Abstract
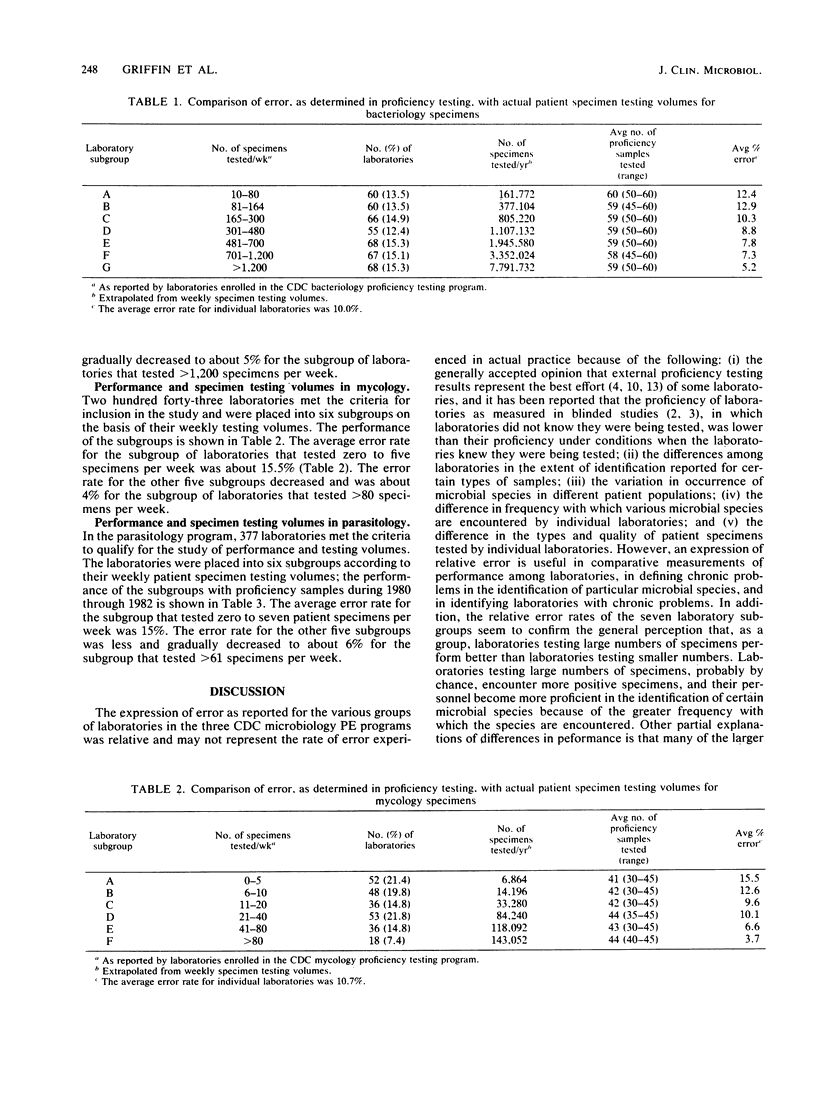
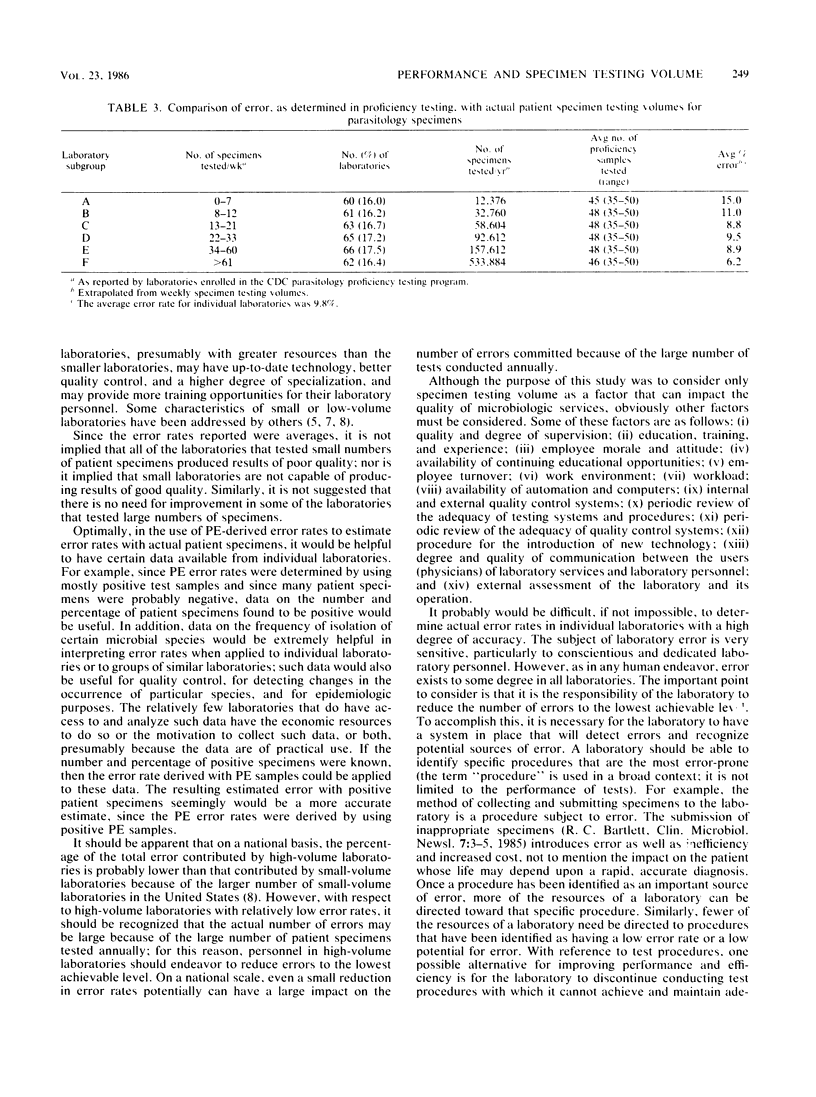
The performance of laboratories enrolled in three of the Centers for Disease Control microbiology performance evaluation programs was studied in relation to the number of actual patient specimens tested by the laboratories. Laboratories were grouped according to the number of specimens tested weekly to compare performance among the groups. Results of the study showed that laboratories, as a group, that tested large numbers of specimens performed better than laboratories that tested small numbers. The infrequency of performing certain tests or identifying certain microbial species can be an important factor in laboratory error. One strategy for laboratories that cannot resolve internal problems associated with low testing volumes would be to limit their testing to procedures that are done well.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black W. A., Dorse S. E. A regional quality control program in microbiology. II. Advantages of simulated clinical specimens. Am J Clin Pathol. 1976 Aug;66(2):407–415. doi: 10.1093/ajcp/66.2.407. [DOI] [PubMed] [Google Scholar]
- Boone D. J., Hansen H. J., Hearn T. L., Lewis D. S., Dudley D. Laboratory evaluation and assistance efforts: mailed, on-site and blind proficiency testing surveys conducted by the Centers for Disease Control. Am J Public Health. 1982 Dec;72(12):1364–1368. doi: 10.2105/ajph.72.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C. W., 3rd, Mehaffey M. A., Cook E. C. Five years of experience with a national external quality control program for the culture and identification of Neisseria gonorrhoeae. J Clin Microbiol. 1983 Nov;18(5):1150–1159. doi: 10.1128/jcm.18.5.1150-1159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft H. S., Bunker J. P., Enthoven A. C. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979 Dec 20;301(25):1364–1369. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- Martin R. J. Quality assurance and clinical microbiology. Med Lab Sci. 1983 Jul;40(3):269–274. [PubMed] [Google Scholar]
- Mehaffey M. A., Cook E. C., Griffin C. W., 3rd Preparation and stability of freeze-dried Neisseria gonorrhoeae cultures used for external quality control. J Clin Microbiol. 1984 Dec;20(6):1126–1129. doi: 10.1128/jcm.20.6.1126-1129.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer M., Widelock D., Blatt S., Wilson E. The clinical laboratory improvement program in New York City. I. Methods of evaluation and results of performance tests. Health Lab Sci. 1967 Apr;4(2):72–89. [PubMed] [Google Scholar]
- Whitby J. L., Black W. A., Richardson H., Wood D. E. System for laboratory proficiency testing in bacteriology: organisation and impact on microbiology laboratories in health care facilities funded by the Ontario Government. J Clin Pathol. 1982 Jan;35(1):94–100. doi: 10.1136/jcp.35.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow K. J., Campbell D. J. Assessment of technologist workload as a factor in quality of laboratory performance. Am J Clin Pathol. 1983 May;79(5):609–610. doi: 10.1093/ajcp/79.5.609. [DOI] [PubMed] [Google Scholar]


