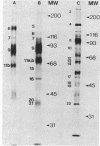
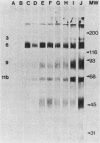
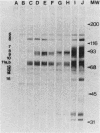
Abstract
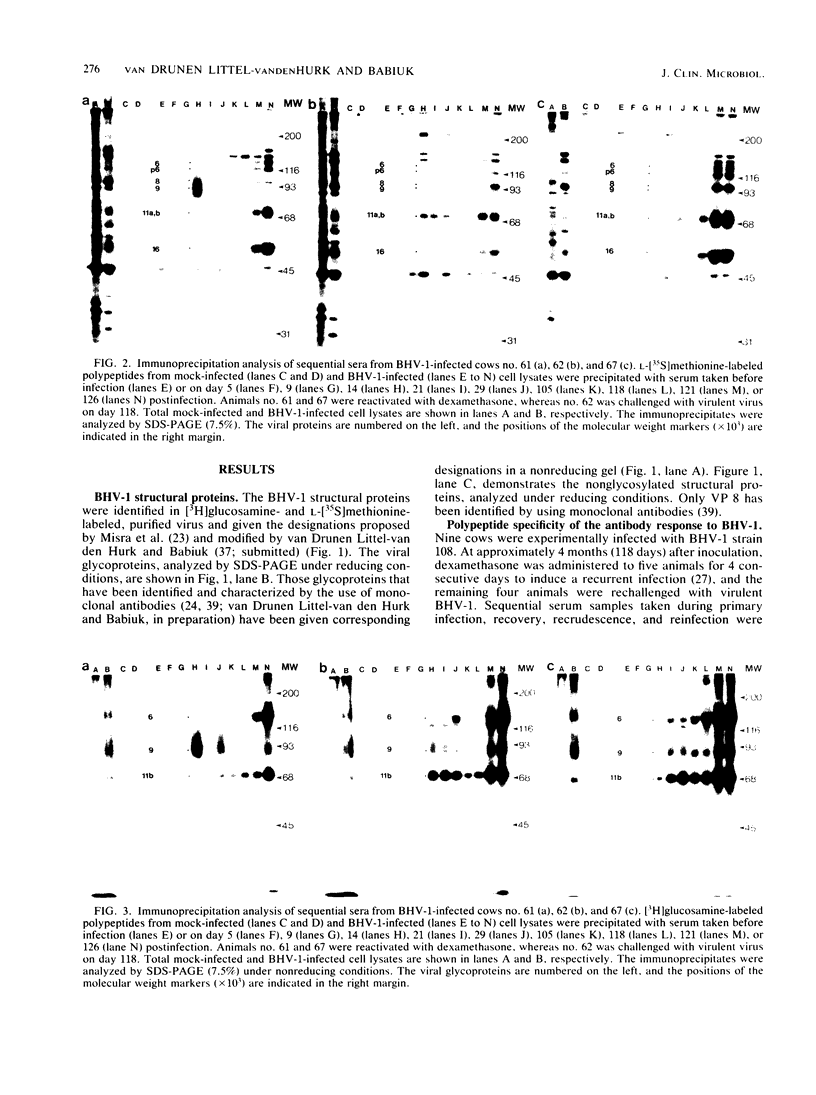
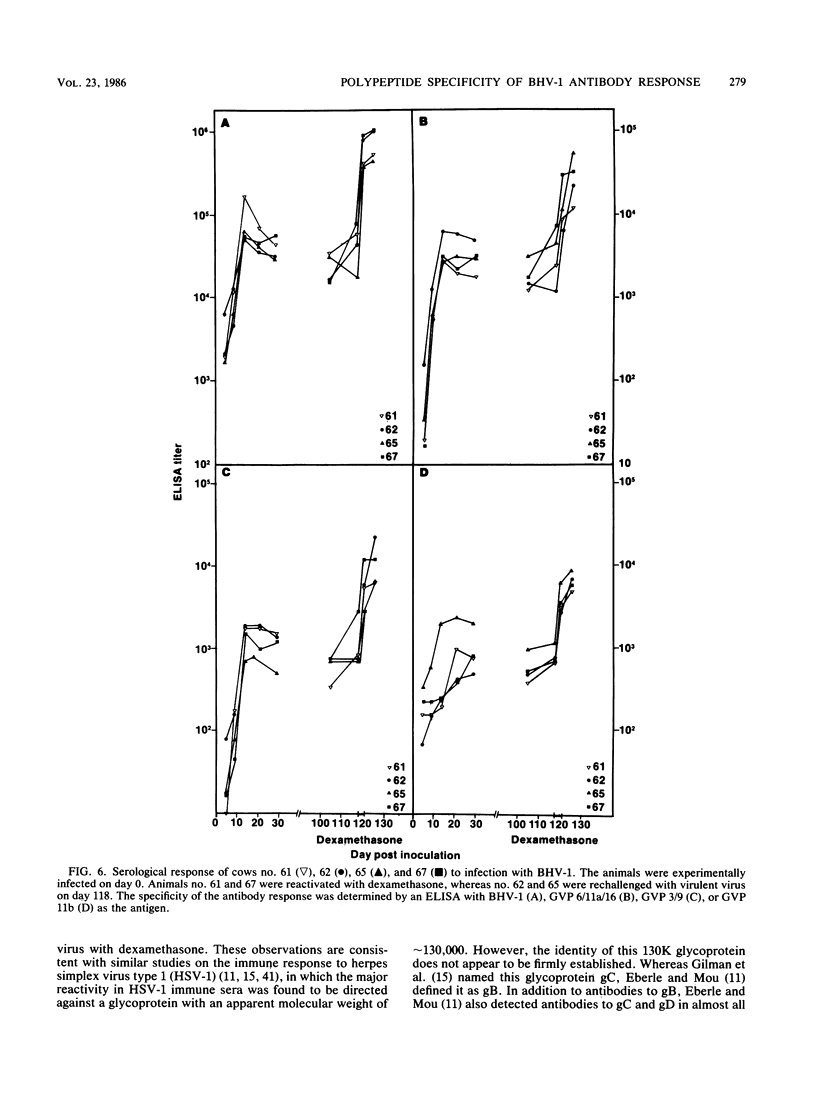
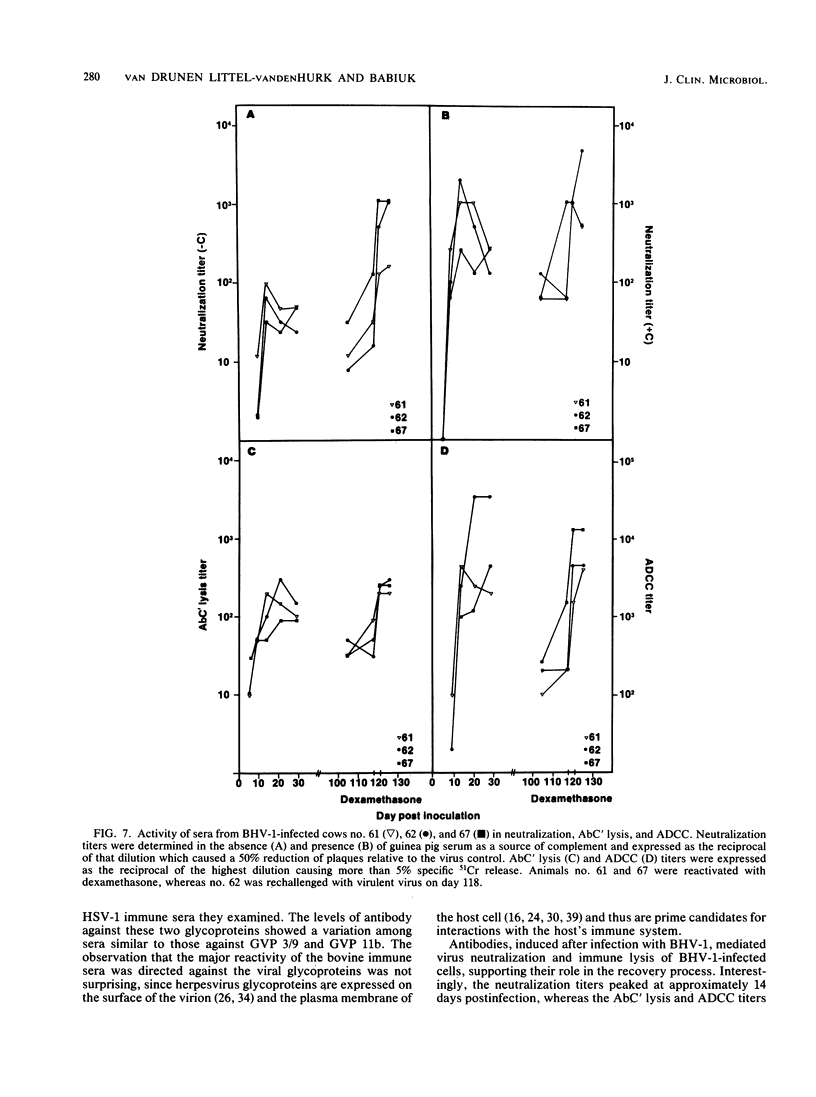
The polypeptide specificities and defense mechanisms of the humoral immune response to bovine herpesvirus 1 were analyzed. Sequential serum samples taken from cows which were experimentally infected with bovine herpesvirus 1 were tested for their reactivity with individual bovine herpesvirus 1 polypeptides by immunoprecipitation, immunoblotting, and enzyme-linked immunosorbent assay. All bovine immune sera reacted with each of the three major bovine herpesvirus 1 glycoproteins, GVP 6/11a/16, GVP 3/9, and GVP 11b, during primary as well as recurrent infection. Among these glycoproteins, GVP 6/11a/16 induced the earliest and most consistent immune response. The levels of antibody to GVP 3/9 and GVP 11b varied among the animals, and they were slightly lower than the level of antibody to GVP 6/11a/16. Antibodies to several nonglycosylated polypeptides and two additional glycoproteins were also detected with the immunoblot assay. However, these antibodies were usually apparent only during recurrent infection, whereas they were undetectable or low during primary infection. The antibodies in the sera from all animals mediated virus neutralization and destruction of virus-infected cells by two immune mechanisms, e.g., antibody- and complement-mediated lysis and antibody-dependent cell-mediated cytotoxicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Rawls W. E., Bacchetti S. Glycoproteins of herpes simplex virus type 2 as defined by monoclonal antibodies. J Virol. 1982 Oct;44(1):344–355. doi: 10.1128/jvi.44.1.344-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Babiuk L. A. Viral-bacterial pneumonia in calves: effect of bovine herpesvirus-1 on immunologic functions. J Infect Dis. 1985 May;151(5):937–947. doi: 10.1093/infdis/151.5.937. [DOI] [PubMed] [Google Scholar]
- Bishop G. A., Marlin S. D., Schwartz S. A., Glorioso J. C. Human natural killer cell recognition of herpes simplex virus type 1 glycoproteins: specificity analysis with the use of monoclonal antibodies and antigenic variants. J Immunol. 1984 Oct;133(4):2206–2214. [PubMed] [Google Scholar]
- Bolton D. C., Zee Y. C., Ardans A. A. Identification of envelope and nucleocapsid proteins of infectious bovine rhinotracheitis virus by SDS-polyacrylamide gel electrophoresis. Vet Microbiol. 1983 Feb;8(1):57–68. doi: 10.1016/0378-1135(83)90019-6. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Davies D. H., Carmichael L. E. Role of cell-mediated immunity in the recovery of cattle from primary and recurrent infections with infectious bovine rhinotracheitis virus. Infect Immun. 1973 Oct;8(4):510–518. doi: 10.1128/iai.8.4.510-518.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Mou S. W. Relative titers of antibodies to individual polypeptide antigens of herpes simplex virus type 1 in human sera. J Infect Dis. 1983 Sep;148(3):436–444. doi: 10.1093/infdis/148.3.436. [DOI] [PubMed] [Google Scholar]
- Eberle R., Russell R. G., Rouse B. T. Cell-mediated immunity to herpes simplex virus: recognition of type-specific and type-common surface antigens by cytotoxic T cell populations. Infect Immun. 1981 Dec;34(3):795–803. doi: 10.1128/iai.34.3.795-803.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J. D., Marron A. E., Kucera C. J. Local and systemic cellular and antibody immune responses of cattle to infectious bovine rhinotracheitis virus vaccines administered intranassally or intramuscularly. Am J Vet Res. 1978 May;39(5):753–760. [PubMed] [Google Scholar]
- Gilman S. C., Docherty J. J., Rawls W. E. Antibody responses in humans to individual proteins of herpes simplex viruses. Infect Immun. 1981 Dec;34(3):880–887. doi: 10.1128/iai.34.3.880-887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Smith J. W. Immune interactions with cells infected with herpes simplex virus: antibodies to radioiodinated surface antigens. J Immunol. 1977 Jan;118(1):114–121. [PubMed] [Google Scholar]
- Grewal A. S., Rouse B. T., Babiuk L. A. Mechanisms of resistant of herpesviruses: comparison of the effectiveness of different cell types in mediating antibody-dependent cell-mediated cytotoxicity. Infect Immun. 1977 Mar;15(3):698–703. doi: 10.1128/iai.15.3.698-703.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J. A., Baker J. A. Bovine herpesvirus IBR-IPV. The antibody virus neutralization reaction. Cornell Vet. 1971 Apr;61(2):320–335. [PubMed] [Google Scholar]
- Jericho K. W., Babiuk L. A. The effect of dose, route and virulence of bovine herpesvirus 1 vaccine on experimental respiratory disease in cattle. Can J Comp Med. 1983 Apr;47(2):133–139. [PMC free article] [PubMed] [Google Scholar]
- Kahrs R. F. Infectious bovine rhinotracheitis: a review and update. J Am Vet Med Assoc. 1977 Nov 15;171(10):1055–1064. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long D., Madara T. J., Ponce de Leon M., Cohen G. H., Montgomery P. C., Eisenberg R. J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984 Feb;43(2):761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V., Gilchrist J. E., Weinmaster G., Qualtiere L., Van den Hurk S., Babiuk L. A. Herpesvirus-induced "early" glycoprotein: characterization and possible role in immune cytolysis. J Virol. 1982 Sep;43(3):1046–1054. doi: 10.1128/jvi.43.3.1046-1054.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshevsky U., Becker Y. Surface glycopeptides in the envelope of herpes simplex virions. Virology. 1972 Oct;50(1):277–279. doi: 10.1016/0042-6822(72)90371-6. [DOI] [PubMed] [Google Scholar]
- Pastoret P. P., Babiuk L. A., Misra V., Griebel P. Reactivation of temperature-sensitive and non-temperature-sensitive infectious bovine rhinotracheitis vaccine virus with dexamethasone. Infect Immun. 1980 Aug;29(2):483–488. doi: 10.1128/iai.29.2.483-488.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Buchan A., Sim C., Watson D. H. Type-specific protein in herpes simplex virus envelope reacts with neutralising antibody. Nature. 1974 May 24;249(455):360–361. doi: 10.1038/249360a0. [DOI] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Herpesvirus antigens on cell membranes detected by centrifugation of membrane-antibody complexes. Science. 1971 Jan 22;171(3968):298–300. doi: 10.1126/science.171.3968.298. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Mechanisms of recovery from Herpesvirus infections -a review. Can J Comp Med. 1978 Oct;42(4):414–427. [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. Antibody-dependent cell-mediated cytotoxicity in cows: comparison of effector cell activity against heterologous erthrocyte and herpesvirus-infected bovine target cells. Infect Immun. 1976 May;13(5):1433–1441. doi: 10.1128/iai.13.5.1433-1441.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. The role of antibody dependent cytotoxicity in recovery from herpesvirus infections. Cell Immunol. 1976 Mar 1;22(1):182–186. doi: 10.1016/0008-8749(76)90019-8. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffy B. E., Davies D. H. Reactivation of a bovine herpesvirus after corticosteroid treatment. Proc Soc Exp Biol Med. 1972 Jul;140(3):974–976. doi: 10.3181/00379727-140-36592. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Glycoproteins specified by herpes simplex virus type 1: their synthesis, processing and antigenic relatedness to HSV -2 glycoproteins. IARC Sci Publ. 1975;(11 Pt 1):49–61. [PubMed] [Google Scholar]
- Zweerink H. J., Stanton L. W. Immune response to herpes simplex virus infections: virus-specific antibodies in sera from patients with recurrent facial infections. Infect Immun. 1981 Feb;31(2):624–630. doi: 10.1128/iai.31.2.624-630.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Antigenic and immunogenic characteristics of bovine herpesvirus type-1 glycoproteins GVP 3/9 and GVP 6/11a/16, purified by immunoadsorbent chromatography. Virology. 1985 Jul 15;144(1):204–215. doi: 10.1016/0042-6822(85)90318-6. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Effect of tunicamycin and monensin on biosynthesis, transport, and maturation of bovine herpesvirus type-1 glycoproteins. Virology. 1985 May;143(1):104–118. doi: 10.1016/0042-6822(85)90100-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Gilchrist J. E., Misra V., Babiuk L. A. Interactions of monoclonal antibodies and bovine herpesvirus type 1 (BHV-1) glycoproteins: characterization of their biochemical and immunological properties. Virology. 1984 Jun;135(2):466–479. doi: 10.1016/0042-6822(84)90201-0. [DOI] [PubMed] [Google Scholar]