere, with methanol as the carbon source. For nitrogen-sufficient growth, the medium was supplemented with 10 mM ammonium or 4 mM glutamine as the nitrogen source (see Materials and methods). Methanosarcina mazei showed significant growth when incubated under nitrogen-limiting conditions, with molecular nitrogen in the gas phase as the sole nitrogen source (Figure 1A). No growth was observed in the controls supplied with nitrogen-depleted medium in an argon/CO2 (80%/20%) or a hydrogen/CO2 (80%/20%) gas atmosphere (Figures 1B and 1C), indicating that growth of M. mazei in a gas atmosphere containing 80% molecular nitrogen resulted from nitrogen fixation. The doubling time under these nitrogen-limiting conditions was 13 h, versus 10 h when grown under nitrogen-sufficient conditions (Figure 1A). In addition to this decrease in growth rate, nitrogen-limited cultures showed a prolonged lag-phase and the cell yield was about twofold lower than under nitrogen-sufficient growth conditions (maximal optical density of OD600 = 0.35 to 0.4 for nitrogen-limiting conditions, versus OD600 = 0.75 for nitrogen-sufficient growth, Figure 1A). This reduced yield was likely caused by the ATP expenditure of N2 fixation and the limited solubility of N2 in the medium. Because the cultures were incubated without shaking, diffusion of N2 into the medium appeared to be insufficient to support high density cultures.
Figure 1.
Growth of M. mazei with different nitrogen availabilities. Cells were grown on 150 mM methanol in 5 ml of minimal medium in a nitrogen gas atmosphere containing 20% CO2 (A), a hydrogen gas atmosphere containing 20% CO2 (B), or an argon gas atmosphere containing 20% CO2 (C). The medium was supplemented with 10 mM ammonium (◆) or contained no additional nitrogen source (■).
Sequencing and nucleotide analysis of the nif gene cluster from M. mazei strain Gö1
The entire genome of M. mazei strain Gö1 has been sequenced by the Göttingen Genomics Laboratory (Deppenmeier et al. 2002). The assembled sequence data were searched for the presence of regions encoding methanogenic or bacterial subunits of nitrogenase. A single nif gene cluster, with an approximate length of 9 kbp, was detected. The fragment comprises seven open reading frames, each preceded by a putative ribosome-binding site and starting with the initiation codon ATG (or, in one case GTG), and terminated by stop codons TAA or TGA. Sequence analysis of this nif gene cluster was performed with the Genetics Computer Group (GCG) software package (Devereux et al. 1984), and revealed five genes with sequence similarities to nitrogen fixation genes nifH, nifD, nifK, nifE and nifN. In addition, two genes located between nifH and nifD showed sequence similarities to bacterial glnB genes (Sibold et al. 1991, Merrick and Edwards 1995, Arcondeguy et al. 2001) and were, therefore, designated nifI1 and nifI2, respectively. Additional open reading frames in the adjacent regions on either side of the cluster have been tentatively identified as a flavine-containing oxidoreductase (upstream of nifH) and a periplasmic molybdate binding protein plus a molybdenum transporter (downstream of nifN). The order of the M. mazei nitrogen fixation genes is the same as in other diazotrophic methanogenic archaea and differs from most bacterial nif gene clusters in that the two nifI genes are located between nifH and nifD (Sibold et al. 1991, Chien and Zinder 1994, 1996, Kessler et al. 1998, Arcondeguy et al. 2001). Phylogenetic analysis of the deduced amino acid sequence of the M. mazei Gö1 nif genes showed that they are most closely related to M. barkeri nif2 genes, suggesting that M. mazei Gö1 has a molybdenum-containing nitrogenase (Table 1).
Table 1.
Comparison of deduced amino acid sequences of Methanosarcina mazei nif genes with those of other methanogenic and nitrogen-fixing microorganisms. Percentage of identity was calculated using the GCG program (Deveraux et al. 1984).
| Gene | M. mazei | M. barkeri | M. acetivorans | Methanococcus | Methanothermobacter | Clostridium | Clostridium | Klebsiella |
| Gö1 | (nif2) | maripalidus | thermoautotrophicus | acetobutylicum | pasteurianum | pneumoniae | ||
| nifH | 100% | 91% | 90% | 60% | 61% | 69% | 68% (nifH1) | 62% |
| glnB′ | 100% | 88% | 90% | 56% | 55% | 49% | – | – |
| glnB″ | 100% | 48% | 76% | 43% | 48% | 48% | – | – |
| nifD | 100% | 87% | 88% | 39% | 39% | 52% | 52% | 40% |
| nifK | 100% | 91% | 91% | 45% | 40% | 51% | 49% | 38% |
| nifE | 100% | 83% | 87% | 45% | 45% | 49% | 53% | 34% |
| nifN | 100% | 75% | 85% | 40% | 36% | 41% | –1 | 26% |
1Sequence not available in public databases.
In addition to the high similarity of M. mazei nitrogenase structural gene products to the nif2 gene cluster of M. barkeri and the nif cluster of M. acetivorans, the genes showed a significantly higher similarity to the corresponding nif gene products of the Gram-positive bacterium C. acetobutylicum than to their archaeal counterparts in M. maripaludis and Methanothermobacter thermoautotrophicus. In addition, it was recently found that the nif cluster of C. acetobutylicum shows the same gene organization, as two nifI genes are located between nifH and nifD (Nölling et al. 2001). These findings suggest that the nitrogenase subunits of the Methanosarcina species are more closely related to the clostridial nitrogenases than to the archaeal nitrogenases of Methanococcus and Methanothermobacter.
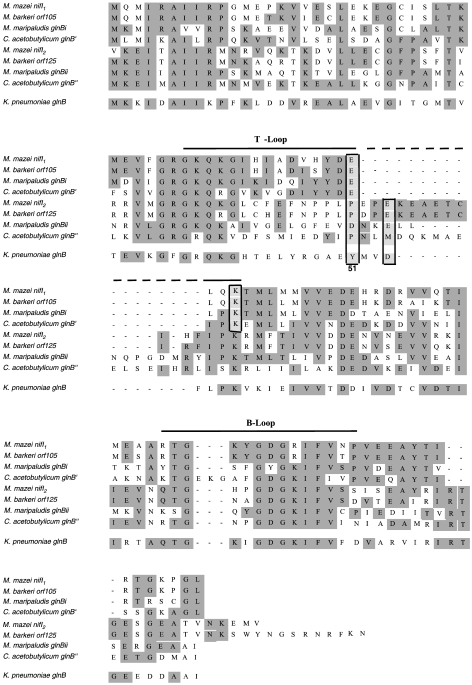
The deduced amino acid sequences of the two glnB-like nifI genes showed considerable similarity to the corresponding gene products from the nif regions of other diazotrophic methanogens and C. acetobutylicum (Figure 2). The strongest similarities were observed in the B-loop region and the C-terminal regions just after the B-loop. In the T-loop regions, only the N-terminal regions showed a strong similarity (amino acids 33 to 42). In general, the GlnB-like proteins encoded by homologs of the glnB family located within nif clusters do not contain the conserved uridylylation site (tyrosine 51) of bacterial GlnB-like proteins in the T-loop, which was originally shown by Sibold et al. (1991), and differ significantly from bacterial GlnB-like proteins in the region N-terminal of amino acid residue 51 (amino acids 43 to 50). These findings suggest that NifI proteins encoded in nif clusters are unlikely to be covalently modified by uridylylation. However, the conserved tyrosine at position 49 in M. mazei NifI1 and its homologs might be a target for modification, if the NifI proteins are modified at all. In addition to amino acid 49, amino acids in positions 3, 16 and 17, and positions 51 and 54 in the T-loop, are conserved within the NifI1 and NifI2 proteins, respectively (Figure 2). The alignment further indicates that the NifI2 protein of M. mazei and its homologs contains additional amino acids in their C-terminal loop region, whereas only the B-loop of C. acetobutylicum NifI1 contains three additional amino acids. Taken together, these nifI genes appear to represent a separate glnB-like family in addition to the GlnB and GlnK homologs. Experimental data indicate that the nifI genes are not involved in the regulation of nif gene expression at the transcriptional level in M. maripaludis (Kessler et al. 1998). However, Leigh and coworkers have recently presented evidence that both nifI genes are required for posttranslational inhibition by ammonium of the M. maripaludis nitrogenase (“ammonium switch-off”) (Kessler and Leigh 1999, Kessler et al. 2001). The nifI2 homologs of M. mazei and M. barkeri differ from all known nifI2 homologs in that both deduced proteins contain additional amino acids in the C-terminal loop region that are not found in PII-like proteins (Figure 2).
Figure 2.
Alignment of the two glnB-like genes of Methanosarcina mazei located in the nif gene cluster, compared with the corresponding glnB-like genes in Methanococcus maripaludis, Methanosarcina barkeri and Clostridium acetobutylicum, and glnB from Klebsiella pneumoniae. The predicted secondary structural elements, the T-loop and the B-loop, are indicated above the sequence. The boxed areas show the tyrosine residue (Tyr51) that is the conserved site of uridylylation in bacteria and the corresponding amino acid residues of the glnB-like genes at position 51.
Transcriptional organization and regulation of the nif gene cluster in M. mazei
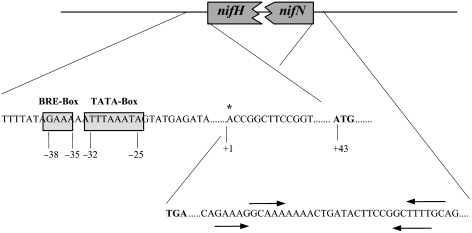
To analyze the promoter region, the nif transcriptional start site was determined by the 5′-RACE method from RNA extracted from cells grown under nitrogen-starvation conditions with molecular nitrogen as the sole nitrogen source. The transcriptional start site was localized 42 bp upstream of the putative translational start site of nifH (Figure 3). A potential archaeal consensus promoter sequence is centered 38 to 25 bp upstream from the 5′ end of the transcriptional start site, and 81 to 68 bp from the translational start codon ATG of nifH. It contains a typical, archaeal factor-B-recognition element (BRE) [GAAA] and a potential TATA-box [TTTAAATA] (Figure 3). No similar promoter sequences were obtained at appropriate locations 5′ of the other genes. Downstream of nifN, a potential transcriptional termination site was identified, containing two potential stem loops followed by a T-rich region (Figure 3). These findings indicate that the nif genes in M. mazei are organized in one operon that includes the two nifI genes.
Figure 3.
Diagram of the putative promoter region upstream of nifH and putative terminator region downstream of nifN in the M. mazei nif gene cluster. Abbreviations and symbols: * = transcriptional start site; TATA-box = archaeal TATA-box promoter element; BRE = factor B recognition element; stem loop structures are marked by arrows indicating the length and orientation of the stems. Sequences are numbered relative to the mRNA initiation site determined by the 5′ RACE method.
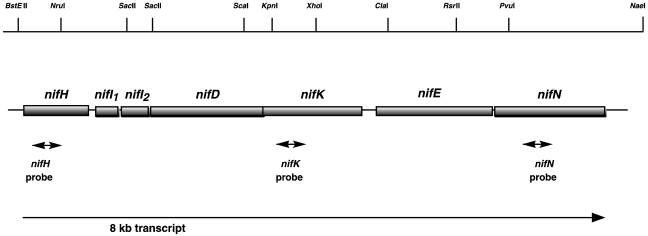
Northern blot analysis was performed to provide further evidence of a nif gene cluster operon organization and to study the potential transcriptional regulation by nitrogen. The RNA was extracted from cells grown under nitrogen-starvation conditions, with molecular nitrogen as the sole nitrogen source, and under nitrogen-sufficient conditions in the presence of 10 mM ammonium. A single transcript was detected in mRNA isolated from cells grown under nitrogen-starvation conditions when an internal fragment derived from nifH was used as the hybridization probe (see Materials and methods). As shown in Figure 4A, the presence of the same single transcript in RNA isolated from cells grown under nitrogen-starvation conditions was observed using internal probes derived from nifK or nifN. The length of this single transcript was about 8 kb, which is in accordance with the expected length of a nifHI1I2DKEN transcript, and indicates that the seven genes are co-transcribed from a single promoter (Figure 5). However, when cells were grown under nitrogen-sufficient conditions, no transcript was detected in the total RNA, suggesting that transcription is regulated by ammonium and only occurs during nitrogen starvation (Figure 4A). Transcriptional regulation by nitrogen availability was further confirmed by RT-PCR (Figure 4B). Products of 415 and 450 bp, corresponding to the expected sizes of the products of the control gene glnK1 (known to be regulated by ammonium (Ehlers et al. 2002)) and the nifH gene, respectively, were amplified from RNA extracted from cells grown under nitrogen-limiting conditions, using the OneStep RT-PCR Kit and the glnK1 or nifH primers. Using the same amounts of RNA extracted from nitrogen-limited cells, the nifK (417 bp) and nifN (438 bp) products were significantly less amplified than the nifH product. Small amounts of the nifH, nifK and nifN products were obtained from RNA extracted from cells grown under nitrogen-sufficient conditions—equivalent to 1, 18 and 50% of the amount obtained from RNA extracted from nitrogen-limited cells, respectively (Figure 4B). The control RT-PCR with 16S rDNA-specific primers confirmed that equal amounts of RNA from nitrogen-deprived cells and nitrogen-sufficient cells were used. Thus, transcription of the nif operon was not completely repressed in response to nitrogen sufficiency and the transcript was rapidly degraded from the 3′ end. The apparent degradation of the transcript also explains the difficulties in detecting the 8 kb transcript in the Northern blot analyses (Figure 4A).
Figure 4.
Transcriptional analysis of the M. mazei nif gene cluster. (A) Northern blot analysis of total RNA isolated from M. mazei cells grown under conditions of nitrogen limitation (N2) and nitrogen sufficiency ( ) using probes for nifH, nifK and nifN. Each lane was loaded with 0.25 µg total RNA from cells grown under nitrogen limitation (-) or nitrogen sufficiency (+); numbers on the left are molecular sizes in kilobases. (B) RT-PCR analysis. Reverse transcription was carried out on 0.1 µg RNA isolated from cells grown under conditions of nitrogen limitation (-) or nitrogen sufficiency (+) using the OneStep RT-PCR Kit from Qiagen and primers as described in Materials and methods. Control PCR reactions with RNA in the absence of reverse transcriptase showed that the isolated RNA preparations were free of genomic DNA. As a control, a 16S rDNA-specific RT-PCR was carried out on 10 ng of RNA from cells from each growth condition. Products of the expected size (450 bp (nifH), 417 bp (nifK), 438 bp (nifN), 415 bp (glnK1) and 420 bp (16S rDNA)) were separated in 1.5% agarose gels and visualized by ethidium bromide staining.
Figure 5.
Organization of the nif gene cluster from M. mazei Gö1. The sizes of the boxes are proportional to the lengths of the genes. Probes used in Northern blot analysis, the single transcript observed, and the restriction sites are indicated.
The organization of the seven genes of the nif gene cluster in one operon in M. mazei is similar to the nif gene organization in M. maripaludis, but differs from the corresponding nif2 gene cluster in M. barkeri, which appears to be organized in two transcriptional units (Chien and Zinder 1996, Kessler et al. 1998). The sequence at the transcriptional start site in front of the nifH gene in M. mazei shows a nearly palindromic sequence ACCGGCTTCCGGT (see Figure 3). For M. maripaludis, Leigh and coworkers identified a palindromic operator sequence located immediately 3′ to the transcriptional start site for nifH (CGGAAAGAAGCTTCCG) (Cohen-Kupiec et al. 1997). Thus, the palindromic sequence in front of the M. mazei nifH gene may have a function in regulatory processes of nif gene transcription. If this is the case, the mechanism of transcriptional regulation of the nif cluster in M. mazei may be more similar to the regulation in M. maripaludis and may differ from that in M. barkeri, which lacks the operator sequence (Chien and Zinder 1996).
Conclusion
We have identified a single ammonium-regulated nif gene cluster in the mesophilic methanogenic archaeon M. mazei strain Gö1 encoding a molybdenum-containing nitrogenase most closely related to the molybenum nitrogenases of M. acetivorans and M. barkeri 227. However, no additional nif gene cluster encoding a potential alternative nitrogenase was observed in the complete M. mazei genome sequence. This is in contrast to the genome sequences of M. acetivorans and M. barkeri, which contain three and at least two sets of nif genes, respectively (Chien et al. 2000, Galagan et al. 2002; ERGO database (Integrated Genomics, Inc., http://www. integratedgenomics.com) and ORNL database (http://genome.ornl.gov/microbial/mbar)). We cannot rule out that M. mazei has lost the genes encoding the alternative nitrogenases. However, because M. mazei has a smaller genome than the two other organisms, the number of nitrogenases might correlate with the size of the genome.
Acknowledgments
We thank Anita Kuhn for her contributions during the initial steps of this work and Andrea Shauger for critical reading of the manuscript. This work was supported by a grant of the “Niedersächsisches Ministerium für Wissenschaft and Kultur” to the Göttingen Genomics Laboratory, and by grants of the DFG and by the Fonds der Chemischen Industrie.
References
- R1.Arcondeguy T., Jack R., Merrick M. The PII signal transduction proteins: pivotal players in microbial nitrogen control. Microbiol. Mol. Biol. Rev. 2001;65:80–105. doi: 10.1128/MMBR.65.1.80-105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R2.Bell S.D., Jackson S.P. Mechanism and regulation of transcription in archaea. Curr. Opin. Microbiol. 2001;4:208–213. doi: 10.1016/s1369-5274(00)00190-9. [DOI] [PubMed] [Google Scholar]
- R3.Bult C.J., White O., Olsen G.J., et al. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii . Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- R4.Chen J.S., Toth J., Kasap M. Genbank submission AF266462. 2001.
- R5.Chien Y.T., Zinder S.H. Cloning, DNA sequencing, and characterization of a nifD-homologous gene from the archaeon Methanosarcina barkeri 227 which resembles nifD1 from the eubacterium Clostridium pasteurianum . J. Bacteriol. 1994;176:6590–6598. doi: 10.1128/jb.176.21.6590-6598.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R6.Chien Y.T., Zinder S.H. Cloning, functional organization, transcript studies, and phylogenetic analysis of the complete nitrogenase structural genes (nifHDK2) and associated genes in the archaeon Methanosarcina barkeri 227. J. Bacteriol. 1996;178:143–148. doi: 10.1128/jb.178.1.143-148.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R7.Chien Y.T., Helmann J.D., Zinder S.H. Interactions between the promoter regions of nitrogenase structural genes (nifHDK2) and DNA-binding proteins from N2- and ammonium-grown cells of the archaeon Methanosarcina barkeri 227. J. Bacteriol. 1998;180:2723–2728. doi: 10.1128/jb.180.10.2723-2728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R8.Chien Y.T., Auerbuch V., Brabban A.D., Zinder S.H. Analysis of genes encoding an alternative nitrogenase in the archaeon Methanosarcina barkeri 227. J. Bacteriol. 2000;182:3247–3253. doi: 10.1128/jb.182.11.3247-3253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R9.Cohen-Kupiec R., Blank C., Leigh J.A. Transcriptional regulation in Archaea: in vivo demonstration of a repressor binding site in a methanogen. Proc. Natl. Acad. Sci. USA. 1997;94:1316–1320. doi: 10.1073/pnas.94.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R10.Dean D.R., Jacobson M.R. Biochemical genetics of nitrogenases. In: Stacey G., Burris R.H., Evans H.J., editors. Biological Nitrogen Fixation. New York: Chapman & Hall; 1992. pp. 763–834. [Google Scholar]
- R11.Dean D.R., Bolin J.T., Zheng L. Nitrogenase metalloclusters: structures, organization, and synthesis. J. Bacteriol. 1993;175:6737–6744. doi: 10.1128/jb.175.21.6737-6744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R12.Deppenmeier U., Blaut M., Mahlmann A., Gottschalk G. Reduced coenzyme F420 H2-dependent heterodisulfide oxidoreductase: a proton translocating redox system in methanogenic bacteria. Proc. Natl. Acad. Sci. USA. 1990;87:9449–9453. doi: 10.1073/pnas.87.23.9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R13.Deppenmeier U., Johann A., Hartsch T., et al. The genome of Methanosarcina mazei: evidence for lateral gene transfer between Bacteria and Archaea. J. Mol. Microbiol. Biotechnol. 2002;4:453–461. [PubMed] [Google Scholar]
- R14.Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R15.Ehlers C., Grabbe R., Veit K., Schmitz R.A. Characterization of GlnK1 from Methanosarcina mazei strain Gö1: complementation of an Escherichia coli glnK mutant strain by M. mazei GlnK1. J. Bacteriol. 2002;184:1028–1040. doi: 10.1128/jb.184.4.1028-1040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R16.Ferry J.G. Enzymology of one-carbon metabolism in methanogenic pathways. FEMS Microbiol. Rev. 1999;23:13–38. doi: 10.1111/j.1574-6976.1999.tb00390.x. [DOI] [PubMed] [Google Scholar]
- R17.Fischer H.M. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 1994;58:352–386. doi: 10.1128/mr.58.3.352-386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R18.Galagan J.E., Nusbaum C., Roy A., et al. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 2002;12:532–542. doi: 10.1101/gr.223902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R19.Georgiadis M.M., Komiya H., Chakrabarti P., Woo D., Kornuc J.J., Rees D.C. Crystallographic structure of the nitrogenase iron protein from Azotobacter vinelandii . Science. 1992;257:1653–1659. doi: 10.1126/science.1529353. [DOI] [PubMed] [Google Scholar]
- R20.Haselkorn R., Buikema W.J. Methanococcus genome. Science. 1996;274:901–902. doi: 10.1126/science.274.5289.901. [DOI] [PubMed] [Google Scholar]
- R21.Hausner W., Wettach J., Hethke C., Thomm M. Two transcription factors related with the eucaryal transcription factors TATA-binding protein and transcription factor IIB direct promoter recognition by an archaeal RNA polymerase. J. Biol. Chem. 1996;271:30144–30148. doi: 10.1074/jbc.271.47.30144. [DOI] [PubMed] [Google Scholar]
- R22.Kessler P.S., Leigh J.A. Genetics of nitrogen regulation in Methanococcus maripaludis . Genetics. 1999;152:1343–1351. doi: 10.1093/genetics/152.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R23.Kessler P.S., Mclarnan J., Leigh J.A. Nitrogenase phylogeny and the molybdenum dependence of nitrogen fixation in Methanococcus maripaludis . J. Bacteriol. 1997;179:541–543. doi: 10.1128/jb.179.2.541-543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R24.Kessler P.S., Blank C., Leigh J.A. The nif gene operon of the methanogenic archaeon Methanococcus maripaludis . J. Bacteriol. 1998;180:1504–1511. doi: 10.1128/jb.180.6.1504-1511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R25.Kessler P.S., Daniel K., Leigh J.A. Ammonium switch-off of nitrogen fixation in the methanogenic archeaon Methanococcus maripaludis: Mechanistic features and requirement for the novel GlnB homologues, NifI1 and NifI2 . J. Bacteriol. 2001;183:882–889. doi: 10.1128/JB.183.3.882-889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R26.Langer D., Zillig W. Putative tfIIs gene of Sulfolobus acidocaldarius encoding an archaeal transcription elongation factor is situated directly downstream of the gene for a small subunit of DNA-dependent RNA polymerase. Nucleic Acids Res. 1993;21:2251. doi: 10.1093/nar/21.9.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R27.Langer D., Hain J., Thuriaux P., Zillig W. Transcription in Archaea: similarity to that in Eucarya. Proc. Natl. Acad. Sci. USA. 1995;92:5768–5772. doi: 10.1073/pnas.92.13.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R28.Lobo A.L., Zinder S.H. Nitrogen fixation by methanogenic bacteria. In: Stacey G., Burris R.H., Evans H.J., editors. Biological Nitrogen Fixation. New York: Chapman & Hall; 1992. pp. 191–211. [Google Scholar]
- R29.Marsh T.L., Reich C.I., Whitelock R.B., Olsen G.J. Transcription factor IID in the Archaea: sequences in the Thermococcus celer genome would encode a product closely related to the TATA-binding protein of eukaryotes. Proc. Natl. Acad. Sci. USA. 1994;91:4180–4184. doi: 10.1073/pnas.91.10.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R30.Merrick M.J., Edwards R.A. Nitrogen control in bacteria. Microbiol. Rev. 1995;59:604–622. doi: 10.1128/mr.59.4.604-622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R31.Nölling J., Breton G., Omelchenko M.V., et al. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum . J. Bacteriol. 2001;183:4823–4838. doi: 10.1128/JB.183.16.4823-4838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R32.Qureshi S.A., Khoo B., Baumann P., Jackson S.P. Molecular cloning of the transcription factor TFIIB homolog from Sulfolobus shibatae . Proc. Natl. Acad. Sci. USA. 1995;92:6077–6081. doi: 10.1073/pnas.92.13.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R33.Rees D.C., Howard J.B. Structural bioenergetics and energy transduction mechanisms. J. Mol. Biol. 1999;293:343–350. doi: 10.1006/jmbi.1999.3005. [DOI] [PubMed] [Google Scholar]
- R34.Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. 2nd Edn. . Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. Molecular cloning: a laboratory manual. [Google Scholar]
- R35.Sibold L., Henriquet M., Possot O., Aubert J.P. Nucleotide sequence of nifH regions from Methanobacterium ivanovii and Methanosarcina barkeri 227 and characterization of glnB-like genes. Res. Microbiol. 1991;142:5–12. doi: 10.1016/0923-2508(91)90091-n. [DOI] [PubMed] [Google Scholar]
- R36.Smith D.R., Doucette-Stamm L.A., Deloughery C., et al. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J. Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R37.Souillard N., Sibold L. Primary structure, functional organization and expression of nitrogenase structural genes of the thermophilic archaebacterium Methanococcus thermolithotrophicus . Mol. Microbiol. 1989;3:541–551. doi: 10.1111/j.1365-2958.1989.tb00200.x. [DOI] [PubMed] [Google Scholar]
- R38.Staden R., Beal K.F., Bonfield J.K. The Staden package. Methods Mol. Biol. 2000;132:115–130. doi: 10.1385/1-59259-192-2:115. [DOI] [PubMed] [Google Scholar]
- R39.Thauer R.K. Biochemistry of methanogenesis: a tribute to Marjory Stephenson 1998 Marjory Stephenson Prize Lecture. Microbiology. 1998;144:2377–2406. doi: 10.1099/00221287-144-9-2377. [DOI] [PubMed] [Google Scholar]
- R40.Thomm M. Die Transkriptionsmaschinerie der Archaea. Biospektrum. 2000;3:179–185. [Google Scholar]
- R41.Young J.P.W. Phylogenetic classification of nitrogen fixing organisms. In: Stacey G., Burris R.H., Evans H.J., editors. Biological Nitrogen Fixation. New York: Chapman & Hall; 1992. pp. 736–762. [Google Scholar]