Summary
The primary mouth forms from ectoderm and endoderm at the extreme anterior of the embryo, a conserved mesoderm-free region. In Xenopus, a very early step in primary mouth formation is loss of the basement membrane between the ectoderm and endoderm. In an unbiased microarray screen, we defined genes encoding the sFRPs Frzb-1 and Crescent as transiently and locally expressed in the primary mouth anlage. Using antisense oligonucleotides and `face transplants', we show that frzb-1 and crescent expression is specifically required in the primary mouth region at the time this organ begins to form. Several assays indicate that Frzb-1 and Crescent modulate primary mouth formation by suppressing Wnt signaling, which is likely to be mediated by β-catenin. First, a similar phenotype (no primary mouth) is seen after loss of Frzb-1/Crescent function to that seen after temporally and spatially restricted overexpression of Wnt-8. Second, overexpression of either Frzb-1 or Dkk-1 results in an enlarged primary mouth anlage. Third, overexpression of Dkk-1 can restore a primary mouth to embryos in which Frzb-1/Crescent expression has been inhibited. We show that Frzb-1/Crescent function locally promotes basement membrane dissolution in the primary mouth primordium. Consistently, Frzb-1 overexpression decreases RNA levels of the essential basement membrane genes fibronectin and laminin, whereas Wnt-8 overexpression increases the levels of these RNAs. These data are the first to connect Wnt signaling and basement membrane integrity during primary mouth development, and suggest a general paradigm for the regulation of basement membrane remodeling.
Keywords: Primary mouth, Xenopus, Wnt, sFRP, Frzb-1, Crescent, Basement membrane, Laminin, Fibronectin
INTRODUCTION
The primary mouth is the initial opening that connects the foregut to the outside of the embryo. It originates from a unique and conserved extreme anterior domain in the embryo, where endoderm directly contacts ectoderm, without intervening mesoderm (Dickinson and Sive, 2007). In vertebrates, the primary mouth becomes the opening to the pharynx as surrounding tissues form the jaws, teeth, tongue and palate, which together constitute the secondary or adult mouth.
We previously defined morphological changes that lead to primary mouth formation in Xenopus laevis during early tailbud and hatching stages (Dickinson and Sive, 2006). The earliest step identified is disappearance of the basement membrane between the ectoderm and endoderm, which occurs at early tailbud stage. Later, during tadpole stages, the presumptive primary mouth ectoderm undergoes invagination to form the `stomodeum'. Subsequently, this invagination deepens, accompanied by a burst of cell death in the ectodermal layer. Ectoderm and endodermal layers intercalate, leading to thinning of the cell layers in the primary mouth anlage. Finally, the thin covering (the `buccopharyngeal membrane') perforates at swimming tadpole stage to open the primary mouth.
Three regions of the embryo are required to induce formation of the primary mouth. These are the deep anterior endoderm, the anterior neural plate and the lateral mesectoderm, including the neural crest (Dickinson and Sive, 2006). These regions are likely to secrete regulatory factors that govern primary mouth development, but these signals and other genes involved are not known, and their identification forms the basis of this paper.
Early during development, substantial data indicate that anterior development in Xenopus and other vertebrates requires the inhibition of β-catenin-mediated Wnt signaling (Agathon et al., 2003; De Robertis, 2006; De Robertis et al., 2000; Kemp et al., 2005; Lewis et al., 2008; Niehrs, 1999). Wnt signaling can be inhibited by several secreted antagonists, which target the Wnt co-receptors Frizzled and LRP6 (Semenov et al., 2008; Yamamoto et al., 2008). The secreted Frizzled Related Proteins (sFRPs) comprise another class of Wnt antagonists, which contain a cysteine-rich domain with homology to the extracellular domain of Frizzled receptors. sFRPs are believed to bind Wnt ligands, thereby preventing their interaction with Frizzleds (Jones and Jomary, 2002; Kawano and Kypta, 2003). Some sFRPs also inhibit other pathways, including BMP signaling (Bovolenta et al., 2008; Lee et al., 2006).
Wnt antagonists are required for anterior specification during primary axis formation. For example, during gastrula stages of X. laevis, dkk-1 and the sFRPs frzb-1, crescent, sfrp-2 and sizzled are expressed in the Spemann organizer and are important for formation of the head (Glinka et al., 1998; Niehrs et al., 2001; De Robertis, 2006; Jones and Jomary, 2002; Kawano and Kypta, 2003). Later, during Xenopus and zebrafish neurulation, Wnt antagonists are expressed anteriorly and are required for formation of the forebrain and placodes (Carmona-Fontaine et al., 2007; Houart et al., 2002). Although it is clear that inhibition of Wnt/β-catenin signaling is important for early stages of anterior patterning, it is not clear whether these antagonists function later during anterior organogenesis, including formation of the primary mouth.
In order to define signaling pathways that regulate primary mouth formation, we used expression microarrays to identify genes with enriched expression in the primary mouth anlage. Through this screen, we isolated two Wnt antagonists, the sFRPs Frzb-1 and Crescent, as potential molecular regulators of primary mouth development. We show that sFRP function is crucial for primary mouth formation, and to locally promote dissolution of the basement membrane. These data are the first to connect Wnt signaling and basement membrane integrity during primary mouth development.
MATERIALS AND METHODS
Embryos
Xenopus laevis embryos were obtained and cultured using standard methods (Sive et al., 2000). Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1994).
Microarray analysis
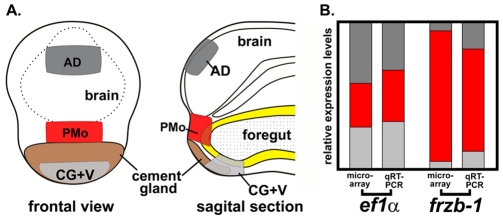
Tissue was collected from three regions of the embryo at stage 25-26. (1) The presumptive primary mouth (PMo), including endoderm and ectoderm, dorsal to the cement gland, ventral to the telencephalon and central to the hatching gland (Fig. 1A, PMo, red). (2) The anterodorsal (neural) region (AD) (Fig. 1A, dark gray), comprising the central telencephalon, excluding the eyes. (3) The ventral region including the cement gland (V+CG) (Fig. 1A, light gray). One hundred dissections were performed for each of three biological replicates and stored in Trizol (Invitrogen) at -80°C. Total RNA was isolated using Trizol extraction followed by a lithium chloride solution (Ambion) precipitation. Total RNA (100 ng) was used to prepare biotinylated cRNA using the Two Cycle cDNA Synthesis Kit (Affymetrix), according to the manufacturer's protocol. Briefly, SuperScript II-directed reverse transcription used a T7-Oligo(dT) Promoter Primer to create first strand cDNA. RNase H-mediated second strand cDNA synthesis was followed by MEGAscript T7 (Ambion) directed in vitro transcription, which generated unmodified cRNA. cRNA was used as a template for a second round of cDNA synthesis, followed by a second in vitro transcription reaction, which incorporated a biotinylated nucleotide analog during cRNA amplification. Samples were prepared for hybridization using 15 μg biotinylated cRNA in a 1×hybridization cocktail. Additional hybridization cocktail components were provided in the Affymetrix GeneChip Hybridization, Wash and Stain Kit. GeneChip arrays (Xenopus) were hybridized in a GeneChip Hybridization Oven at 45°C for 16 hours at 60 rotations per minute. Washing was done using a GeneChip Fluidics Station 450 according to the manufacturer's instructions, using the buffers provided in the Affymetrix GeneChip Hybridization, Wash and Stain Kit. Arrays were scanned on a GeneChip Scanner 3000 and images were extracted and analyzed using GeneChip Operating Software v1.4. The generated CHP and CEL files have been deposited in the Gene Expression Omnibus (NCBI, GSE13377). Expression level differences and statistical significance were calculated using Excel, and both were considered in identifying candidate genes.
Fig. 1.
Schematic summarizing microarray screen for genes whose expression is enriched in the primary mouth anlage. (A) Regions microdissected for RNA collection: anterior dorsal region (AD, dark gray), ventral region including the cement gland (CG+V, light gray) and the presumptive primary mouth (PMo, red). Foregut epithelium is shown in yellow. (B) Expression of frzb-1 and ef1-alpha in the primary mouth (red) relative to flanking regions (88%, microarray; 71%, qRT-PCR).
qRT-PCR
cDNA was prepared using the Sensiscript Kit (Qiagen). qRT-PCR was performed using ABI Prism 7000 or 7900 (ABI). Fluorescence detection chemistry used the SYBR green dye master mix (Roche). Primers sequences are available on request. The relative amount of product was calculated using ΔCT and products normalized to ef1-alpha.
In situ hybridization
cDNAs used to transcribe in situ hybridization probes were frzb-1 [GA# U68059 (Wang et al., 1997a; Wang et al., 1997b)], crescent (also called frzb-2) (Bradley et al., 2000), XCG (Sive et al., 1989) and nrp-1 [GA#, BC084198 (Richter et al., 1990)]. In situ hybridization was performed as described by Sive et al. (Sive et al., 2000), omitting the proteinase K treatment. Double-staining analysis was performed as described previously (Wiellette and Sive, 2003).
Morpholinos and RNA injections
Antisense morpholinos were purchased from Gene Tools. To design frzb-1 morpholinos, we sequenced the start site of frzb-1 in X. laevis (primer sequences are available on request). Morpholinos included two frzb-1 start site morpholinos (morpholino sequences are available on request), a previously published splice blocking crescent morpholino (Shibata et al., 2005) and a standard control morpholino. For rescue assays, 10 mutations were introduced into the start site of the frzb-1 cDNA, using a site-directed mutagenesis kit (Stratagene). One nanogram per embryo of the mutated frzb-1 mRNA produced an overexpression phenotype comparable to the wild-type construct, as described (Wang et al., 1997a).
F0 transgenics and heat shock
For heat-shock inducible transgenics, we used a construct [pSGH2; ISceI-GFP-HSE plasmid (Bajoghli et al., 2004)] with a multimerized heat-shock element (HSE) promoter, a multiple cloning site and I-SceI sites. The genes dkk-1 [GA# AF030434 (Glinka et al., 1998)], wnt-8b (GA# U22173 (Christian et al., 1991)], chordin [GA#, L35764 (Sasai et al., 1994)], frzb-1 and crescent (see above) were inserted into the multiple cloning site (GFP::HSE::gene of interest). The meganuclease method was used to create F0 transgenics as previously described (Pan et al., 2006). Heat shock was achieved by moving embryos from 15°C to 35-37°C at the appropriate stage, and maintaining embryos at this temperature for 2 hours. The construct minus inserted genes served as a control.
Laminin immunohistochemistry and auto-fluorescent rendering
Specimens were embedded in 4% low-melt agarose (SeaPlaque GTG, Cambrex) and sectioned with a 1000 Series Vibratome at 100 μm. Immunohistochemistry was performed as described (Dickinson and Sive, 2006) using a polyclonal anti-laminin antibody (Sigma, L-9393) diluted 1:150, a goat anti-rabbit Alexa Fluor-conjugated antibody (Molecular Probes) diluted 1:500 and a 0.1% propidium iodide (Sigma) counterstain. Embryos were prepared for optical auto-fluorescent sections as described (Dickinson and Sive, 2006).
Extirpations and transplants
Extirpations and transplants were performed in 1.0×MBS (Modified Barth's Solution) in plasticine-lined Petri dishes, using 1-mm diameter capillary tubes pulled to a fine point. Glass bridges were used to hold tissue together while healing. Two types of transplants were performed. (1) `Face transplants', which involve dissecting the ectodermal and endodermal layers of the cement gland and the region just dorsal, which fate maps to the presumptive primary mouth, and transplanting this entire region to a donor embryo in which the same tissue has been removed. (2) `Ectoderm transplants', which involve removing the ectoderm, both superficial and deep, from the presumptive primary mouth and transplanting to a donor embryo in which the same tissue has been removed.
JNK inhibition
Embryos were bathed in a 20 μM solution of SP600125 (Sigma) with 1% DMSO in 0.1% MBS from stage 17 to stage 40 in culture dishes at room temperature.
β-Catenin protein levels and activity
β-Catenin protein levels were analyzed by western blotting. Tissue lysates were prepared from pooled samples of 10 embyros. Primary antibodies were anti-β-catenin (Zymed, 71-2700) and anti-β-actin (Sigma, A5441), both diluted 1:1000. Secondary antibodies were HRP-conjugated anti-mouse or anti-rabbit IgG (Cell Signaling), and detection was by chemiluminescence using LumniGLO Reagent and Peroxide (Cell Signaling). The bands were quantified by densitometry using Photoshop (Adobe).
β-catenin activity was measured using the TOPFLASH system. Briefly, embryos were injected with 10 pg of the TOPFLASH construct (Clevers and van de Wetering, 1997), 10 pg pRL-SV40/TK as a reference plasmid, and 1 ng of frzb-1 RNA. Luciferase assays were performed using the Dual-Luciferase Assay Kit (Promega). The primary mouth and surrounding area were dissected at stage 20-22, suspended in 50 μl of 1×Passive Lysis Buffer and stored at -80°C. Luciferase was detected on a luminometer (Molecular Devices). All values were expressed as relative luciferase units (Firefly luciferase activity/Renilla luciferase activity) and scaled and plotted with Microsoft Excel.
Scoring primary mouth formation
We evaluated primary mouth development by examining the perforated opening or by examining the stomodeum, an invagination that forms prior to the opening. Perturbing primary mouth development, in the most severe cases, results in neither a stomodeum nor a primary mouth. In less severe cases, a stomodeum forms with no opening. The stomodeum can also vary in size, which indicates how large the mouth opening will be.
RESULTS
The sFRPs frzb-1 and crescent are expressed in the primary mouth anlage
In order to define genes required for primary mouth formation, we identified those that are differentially expressed in the future primary mouth, relative to surrounding regions. We used an unbiased expression microarray approach and microdissected tissue at stage 24-26, as described in the Materials and methods. Thirteen genes were identified, whose expression was highly enriched in the primary mouth (Fig. 1A; see also Table S1 in the supplementary material), and validated by qRT-PCR and in situ hybridization (see Fig. S1A,B in the supplementary material). One of these genes was frzb-1, which displayed 18-fold-enriched expression in the primary mouth relative to the other regions. Relative to other regions used for comparison, the level of frzb-1 expression in the primary mouth is 88% as measured by microarray, and 71% by qRT-PCR (Fig. 1B).
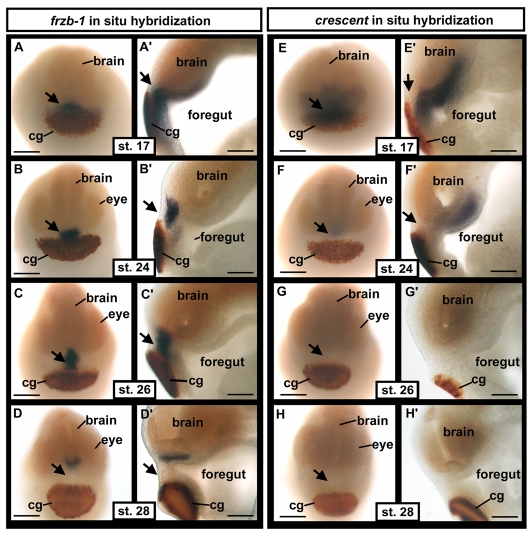
In situ hybridization showed that frzb-1 was expressed in the prechordal plate, presumptive anterior pituitary and primary mouth, as well as in the endoderm lying beneath the cement gland, at neurula stages (stage 17-20; Fig. 2A,A′). At early tailbud stages (stage 24 and 26), expression appeared strong in the deep ectoderm of the future anterior pituitary and primary mouth, while fainter expression persisted in underlying endoderm (Fig. 2B-C′). At stage 28, frzb-1 mRNA was present in the developing anterior pituitary (Fig. 2D,D′). By tadpole, stage 32, expression appeared to be absent in the head (data not shown). Increased probe concentrations and longer incubation times revealed a low level of frzb-1 expression in the brain (data not shown), consistent with expression values from the microarray analysis.
Fig. 2.
In situ hybridization of frzb-1 and crescent mRNA during neurula and early tailbud stages. (A-H′) In situ hybridization of frzb-1 (A-D) and crescent (E-H) during neurula and early tailbud stages. frzb-1 and crescent are stained purple/blue, the cement gland marker (XCG), dark red and the CNS-specific marker (nrp1), light orange. Arrows indicate the presumptive primary mouth. cg, cement gland. A-H are frontal views, scale bars: 200 μm; A′-H′ are sagittal sections with anterior to the left, scale bars: 130 μm.
Frzb-1 belongs to a large class of Wnt antagonists, the secreted frizzled related proteins (sFRPs). Because our microarray screen consisted of only one time point, and primary mouth formation takes place over many hours, we examined the expression of other sFRPs, including sFRP1, sFRP2, sFRP5 and crescent (see Fig. S2A-L in the supplementary material), to detect redundantly expressed genes. Only crescent was expressed in or near the future primary mouth (Fig. 2E-H′). At stage 17, crescent was expressed in a broader domain than was frzb-1, including the prechordal plate, the presumptive anterior pituitary and primary mouth, and in tissue lying beneath the cement gland (Fig. 2E,E′). At stages 20-24, crescent expression was primarily confined to the prechordal plate, posterior and anterior to the presumptive primary mouth and the anterior pituitary (Fig. 2F,F′). By stage 26, crescent mRNA was no longer detected in the head region (Fig. 2E-H′), although microarray analysis showed very low levels of crescent expression in the future primary mouth at stage 26 (data not shown).
These data indicate that during early neurula stages, crescent and frzb-1 have overlapping expression patterns in the presumptive primary mouth, consistent with a role in the development of this organ.
Frzb-1 and Crescent are required for primary mouth formation
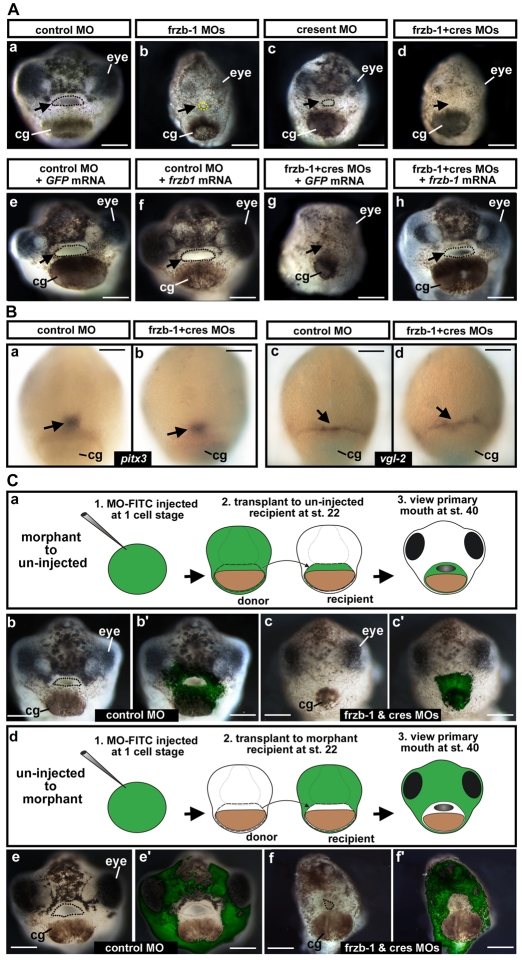
We investigated whether Frzb-1/Crescent function was necessary for primary mouth formation, and whether the function of these sFRPs was redundant, using antisense morpholinos against frzb-1 and/or crescent, as detailed in the Materials and methods. Injection of frzb-1 morpholinos alone resulted in embryos with a small stomodeum (invagination, dotted yellow) but no primary mouth opening, whereas the same amount of control morpholino had no effect on the primary mouth opening (dotted black; Fig. 3A, parts a,b). The injection of crescent morpholinos resulted in a smaller primary mouth opening relative to controls (Fig. 3A, part c). However, co-injection of frzb-1 and crescent morpholinos resulted in morphants with neither stomodeum nor primary mouth opening (Fig. 3A, part d). The specificity of the phenotype was confirmed by rescue with low levels (200 pg) of mutated frzb-1 mRNA that does not hybridize to the morpholino (see Materials and methods). When injected into control embryos, this amount of frzb-1 mRNA did not alter primary mouth morphology (3A, parts e,f). Ninety-three percent of embryos injected with frzb-1 and crescent morpholinos together with the control GFP mRNA had neither a stomodeum nor a primary mouth opening (Fig. 3A, part g). This phenotype was rescued in 86% of morphant embryos injected with frzb-1 mRNA (Fig. 3A, part h). Although these embryos were not completely normal, possibly because of the effects of crescent loss of function (Shibata et al., 2005), the primary mouth opening was relatively normal in size. crescent mRNA alone was not able to rescue the primary mouth defect (data not shown), and gave a cyclopic phenotype that resulted from effects on prechordal plate migration (Pera and De Robertis, 2000).
Fig. 3.
frzb-1/crescent loss-of-function analysis. (A) frzb-1 and crescent loss of function using antisense morpholinos, and rescue by frzb-1 mRNA in whole embryos. Frontal views are shown, assayed at stage 40 in two to four independent experiments. cg, cement gland. Scale bars: 250 μm. Open primary mouth, black dotted line; closed stomodeum, yellow dotted line. (a-d) Morpholino injection (60-80 ng/embryo). (a) Control morpholino results in a normal primary mouth (100%, n=121). (b) Two frzb-1 start site morpholinos result in a very small stomodeum (97%, n=57). (c) A crescent splice blocking morpholino results in a smaller primary mouth (100%, n=50). (d) frzb-1 morpholinos (15 ng/embryo of each) and crescent morpholino (30 ng/embryo) result in neither stomodeum nor primary mouth (99%, n=66). (e-h) Rescue: 60 ng morpholino and 200 pg mRNA was injected/embryo. (e) Control morpholinos plus GFP mRNA have no effect (100%, n=45). (f) Control morpholinos with frzb-1 mRNA results in a normal primary mouth (100%, n=33). (g) frzb-1/crescent morpholinos plus GFP mRNA result in neither a stomodeum nor a primary mouth (94%, n=32). (h) frzb-1/crescent morpholinos plus frzb-1 mRNA results in a primary mouth opening (86%, n=55). Arrows indicate the primary mouth or region where it would form. (B) The primary mouth anlage is correctly specified. (a,b) Analysis of pitx3 expression by in situ hybridization in (a) control and (b) frzb-1/crescent morphant embryos. Note that pitx3 expression is present in the morphant. Scale bars: 200 μm. (c,d) Analysis of vgl-2 expression by in situ hybridization in (c) control and (d) frzb-1/crescent morphant embryos. Note that vgl-2 expression it is present in the morphant. Arrows indicate the primary mouth anlage. Scale bars: 200 μm. (C) Localizing morphant and wild-type tissue using face transplants. (a) Schematic of experimental design: donor morphant tissue (FITC labeled) was transplanted to uninjected sibling recipients. (b) The primary mouth is normal when donor tissue is derived from embryos injected with control morpholinos (100%, n=9). (b′) Overlay of b with FITC fluorescence, indicating the location of the donor tissue in the recipient. (c) When donor tissue is derived from frzb-1/crescent morphants, 83% of recipients do not form a primary mouth opening and 17% form a small stomodeum (n=12). These embryos also have abnormalities in surrounding tissues, pigment cells do not migrate normally and the face appears thinner. (c′) Overlay of c with FITC fluorescence. (d) Schematic of experimental design: donor wild-type tissue was transplanted to morphants. (e) The primary mouth is normal when recipients are injected with standard control morpholinos (100%, n=7). (e′) Overlay of e with FITC fluorescence. (f) When recipients are frzb-1/crescent morphants, a primary mouth is present (80%, n=15). Although the primary mouth is not a normal shape, a deep invagination forms, followed by perforation. (f′) Overlay of f with FITC fluorescence.
We did not observe abnormal levels of cell death or proliferation in the primary mouth region of morphants at stage 23-24 (see Fig. S3B, parts a-d in the supplementary material). Importantly, despite the absence of the primary mouth at stage 40, this region was correctly specified at stage 23-24, as indicated by the expression of two markers specifically expressed in the primary mouth anlage, pitx3 and vgl-2 (Fig. 3B, parts a-d). Furthermore, the morphology of the morphants appeared to be relatively normal at stage 23-24, with structures around the future primary mouth present, including the eyes and cement gland (see Fig. S3A, part a in the supplementary material). At later times, frzb-1/crescent morphants show a phenotype consistent with a function in primary axis formation (Bradley et al., 2000; Pera and De Robertis, 2000) (see Fig. S3A, parts b-h in the supplementary material). In agreement with a requirement for Frzb-1/Crescent, removal of the frzb-1 expression domain at early tailbud by extirpation resulted in neither a stomodeum nor a primary mouth (Fig. S4 in the supplementary material).
These data show that expression of both frzb-1 and crescent is required for primary mouth formation, with frzb-1 loss of function giving a stronger phenotype than does crescent loss of function. The function of these genes is required after initial primary mouth specification.
A specific requirement for Frzb-1 and Crescent in the primary mouth region during tailbud stages
Because frzb-1/crescent morphants showed a whole embryo phenotype, we investigated whether gene function is required in the forming primary mouth, or whether the primary mouth phenotype is secondary to earlier defects. To answer this, we performed face transplants to localize morphant tissue specifically to the future primary mouth during early tailbud stages (stage 23-24; see Fig. 3B, part a, and 3C, part a; see also Materials and methods). In the first transplant, donor tissue originated from morpholino-injected embryos, while recipients were uninjected sibling embryos (Fig. 3B, part a). Using morphant donor tissue, 83% of embryos did not form a stomodeum or a primary mouth opening and 17% had a smaller stomodeum and no opening; controls all had normal primary mouth morphology (Fig. 3B, parts b-c′). We note that localized loss of Frzb-1/Crescent results in a smaller surrounding face, suggesting that this region may organize other aspects of face development.
In the second transplant, donor tissue originated from uninjected embryos and the recipients were embryos injected with frzb-1 and crescent or control morpholinos (Fig. 3C, part d). All of the controls and 80% of the morphant recipients had a primary mouth opening, albeit of variable size and shape (Fig. 3B, parts e-f′).
These results indicate that Frzb-1 and Crescent are necessary locally in tissue that will form the primary mouth, from the time this organ begins to develop.
Wnt overexpression and loss of Frzb-1/Crescent give similar phenotypes
Previous studies have shown that both frzb-1 and crescent can antagonize Wnt-8 (Bradley et al., 2000; Leyns et al., 1997; Schneider and Mercola, 2001; Wang et al., 1997a). However, sFRPs can also interact with other signaling pathways, such as BMP (reviewed by Bovolenta et al., 2008). In order to determine whether the Wnt signaling pathway is targeted during primary mouth formation, we investigated whether modulators of this pathway could phenocopy the effects of changing Frzb-1/Crescent expression.
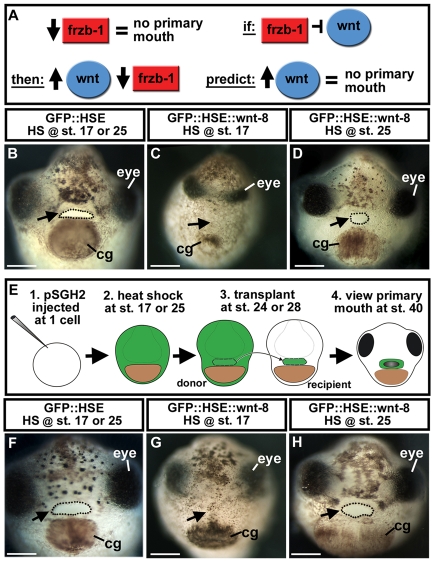
First, we tested whether an increase in Wnt signaling would phenocopy the effects of frzb-1/crescent morpholinos. This would be predicted if these sFRPs targeted Wnt signaling (Fig. 4A). Because overexpression of wnt-8 during early development has profound effects on axial patterning, we restricted wnt-8 expression to neurula and tailbud stages by driving the expression of this gene in transgenic embryos under the control of a heat-shock element (HSE) promoter (GFP::HSE::wnt-8). Two periods of heat shock were administered to determine whether the effects of Wnt-8 overexpression correlated with the expression of frzb-1 and crescent in the primary mouth anlage. Maximal overexpression was expected at the end of the 2-hour heat shock. Overexpression of wnt-8, during the time of frzb-1 and crescent expression (stage 17-24), resulted in embryos with a reduced head, and neither a stomodeum nor a primary mouth opening (Fig. 4B,C). This phenotype resembled that observed with loss of Frzb-1/Crescent function in the whole embryo (see Fig. 3A-D). When wnt-8 was overexpressed later (stage 25-28), after frzb-1 and crescent expression is downregulated in the primary mouth anlage, a normal or slightly smaller primary mouth opening formed (Fig. 4D).
Fig. 4.
Temporal and spatial overexpression of wnt-8 under control of a heat-shock promoter element (HSE) and using ectoderm transplants. Frontal views are shown, assayed at stage 40 in two to three independent experiments. Open primary mouth, black dotted line; cg, cement gland; pSGH2, ISceI-GFP-HSE plasmid. Arrows indicate the primary mouth or region where it would form. Scale bars: 250 μm. (A) Schematic showing prediction that if Frzb-1/Cresent inhibits Wnt signaling, increased Wnt-8 would phenocopy loss of Frzb-1/Crescent. (B) Injected with GFP::HSE followed by heat shock at either stage 17 or 25 has no effect on primary mouth formation (90%, n=50). (C) Injection of GFP::HSE::wnt-8 and heat shock administered at stage 17 results in neither a stomodeum nor a primary mouth (97%, n=65). (D) Injection of GFP::HSE::wnt-8 and heat shock administered later (stage 25-28) results in a normal primary mouth (47%) or a slightly smaller one (53%, n=32). (E) Schematic of experimental design using ectoderm transplants. (F) Control (GFP::HSE): when heat shock was administered at stage 17 and transplants performed at stage 24, recipients form a primary mouth (100%, n=12). The same is true if heat shock is administered at stage 24 and transplants are performed at stage 28 (n=10). (G) GFP::HSE::wnt-8: when heat shock was administered at stage 17 and transplants performed at stage 24, 83% of the recipients do not form a stomodeum or a primary mouth (n=12). (H) When the experiment in G was performed later, 88% formed a normal primary mouth (n=9).
Because Wnt-8 has many functions in the whole embryo, we examined the effect of overexpression exclusively in the presumptive primary mouth by performing `ectoderm transplants'. These transplants involved a smaller region than the face transplants described (see Materials and methods) to limit the cells exposed to Wnt-8 (Fig. 4E). Expression was controlled temporally using the HSE promoter. Donor embryos were heat shocked at stage 17-24 or stage 25-28, and were transplanted into host embryos at stage 24 or stage 28, respectively. In recipient embryos, where heat shock was administered at stage 17, and Wnt-8 expressing tissue was transplanted at stage 24, neither a stomodeum nor a primary mouth opening formed (Fig. 4F,G). The timing of this experiment correlates with the time of frzb-1/crescent expression in the presumptive primary mouth. When heat shock was administered later (stage 24) and wnt-8 expressing tissue was transplanted at stage 28 (when frzb-1 and crescent are no longer expressed in the future primary mouth) a normal opening formed (Fig. 4H).
These data show that temporally and spatially restricted Wnt-8 overexpression phenocopies loss of Frzb-1/Crescent function, suggesting that these sFRPs target the Wnt pathway. Because Wnt-8 predominantly activates β-catenin-mediated signaling (Darken and Wilson, 2001), these data suggest a role for Frzb-1 and Crescent in modulating the Wnt/β-catenin pathway.
Frzb-1 and Crescent may inhibit the function of one or several Wnt proteins during primary mouth formation. Data gathered in our microarray screen indicate that the expression of wnt-8, wnt-8b, wnt-3a, wnt-2 and wnt-4 is lower in the primary mouth than in the surrounding regions (see Fig. S1 in the supplementary material). This suggests that other mechanisms may downregulate Wnt gene expression at the RNA level.
Overexpression of Dkk-1 or Frzb-1 lead to similar phenotypes
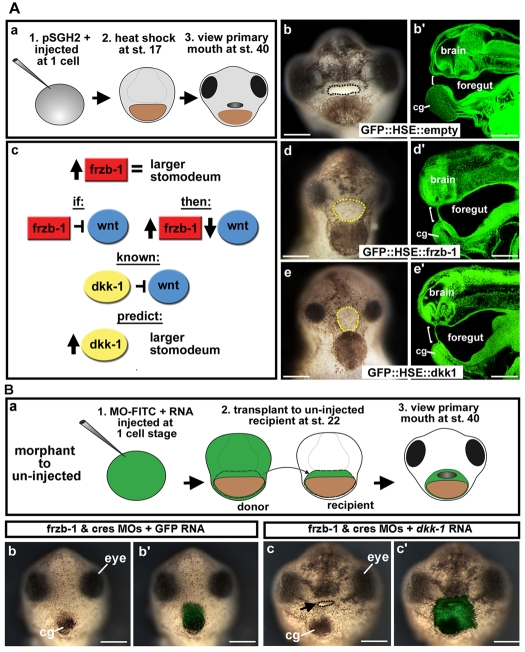
We extended this analysis by asking whether Dkk-1 overexpression, which inhibits Wnt signaling (Yamamoto et al., 2008), phenocopies the effects of Frzb-1 or Crescent overexpression. Using a heat-shock element (HSE) promoter, we overexpressed frzb-1 (GFP::HSE::frzb-1) during late neurula and early tailbud stages (Fig. 5A, part a) in transgenic embryos. This resulted in a very large stomodeum (Fig. 5A, part b-d′), indicating that Frzb-1 is sufficient to expand the size of the primary mouth, consistent with the mRNA overexpression effects (Pera and De Robertis, 2000). A similar, yet less profound, phenotype is observed when Crescent is overexpressed at neurula and tailbud stages (data not shown). Overexpressing Dkk-1 under a heat-shock element promoter (GFP::HSE::dkk-1) in transgenic embryos, during neurula and tailbud stages, to prevent an early patterning phenotype (Glinka et al., 1998) mimics Frzb-1 overexpression (Fig. 5A, part c). These results further suggest that Frzb-1/Crescent function to inhibit Wnt signaling, similar to Dkk-1.
Fig. 5.
Frzb-1 and Dkk-1 regulate the same pathway. (A) Temporal overexpression of frzb-1 and dkk-1 during neurula (stage 17). b′,d′,e′ are sagittal optical sections (anterior to the left). Assayed at stage 40, two independent experiments. Open primary mouth, black dotted line; closed stomodeum, yellow dotted line; bracket indicates the position of the primary mouth or stomodeum; pSGH2, ISceI-GFP-HSE plasmid. Scale bars: 250 μm. (a) Schematic of experimental design. (b) Control (GFP::HSE) results in a normal primary mouth (90%, n=66), frontal view. (b′) Embryo treated as in b with a normal primary mouth. (c) Schematic showing the prediction that increased Frzb-1 would mimic overexpression of Dkk-1. (d) Injection of GFP::HSE::frzb-1 results in a larger stomodeum, frontal view (48%, n=50). (d′) Embryo treated as in d, showing increased stomodeal surface. (e) Injection of GFP::HSE::dkk1 results in a larger stomodeum, frontal view (53%, n=38). (e′) Embryo treated as in e has an increased stomodeal surface. (B) Rescue of the primary mouth in frzb-1/crescent morphants with dkk-1 mRNA. Frontal views, assayed at stage 40 in duplicate. Black arrow, primary mouth; cg, cement gland. Scale bars: 250 μm. (a) Schematic of experimental design. (b) The primary mouth is absent when donor tissue is derived from embryos injected with frzb-1/crescent morpholinos and GFP mRNA (90%, n=10). (b′) Overlay of b with FITC fluorescence, indicating the location of the donor tissue in the recipient. (c) When donor tissue is derived from embryos injected with frzb-1/crescent morpholinos and dkk-1 mRNA, 66% of recipients form a primary mouth and 33% form a large stomodeum with no opening (n=12). (c′) Overlay of c with FITC fluorescence.
Another prediction is that Dkk-1 overexpression would rescue the frzb-1/crescent morphant phenotype. We tested this by injecting dkk-1 or GFP mRNA together with frzb-1/crescent morpholinos and performing face transplants (Fig. 5B, part a) to localize the control or morphant tissue. Ninety percent of embryos receiving donor tissue containing the morpholinos and control GFP mRNA form neither a stomodeum nor a primary mouth, and 10% form a small stomodeum and no opening (Fig. 5B, parts b,b′). Using donor tissue containing the morpholinos and dkk-1 mRNA, 66% of embryos form a primary mouth opening, while 33% of the embryos have a large stomodeum, but no opening (Fig. 5B, parts c,c′). Other controls, including donor tissue containing dkk-1 mRNA plus control morpholinos, or GFP mRNA plus control morpholinos, almost always formed a primary mouth opening (75% and 100%, respectively; data not shown). These results show that overexpression of dkk-1 mRNA can restore primary mouth formation to frzb-1/crescent morphants.
Together, these results indicate that Frzb-1/Crescent antagonize Wnt signaling in a similar manner to Dkk-1 during primary mouth formation. Dkk-1 predominantly inhibits Wnt/β-catenin signaling (Semenov et al., 2001), although recent evidence suggests that this protein can also regulate non-canonical Wnt signaling (Cha et al., 2008).
Frzb-1/Crescent do not target the JNK or BMP signaling pathways
sFRPs can antagonize Wnt signaling mediated by both β-catenin and c-Jun NH2-terminal kinase [JNK; planar cell polarity (PCP)], as well as the BMP pathway (reviewed by Bovolenta et al., 2008). We therefore investigated whether perturbation of the Wnt/PCP and BMP pathways gave a similar primary mouth phenotype to that caused by Frzb-1 overexpression.
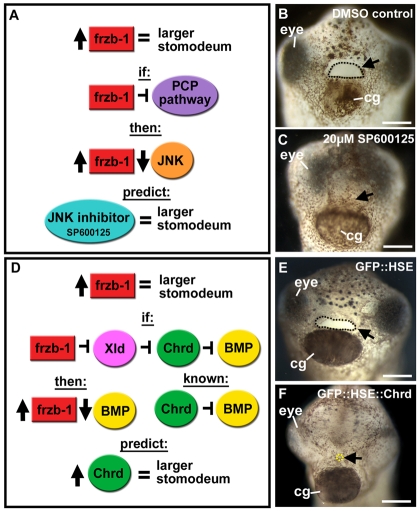
Both Frzb-1 and Crescent can inhibit Wnt/PCP signaling (Qian et al., 2007; Shibata et al., 2005) by activation of the JNK pathway. If Frzb-1 functions to inhibit Wnt/PCP signaling in the primary mouth, inhibition of JNK would phenocopy the Frzb-1 overexpression phenotype (Fig. 6A). However, treatment of late neurula stage embryos (stage 17) with a JNK chemical inhibitor, SP600125 (Han et al., 2001), led to an absence of a stomodeum and a primary mouth opening, which was opposite to the Frzb-1 gain-of-function phenotype (Fig. 6B,C). Thus, although the Wnt/PCP pathway seems to be important for primary mouth formation, Frzb-1 probably does not regulate this pathway. In addition to acting through the JNK pathway, non-canonical Wnt signaling can also use other signaling pathways, including those involving calcium and Src (van Amerongen et al., 2008). It is possible that Frzb-1 and Crescent can target these other pathways during primary mouth formation.
Fig. 6.
Frzb-1 does not regulate Wnt/PCP or Bmp signaling. Frontal views, assayed at stage 40, from 2-3 independent experiments. Open primary mouth, black dotted line; closed stomodeum, yellow dotted; cg, cement gland. Black arrows indicate the primary mouth or region where it would form. Scale bars: 250 μm. (A) Schematic showing the prediction that if frzb-1 inhibits the PCP pathway, JNK inhibition would mimic frzb-1 overexpression. (B) Treatment with 1% DMSO results in a normal primary mouth (100%, n=15). (C) Treatment with 20 μm SP600125 results in neither a stomodeum nor a primary mouth (100%, n=15). (D) Schematic showing the prediction that if frzb-1 inhibits BMP signaling, increased Chordin would phenocopy frzb-1 overexpression. (E) The control GFP::HSE results in a normal primary mouth (100%, n=30). (F) GFP::HSE:chrd results in a small stomodeum (63%, n=32).
The sFRP Sizzled inhibits the Xolloid-like protease, which is essential for BMP regulation (Lee et al., 2006). If Frzb-1 acts similarly and inhibits Xolloid-like in the primary mouth anlage, then overexpression of the BMP inhibitor Chordin should phenocopy the Frzb-1 overexpression phenotype (Fig. 6D). However, contrary to this prediction, overexpression of Chordin under the HSE promoter (GFP::HSE::chrd) in transgenic embryos led to a very small stomodeum and no opening (Fig. 6E,F). A similar phenotype was observed by overexpression of a dominant-negative BMP receptor during neurula stages (data not shown). Therefore, although the BMP signaling pathway is likely to be important for primary mouth development, it does not appear to be a target of Frzb-1 and Crescent.
Frzb-1 is likely to be the major sFRP regulating primary mouth formation owing to the higher level and longer period of expression of the mRNA in the presumptive primary mouth. Therefore, we tested whether increased frzb-1 mRNA could decrease Wnt signaling in the primary mouth region by using β-catenin protein and activity as a readout. We found that overexpression of Frzb-1 significantly decreased β-catenin protein and activity levels in the presumptive primary mouth and flanking tissues (see Fig. S5 in the supplementary material). Furthermore, the microarray screen reveals that β-catenin, and Wnt ligands associated with the Wnt/β-catenin pathway, are expressed at lower levels in the presumptive primary mouth than in surrounding regions (see Fig. S1C in the supplementary material).
Together, these data suggest that Frzb-1 and Crescent primarily act to inhibit β-catenin-mediated Wnt signaling, rather than the Wnt/PCP or BMP pathways, during primary mouth formation.
Loss of Frzb-1 and Crescent, or increased Wnt-8 expression, results in a persistent basement membrane
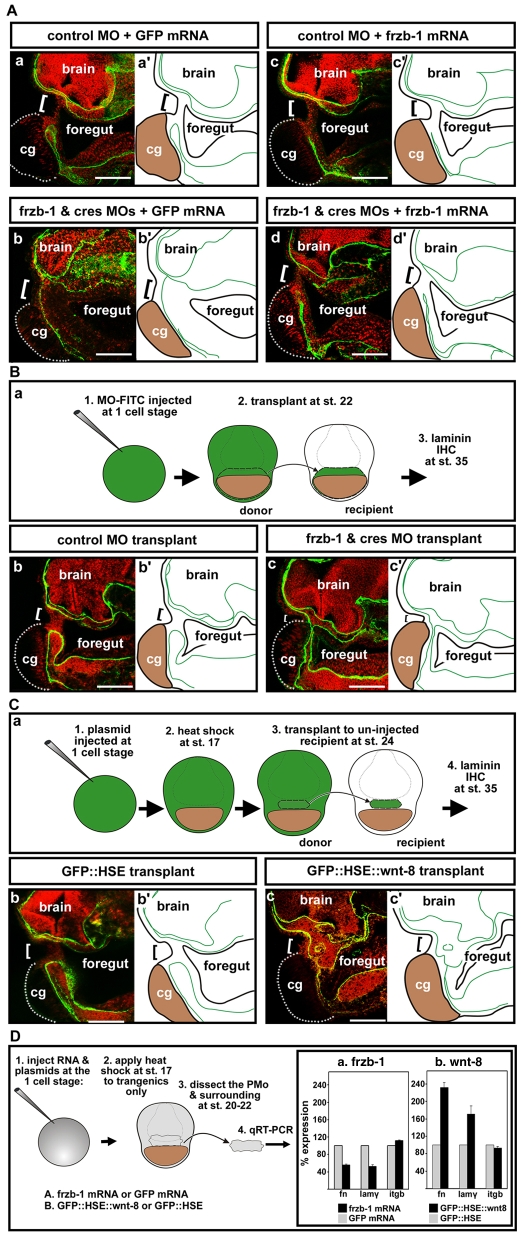
One of the earliest morphological changes during primary mouth formation is loss of the basement membrane (Dickinson and Sive, 2006) between ectoderm and endoderm when frzb-1 and crescent are expressed. We therefore hypothesized that Frzb-1/Crescent regulate basement membrane dissolution. Consistent with this, in frzb-1/crescent morphants, expression of Laminin, a basement membrane protein, persisted in the presumptive primary mouth, whereas control embryos lacked Laminin in this region (Fig. 7A, parts a-b′). This phenotype was specific, as co-injecting a small amount of frzb-1 mRNA restored the loss of Laminin staining to 70% of the morphants (Fig. 7A, parts d,d′). This level of frzb-1 mRNA did not alter primary mouth morphology (not shown), nor normal basement membrane loss (Fig. 7A, parts c,c′).
Fig. 7.
Laminin persists in the primary mouth region when Frzb-1/Crescent are depleted or when wnt-8 is overexpressed. Sagittal sections (anterior to the left) assayed at stage 35-37, in 2-3 independent experiments. Laminin is immunolabed green, nuclear propidium iodide, red; cement gland is outlined by a dotted gray line. Panels denoted by primes are tracings of Laminin immunolabeling (green). Bracket indicates the presumptive primary mouth; cg, cement gland. Scale bars: 170 μm. (A) Laminin persistence in frzb-1/crescent morphants is specific. (a) Control morpholino and GFP mRNA results in a normal absence of Laminin in the presumptive primary mouth (100%, n=10). (b) In frzb-1/crescent morphants (also injected with control GFP mRNA), Laminin persists in the primary mouth region (87%, n=10). Note that similar phenotypes were seen in morphants injected without GFP mRNA. (c) Control morpholino and frzb-1 mRNA (200 pg) results in a normal absence of Laminin in the presumptive primary mouth (n=10). (d) Co-injection of frzb-1/crescent morpholinos and frzb-1 mRNA restores the absence of Laminin in 70% of morphants (n=10). (B) Laminin persists in embryos locally depleted of frzb-1/crescent. (a) Schematic of the experimental design. (b) Recipients receiving tissue injected with control morpholino and GFP mRNA have a normal absence of Laminin (100%, n=7). (c) Eighty-nine percent of recipients receiving tissue from frzb-1/crescent morphants have persistent Laminin (n=9). (C) Temporal and spatial overexpression of Wnt-8 results in persistent Laminin. (a) Schematic of the experimental design. (b) Recipients receiving tissue injected with GFP::HSE have a normal absence of Laminin (91%, n=11). (c) Seventy-five percent of recipients receiving tissue from embryos injected with GFP::HSE::wnt-8 have persistent Laminin (n=12). (D) Wnt signaling regulates the expression of basement membrane components. Schematic depicts experimental design. Results are an average of two independent experiments. (a) Increased frzb-1 results in fibronectin mRNA that is 45% of the control level, laminin-γ1 mRNA that is 48% of the control level and β1-integrin that is 112% of the control level (n=20). (b) Temporally increased wnt-8 results in fibronectin mRNA that is 232% of the control level, laminin-γ1 mRNA that is 170% of the control level and β1-integrin mRNA that is 93% of the control levels (n=20).
In order to address the spatial requirement for frzb-1/crescent expression during basement membrane dissolution, we localized morpholinos by performing face transplants (Fig. 7B, part a; see also Materials and methods). Transplant of frzb-1/crescent morphant tissue to uninjected recipients led to a persistence of Laminin staining in the primary mouth region, whereas control transplants showed the normal absence of Laminin (Fig. 7B, parts b-c′). These data indicate that Frzb-1/Crescent expression is specifically required in the presumptive primary mouth for basement membrane dissolution.
We further investigated whether Wnt signaling can regulate basement membrane breakdown by performing ectoderm transplants (Fig. 7C, part a; see also Materials and methods), where a small piece of presumptive primary mouth ectoderm was transplanted from donor embryos transgenic for wnt-8::HSE::GFP to uninjected recipient embryos (Fig. 8C, part a). With donor tissue overexpressing Wnt-8, Laminin immunoreactivity persisted in the primary mouth region compared with controls (Fig. 7C, parts b-c′). Thus, dissolution of the basement membrane in the presumptive primary mouth region requires the inhibition of Wnt signaling.
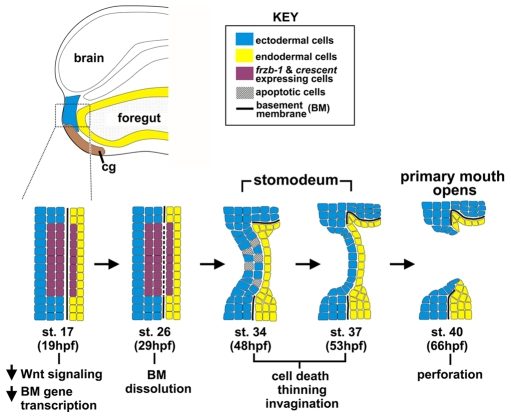
Fig. 8.
Model of frzb-1/crescent function in primary mouth formation. At neurula stages (stage 17-22), frzb-1 and crescent are expressed in a subset of cells that will form the primary mouth (purple). In these cells, frzb-1 and crescent inhibit Wnt signaling, which in turn prevents the continued synthesis of proteins Laminin and Fibronectin. Without such proteins, the basement membrane integrity is lost and by early tailbud stages (stage 24-26) it disappears. The dissolution of the basement membrane leads to subsequent stages of primary mouth development including cell death, invagination, thinning and finally perforation. frzb-1/crescent-expressing cells are colored purple; ectoderm, blue; endoderm, yellow. The basement membrane (BM) is indicated by a black line; cg, cement gland.
We next tested whether Wnt signaling modulates basement membrane proteins at the level of RNA expression (Fig. 7D). Specifically, we expressed frzb-1 mRNA or a wnt-8 construct under the control of the HSE promoter and isolated the presumptive primary mouth region at early tailbud (stage 20-22), prior to basement membrane dissolution. Increased frzb-1 mRNA resulted in fibronectin and laminin-γ1 mRNA levels that were approximately 50% of the control levels, with little effect on integrin-β1 mRNA levels (Fig. 7D, part a). Conversely, overexpression of Wnt-8 from mid-neurula (stage 17) to harvest, resulted in fibronectin mRNA levels that were 232% of the control level, and laminin mRNA levels that were 170% of the control level, while having little effect on integrin-β1 mRNA levels (Fig. 7D, part b). These results indicate that Frzb-1 can regulate basement membrane dissolution by downregulating laminin and fibronectin mRNA expression.
Are other steps of primary mouth morphogenesis impacted by the loss of Frzb1/Crescent function? We examined apoptosis in the frzb-1/crescent morphants at stage 34-35 and could not detect the burst of cell death normally observed (data not shown). However, we cannot rule out the possibility that cell death does occur, but at a different time in the morphants compared with the wild type. Consistent with the absence of cell death, histology revealed that the stomodeum does not thin in frzb-1/crescent morphants, as it does in normal embryos (see Fig. 7A, part b, 7B, part c). Together, these results suggest that Frzb-1/Crescent function, and possibly basement membrane dissolution, is necessary for subsequent steps in primary mouth development.
DISCUSSION
A local sFRP expression domain is required for primary mouth development
Formation of the primary mouth requires multiple signaling regions, including endoderm of the anterior foregut, lateral tissue including branchial arches, and anterior dorsal tissue that will form the telencephalon and placodes (Dickinson and Sive, 2006). The present study identifies a fourth domain, which expresses the sFRPs frzb-1 and, to a lesser extent, crescent. Removal of this domain ablates primary mouth formation, and antisense assays show that this is due to loss of Frzb-1 and Crescent expression, rather than because the domain contains the future primary mouth. In Xenopus, this domain expresses both frzb-1 and other secreted Wnt inhibitors, including dkk-1 and wnt inhibitory factor-1 (wif-1), suggesting that there is a requirement for low Wnt function in this region (Glinka et al., 1998; Hsieh et al., 1999) (see Fig. S1 in the supplementary material). Interestingly, when frzb-1/crescent expression was inhibited locally in the future primary mouth region, the entire face was smaller than controls, suggesting that the frzb-1/crescent domain may organize other aspects of the face.
Roles of early and late Wnt signaling modulation
Wnt inhibition is an important aspect of early anterior determination (Agathon et al., 2003; De Robertis, 2006; De Robertis et al., 2000; Kemp et al., 2005; Lewis et al., 2008; Niehrs, 1999). Our data indicate that local inhibition of Wnt signaling is also crucial to primary mouth formation at the extreme anterior of the embryo. Gene expression analyses indicate that a primary anteroposterior axis is in place by the end of gastrulation (Gamse and Sive, 2001; Zaraisky et al., 1995). Primary mouth specification begins many hours later, by late neurula/early tailbud, when a discrete region can be fate mapped as the future primary mouth, when region-specific gene expression is activated, and when the basement membrane between the ectoderm and endoderm breaks down locally (Dickinson and Sive, 2006). Our data demonstrate that inhibition of Wnt signaling is required for anterior organogenesis after the primary anteroposterior pattern is formed. It is not clear whether this represents a sustained requirement for inhibition of Wnt signaling to maintain `anteriorness', or a new period that involves more local inhibition of this signaling pathway.
Frzb-1/Crescent regulate β-catenin-mediated Wnt signaling
Several assays indicate that Frzb-1 and Crescent antagonize Wnt signaling in the primary mouth anlage. For example, overexpression of Dkk-1, a Wnt inhibitor, gives a similar phenotype to overexpression of Frzb-1/Crescent, and can substitute for these sFRPs in regulating primary mouth formation. Our data suggest that these sFRPs target β-catenin-mediated Wnt signaling. Thus, overexpression of Wnt-8, a known Wnt/β-catenin ligand, mimics the phenotype seen after loss of Frzb-1/Crescent, whereas Frzb-1 overexpression decreases β-catenin levels in the presumptive primary mouth (see Fig. S5 in the supplementary material). Further indication that Frzb-1/Crescent targets Wnt/β-catenin signaling in the primary mouth region comes from the examination of putative promoters for the basement membrane genes laminin and fibronectin, whose expression is inhibited by Frzb-1/Crescent. In the X. tropicalis genome, one TCF/LEF/β-catenin-binding site lies within 5 kb upstream of each of the fibronectin and the laminin start sites (data obtained using Transfac software; not shown). These data suggest that Wnt signaling regulates the transcription of basement membrane genes, via the modulation of β-catenin.
Frzb-1/Crescent do not appear to target either the Wnt/PCP pathway, or the BMP pathway, as neither BMP nor JNK inhibition could mimic the Frzb-1 overexpression phenotype. However, the inhibition of BMP and JNK results in a very small or absent primary mouth, suggesting that both pathways regulate other aspects of primary mouth development.
Basement membrane dynamics and Wnt signaling
Basement membrane dissolution is important in many developmental contexts and may be necessary for invagination, cell death and intercalation (Davidson et al., 2004; Ingber, 2006; Svoboda and O'Shea, 1987). For example, during cavitation of embryoid bodies, loss of contact with the basement membrane initiates cell death (Murray and Edgar, 2000). Basement membrane breakdown is required for changes in cell polarity and movement during chick gastrulation (Nakaya et al., 2008). Basement membrane dissolution may promote subsequent steps in primary mouth formation; however, this possibility has not yet been addressed.
This study provides the first connection between Wnt signaling and basement membrane modulation during primary mouth development in any species. This connection has not been made extensively in any embryonic context. In organ culture of mouse lung, addition of Dkk-1 protein resulted in depressed levels of Fibronectin protein and aberrant lung branching (De Langhe et al., 2005). In addition to in Xenopus, sFRPs and other Wnt inhibitors are expressed in anterior domains at a time that could influence primary mouth development in zebrafish, chick and mouse (Chapman et al., 2004; Duprez et al., 1999; Hoang et al., 1998; Houart et al., 2002; Tylzanowski et al., 2004).
In cancer, basement membrane breakdown is pivotal to metastasis (Spaderna et al., 2006), and connections have been made between Wnt signaling and the expression of basement membrane components. For example, β-catenin controls the expression of laminin during tumor progression (Hlubek et al., 2001), and increased frzb-1 (sFRP3) expression correlates with decreased Fibronectin protein (Guo et al., 2008).
It is not clear whether inhibition of Wnt signaling is the sole mediator of basement membrane dissolution in the primary mouth region. Other mechanisms downstream of Frzb1/Crescent, or in an independent pathway, may contribute. These could include proteolytic degradation of extracellular matrix components by metalloproteases (Page-McCaw et al., 2007), and we are presently investigating this possibility.
Model: local repression of Wnt signaling leads to basement membrane dissolution in the primary mouth anlage
Previous data have shown that loss of the basement membrane in the primary mouth region is a very early step in the formation of this organ (Dickinson and Sive, 2006). Our data identify a molecular mechanism that locally regulates basement membrane dissolution (Fig. 8). Specifically, the Wnt antagonists Frzb-1 and Crescent act redundantly to inhibit β-catenin activity in cells adjacent to the basement membrane in the primary mouth anlage. We suggest that the transcription of pivotal basement membrane genes, including laminin and fibronectin, is dependent on β-catenin, and therefore decreases after Wnt inhibition. After synthesis of basement membrane proteins ceases, the basement membrane breaks down. Without Wnt inhibition, the basement membrane is maintained. Loss of the basement membrane may be required for subsequent steps in primary mouth development, including invagination, cell death, intercalation and perforation. However, these putative connections have not been explored. In addition to the local inhibition of Wnt signaling, other mechanisms may regulate the fine spatial control of basement membrane dissolution. We suggest that the modulation of Wnt signaling is a widespread regulator of basement membrane remodeling during development.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/7/1071/DC1
Supplementary Material
We thank members of the Sive lab, especially J. Gutzman, E. Graeden, A. Shkumatava and S. J. Hong for helpful comments and discussion, and V. Apekin for frog care and husbandry. For providing plasmids, we thank Thomas Czerny (pSGH2), Thomas Hollemann (pitx-3), Sally Moody (six-1) and Peter Good (nrp-1). Thanks to the Whitehead Center for Microarray Technology, especially Jen Love. Imaging was conducted using the W. M. Keck Foundation Biological Imaging Facility at the Whitehead Institute. Supported by NIH grant R21 DE017758-01 and, in the initial stages, by NSF grant IBN-0425030 to H.L.S., and a National Sciences and Engineering Research Council of Canada (NSERC) fellowship to A.J.G.D. Deposited in PMC for release after 12 months.
References
- Agathon, A., Thisse, C. and Thisse, B. (2003). The molecular nature of the zebrafish tail organizer. Nature 424, 448-452. [DOI] [PubMed] [Google Scholar]
- Bajoghli, B., Aghaallaei, N., Heimbucher, T. and Czerny, T. (2004). An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev. Biol. 271, 416-430. [DOI] [PubMed] [Google Scholar]
- Bovolenta, P., Esteve, P., Ruiz, J. M., Cisneros, E. and Lopez-Rios, J. (2008). Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121, 737-746. [DOI] [PubMed] [Google Scholar]
- Bradley, L., Sun, B., Collins-Racie, L., LaVallie, E., McCoy, J. and Sive, H. (2000). Different activities of the frizzled-related proteins frzb2 and sizzled2 during Xenopus anteroposterior patterning. Dev. Biol. 227, 118-132. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine, C., Acuna, G., Ellwanger, K., Niehrs, C. and Mayor, R. (2007). Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev. Biol. 309, 208-221. [DOI] [PubMed] [Google Scholar]
- Cha, S. W., Tadjuidje, E., Tao, Q., Wylie, C. and Heasman, J. (2008). Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135, 3719-3729. [DOI] [PubMed] [Google Scholar]
- Chapman, S. C., Brown, R., Lees, L., Schoenwolf, G. C. and Lumsden, A. (2004). Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev. Dyn. 229, 668-676. [DOI] [PubMed] [Google Scholar]
- Christian, J. L., McMahon, J. A., McMahon, A. P. and Moon, R. T. (1991). Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 111, 1045-1055. [DOI] [PubMed] [Google Scholar]
- Clevers, H. and van de Wetering, M. (1997). TCF/LEF factor earn their wings. Trends Genet. 13, 485-489. [DOI] [PubMed] [Google Scholar]
- Darken, R. S. and Wilson, P. A. (2001). Axis induction by wnt signaling: target promoter responsiveness regulates competence. Dev. Biol. 234, 42-54. [DOI] [PubMed] [Google Scholar]
- Davidson, L. A., Keller, R. and DeSimone, D. W. (2004). Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev. Dyn. 231, 888-895. [DOI] [PubMed] [Google Scholar]
- De Langhe, S. P., Sala, F. G., Del Moral, P. M., Fairbanks, T. J., Yamada, K. M., Warburton, D., Burns, R. C. and Bellusci, S. (2005). Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev. Biol. 277, 316-331. [DOI] [PubMed] [Google Scholar]
- De Robertis, E. M. (2006). Spemann's organizer and self-regulation in amphibian embryos. Nat. Rev. Mol. Cell Biol. 7, 296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis, E. M., Larrain, J., Oelgeschlager, M. and Wessely, O. (2000). The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 1, 171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, A. J. and Sive, H. (2006). Development of the primary mouth in Xenopus laevis. Dev. Biol. 295, 700-713. [DOI] [PubMed] [Google Scholar]
- Dickinson, A. and Sive, H. (2007). Positioning the extreme anterior in Xenopus: cement gland, primary mouth and anterior pituitary. Semin. Cell Dev. Biol. 18, 525-533. [DOI] [PubMed] [Google Scholar]
- Duprez, D., Leyns, L., Bonnin, M. A., Lapointe, F., Etchevers, H., De Robertis, E. M. and Le Douarin, N. (1999). Expression of Frzb-1 during chick development. Mech. Dev. 89, 179-183. [DOI] [PubMed] [Google Scholar]
- Gamse, J. T. and Sive, H. (2001). Early anteroposterior division of the presumptive neurectoderm in Xenopus. Mech. Dev. 104, 21-36. [DOI] [PubMed] [Google Scholar]
- Glinka, A., Wu, W., Delius, H., Monaghan, A. P., Blumenstock, C. and Niehrs, C. (1998). Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357-362. [DOI] [PubMed] [Google Scholar]
- Guo, Y., Xie, J., Rubin, E., Tang, Y. X., Lin, F., Zi, X. and Hoang, B. H. (2008). Frzb, a secreted Wnt antagonist, decreases growth and invasiveness of fibrosarcoma cells associated with inhibition of Met signaling. Cancer Res. 68, 3350-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Z., Boyle, D. L., Chang, L., Bennett, B., Karin, M., Yang, L., Manning, A. M. and Firestein, G. S. (2001). c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Invest. 108, 73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlubek, F., Jung, A., Kotzor, N., Kirchner, T. and Brabletz, T. (2001). Expression of the invasion factor laminin gamma2 in colorectal carcinomas is regulated by beta-catenin. Cancer Res. 61, 8089-8093. [PubMed] [Google Scholar]
- Hoang, B. H., Thomas, J. T., Abdul-Karim, F. W., Correia, K. M., Conlon, R. A., Luyten, F. P. and Ballock, R. T. (1998). Expression pattern of two Frizzled-related genes, Frzb-1 and Sfrp-1, during mouse embryogenesis suggests a role for modulating action of Wnt family members. Dev. Dyn. 212, 364-372. [DOI] [PubMed] [Google Scholar]
- Houart, C., Caneparo, L., Heisenberg, C., Barth, K., Take-Uchi, M. and Wilson, S. (2002). Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron 35, 255-265. [DOI] [PubMed] [Google Scholar]
- Hsieh, J. C., Kodjabachian, L., Rebbert, M. L., Rattner, A., Smallwood, P. M., Samos, C. H., Nusse, R., Dawid, I. B. and Nathans, J. (1999). A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398, 431-436. [DOI] [PubMed] [Google Scholar]
- Ingber, D. E. (2006). Mechanical control of tissue morphogenesis during embryological development. Int. J. Dev. Biol. 50, 255-266. [DOI] [PubMed] [Google Scholar]
- Jones, S. E. and Jomary, C. (2002). Secreted Frizzled-related proteins: searching for relationships and patterns. BioEssays 24, 811-820. [DOI] [PubMed] [Google Scholar]
- Kawano, Y. and Kypta, R. (2003). Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116, 2627-2634. [DOI] [PubMed] [Google Scholar]
- Kemp, C., Willems, E., Abdo, S., Lambiv, L. and Leyns, L. (2005). Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev. Dyn. 233, 1064-1075. [DOI] [PubMed] [Google Scholar]
- Lee, H. X., Ambrosio, A. L., Reversade, B. and De Robertis, E. M. (2006). Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell 124, 147-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. L., Khoo, P. L., De Young, R. A., Steiner, K., Wilcock, C., Mukhopadhyay, M., Westphal, H., Jamieson, R. V., Robb, L. and Tam, P. P. (2008). Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development 135, 1791-1801. [DOI] [PubMed] [Google Scholar]
- Leyns, L., Bouwmeester, T., Kim, S. H., Piccolo, S. and De Robertis, E. M. (1997). Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88, 747-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, P. and Edgar, D. (2000). Regulation of programmed cell death by basement membranes in embryonic development. J. Cell Biol. 150, 1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya, Y., Sukowati, E. W., Wu, Y. and Sheng, G. (2008). RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat. Cell Biol. 10, 765-775. [DOI] [PubMed] [Google Scholar]
- Niehrs, C. (1999). Head in the WNT: the molecular nature of Spemann's head organizer. Trends Genet. 15, 314-319. [DOI] [PubMed] [Google Scholar]
- Niehrs, C., Kazanskaya, O., Wu, W. and Glinka, A. (2001). Dickkopf1 and the Spemann-Mangold head organizer. Int. J. Dev. Biol. 45, 237-240. [PubMed] [Google Scholar]
- Nieuwkoop, P. D. and Faber, J. (1994). Normal Table of Xenopus laevis (Daudin). New York: Garland Publishing.
- Page-McCaw, A., Ewald, A. J. and Werb, Z. (2007). Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, F. C., Chen, Y., Loeber, J., Henningfeld, K. and Pieler, T. (2006). I-SceI meganuclease-mediated transgenesis in Xenopus. Dev. Dyn. 235, 247-252. [DOI] [PubMed] [Google Scholar]
- Pera, E. M. and De Robertis, E. M. (2000). A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb-1. Mech. Dev. 96, 183-195. [DOI] [PubMed] [Google Scholar]
- Qian, D., Jones, C., Rzadzinska, A., Mark, S., Zhang, X., Steel, K. P., Dai, X. and Chen, P. (2007). Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306, 121-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, K., Good, P. J. and Dawid, I. B. (1990). A developmentally regulated, nervous system-specific gene in Xenopus encodes a putative RNA-binding protein. New Biol. 2, 556-565. [PubMed] [Google Scholar]
- Sasai, Y., Lu, B., Steinbeisser, H., Geissert, D., Gont, L. K. and De Robertis, E. M. (1994). Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79, 779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, V. A. and Mercola, M. (2001). Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 15, 304-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov, M. V., Tamai, K., Brott, B. K., Kuhl, M., Sokol, S. and He, X. (2001). Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11, 951-961. [DOI] [PubMed] [Google Scholar]
- Semenov, M. V., Zhang, X. and He, X. (2008). DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J. Biol. Chem. 283, 21427-21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, M., Itoh, M., Hikasa, H., Taira, S. and Taira, M. (2005). Role of crescent in convergent extension movements by modulating Wnt signaling in early Xenopus embryogenesis. Mech. Dev. 122, 1322-1339. [DOI] [PubMed] [Google Scholar]
- Sive, H. L., Hattori, K. and Weintraub, H. (1989). Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell 58, 171-180. [DOI] [PubMed] [Google Scholar]
- Sive, H. L., Grainger, R. M. and Harland, R. M. (2000). Early Development of Xenopus Laevis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Spaderna, S., Schmalhofer, O., Hlubek, F., Berx, G., Eger, A., Merkel, S., Jung, A., Kirchner, T. and Brabletz, T. (2006). A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 131, 830-840. [DOI] [PubMed] [Google Scholar]
- Svoboda, K. K. and O'Shea, K. S. (1987). An analysis of cell shape and the neuroepithelial basal lamina during optic vesicle formation in the mouse embryo. Development 100, 185-200. [DOI] [PubMed] [Google Scholar]
- Tylzanowski, P., Bossuyt, W., Thomas, J. T. and Luyten, F. P. (2004). Characterization of Frzb-Cre transgenic mouse. Genesis 40, 200-204. [DOI] [PubMed] [Google Scholar]
- van Amerongen, R., Mikels, A. and Nusse, R. (2008). Alternative wnt signaling is initiated by distinct receptors. Sci. Signal. 1, re9. [DOI] [PubMed] [Google Scholar]
- Wang, S., Krinks, M., Lin, K., Luyten, F. P. and Moos, M., Jr (1997a). Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88, 757-766. [DOI] [PubMed] [Google Scholar]
- Wang, S., Krinks, M. and Moos, M., Jr (1997b). Frzb-1, an antagonist of Wnt-1 and Wnt-8, does not block signaling by Wnts -3A, -5A, or -11. Biochem. Biophys. Res. Commun. 236, 502-504. [DOI] [PubMed] [Google Scholar]
- Wiellette, E. L. and Sive, H. (2003). vhnf1 and Fgf signals synergize to specify rhombomere identity in the zebrafish hindbrain. Development 130, 3821-3829. [DOI] [PubMed] [Google Scholar]
- Yamamoto, H., Sakane, H., Yamamoto, H., Michiue, T. and Kikuchi, A. (2008). Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev. Cell 15, 37-48. [DOI] [PubMed] [Google Scholar]
- Zaraisky, A. G., Ecochard, V., Kazanskaya, O. V., Lukyanov, S. A., Fesenko, I. V. and Duprat, A. M. (1995). The homeobox-containing gene XANF-1 may control development of the Spemann organizer. Development 121, 3839-3847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.