Abstract
The purinergic receptor, P2X7, has recently emerged as an important component of the innate immune response against microbial infections. Ligation of P2X7 by ATP can stimulate inflammasome activation and secretion of proinflammatory cytokines, but it can also lead directly to killing of intracellular pathogens in infected macrophages and epithelial cells. Thus, while some intracellular pathogens evade host defense responses by modulating with membrane trafficking or cell signaling in the infected cells, the host cells have also developed mechanisms for inhibiting infection. This review will focus on the effects of P2X7 on control of infection by intracellular pathogens, microbial virulence factors that interfere with P2X7 activity, and recent evidence linking polymorphisms in human P2X7 with susceptibility to infection.
Keywords: Apoptosis, PLD, Ecto-ATPases, Purinergic receptors, ATP, SNPs
Introduction
Host organisms and their cells have evolved a large array of mechanisms for controlling infection. Simultaneously, many pathogens have also attempted to circumvent the defense mechanisms. Thus, pathogens can subvert macrophage antimicrobial function, manipulating intracellular signaling pathways [1–3], interfering with membrane trafficking or the cell cycle [4], and modifying host metabolism [5].
The ultimate effect of microbial invasion depends on tissues infected and the microbial strategies of survival, with different strategies of pathogen adaptation being associated with varying degrees of damage of host tissues [6, 7]. Conversely, the host has also evolved defense mechanisms for resisting infection. In this review we will discuss how pathogens and host immune systems have co-evolved to thwart each other’s attacks, focusing on the effects of purinergic signaling on infection and the immune response. We will thus consider effects of P2X7 and the pathogen on fusion between host cell phagosomes and lysosomes, production of reactive oxygen species (ROS) by the host, and modulation of host cell apoptosis. Finally, we will discuss microbial enzymes that deplete the P2X7 ligand and polymorphisms in human P2X7 that influence the ability to control infection.
Interference with membrane trafficking
Lysosomes are dynamic organelles that receive and degrade macromolecules from the secretory, endocytic, autophagic, and phagocytic membrane-trafficking pathways. Many pathogens that hijack the endocytic pathways to enter cells have evolved mechanisms to avoid being degraded by lysosomes [8]. Bacteria such as Salmonella and Mycobacterium arrest the maturation of the phagosome at specific stages of the phagolysosomal route [9, 10]. Intracellular survival of Chlamydia depends in part on the ability of the microorganism to inhibit phagolysosomal fusion and subsequently survive and proliferate within a membrane-bound compartment called the inclusion [11]. Toxoplasma parasites infect virtually all mammalian cells, including macrophages, by active invasion [12]. The vacuole that surrounds Toxoplasma lacks the membrane proteins that normally deliver the endosomes to the cell fusion machinery. Thus, the parasitophorous vacuole remains at a neutral pH, allowing the parasite to survive [13]. Similarly, intracellular survival of Burkholderia cenocepacia in macrophages is associated with the pathogen’s ability to delay maturation of vacuoles that harbor these bacteria [14].
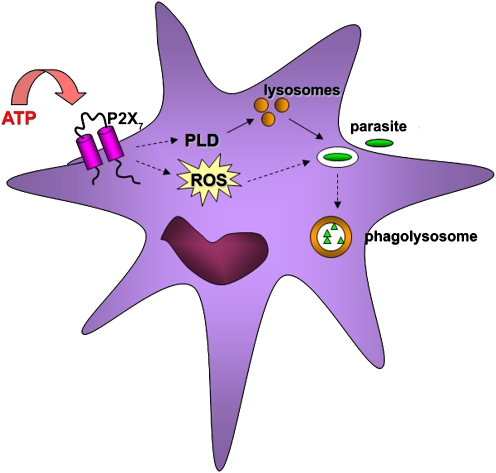
But the host immune system has also evolved to counteract the evasion strategies of these pathogens; and binding of extracellular nucleotides to purinergic receptors, especially the P2X7 receptor, can block development of pathogens that survive in an intracellular vacuole. Thus, treatment of infected macrophages with ATP kills Mycobacterium tuberculosis or Mycobacterium bovis, through a process requiring phospholipase D (PLD) activation, fusion between lysosomes and mycobacterial vacuoles, and acidification of the vacuoles [15, 16]. Likewise, P2X7-dependent PLD activation and fusion between vacuoles and lysosomes is involved in inhibition of Chlamydia trachomatis growth in macrophages [17]. These results have been extended by recent studies, which show that P2X7 activation also inhibits chlamydial infection in a cervical epithelial cell line and in vaginally infected mice [18]. Activation of PLD may be a general mechanism of elimination of parasites that normally reside within intracellular vacuoles that avoid fusion with lysosomes [19] (Fig. 1). Consistent with this view, we have observed that extracellular ATP decreases the parasite load in Toxoplasma gondii-infected macrophages and that this effect is mediated by P2X7-mediated acidification of the parasitophorous vacuole (manuscript in preparation).
Fig. 1.
Pathogen clearance by infected cells. Ligation of P2X7 by extracellular ATP can promote elimination of intracellular pathogens (left side). P2X7 signaling can lead to PLD activation and/or ROS production, both of which can lead to killing of the pathogens. PLD has its effect primarily through stimulation of fusion between parasitophorous vacuoles and lysosomes, and subsequent killing of pathogens in acidic phagolysosomes (right side)
Avoidance of the toxic effects of reactive oxygen species
Production of ROS such as H2O2 was considered for many years as an unfortunate, deleterious consequence of aerobic metabolism. However, it has now become clear that ROS can also participate as physiological mediators in cell signaling pathways [20, 21]. Moreover, ROS can contribute to pathogen killing, and the role of ROS in nucleotide-mediated cell signaling is attracting growing attention. Hence, nucleotide receptors have been implicated in modulating the production of superoxide, H2O2, and other ROS by several cell types [22–26]. Ferrari et al. [27] had previously described that nuclear factor (NF)-κB activation by P2X7 ligation is sensitive to antioxidants, suggesting that ROS might contribute to this outcome. In addition, nucleotide receptor signaling in murine macrophages is linked to ROS generation [26], and ATP treatment can activate an ROS-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages [28] (Fig. 1). Interestingly, one of the mechanisms by which ATP triggers apoptosis in macrophages may also involve ROS, probably via NOX2 [29].
Leishmania parasites enter into macrophages by phagocytosis. But unlike the pathogens cited above, Leishmania does not seek to inhibit fusion between entry vacuoles and lysosomes. Instead, Leishmania amastigotes display the interesting ability to survive and replicate within the hostile, low-pH environment of phagolysosomes [1]. Leishmania promastigotes interfere with reactive oxygen and nitrogen species responses in phagocytes [1].
We have observed that Leishmania infection of macrophages modulates P2X7 activity and that extracellular ATP treatment reduces the parasite load via P2X7 activation (submitted). In addition, we observed an increase in ROS levels in infected macrophages after treatment with ATP and increased parasite survival in ATP-treated macrophages treated with antioxidants (unpublished data). These findings suggest that ROS production by the immune system may contribute to clearance of parasites such as Leishmania that survive within phagolysosomes.
Prevention of host cell apoptosis
Intracellular pathogens obtain many of their nutrients from the host cell and also require that their host cells survive long enough for the pathogen to complete its infectious cycle (reviewed in [30, 31–34]). Apoptosis is a widespread mechanism that is central to the maintenance of cellular homeostasis in all tissues, including the immune system [35]. Apoptosis, or the lack of apoptosis, contributes to the pathogenesis of a number of diseases, including acquired immunodeficiency syndrome, autoimmune disease, and, in particular, cancer [36, 37]. One may argue that the natural tendency of infected cells would be to die, mainly in response to the stress represented by the infection, and that therefore any successful intracellular pathogen should delay host cell apoptosis as long as possible.
In fact, Heussler et al. [38, 39] showed that the intracellular apicomplexan parasite Theileria parva protects infected T cells from apoptosis through activation of the transcription factor NF-κB. Another apicomplexan parasite, Toxoplasma, also modulates activity of NF-κB as a way of protecting infected cells against apoptosis [40]. Similar strategies are also used by several bacteria, and NF-κB activation has been shown to insure host cell survival during Rickettsia rickettsii infection [41]. However, although Chlamydia infection renders host cells resistant to apoptosis, the evidence linking NF-κB activation with Chlamydia infection has been more controversial [33, 34]. Other parasites that protect the host cells against apoptosis include T. cruzi, Leishmania, and Plasmodium [42–44].
Since P2X7 ligation can lead to apoptosis or necrosis of uninfected macrophages and epithelial cells [45, 46], it should come as no surprise that some intracellular pathogens also inhibit P2X7-mediated cell death. In fact, inhibition of P2X7 signaling appears to be critical for propagation of some infections, since P2X7-mediated host cell death has a larger impact on development of intracellular pathogens than host cell death induced through other surface receptors. Thus, treatment of M. tuberculosis-infected macrophages with ATP results in killing of the intracellular mycobacteria [47, 48]. Treatment of monocytes infected with bacille Calmette-Guérin (BCG) with H2O2 or ATP kills the monocytes, but only ATP treatment of the infected monocytes kills the mycobacteria [48]. In a comparison with other conditions that can induce lysis of macrophages, such as complement-mediated cytolysis, Fas ligation, and CD69 activation, only ATP treatment results in death of both host cells and intracellular mycobacteria [47].
Infection by several pathogens has now been shown to inhibit ATP-induced apoptosis of the host cell, including mycobacteria, chlamydiae, Porphyromonas gingivalis, and Leishmania [45, 49–51]. However, the mechanism by which these pathogens protect the host cell has been characterized mainly for mycobacteria and P. gingivalis [49, 50], both of which secrete an enzyme, nucleoside diphosphate kinase (NDK), that consumes extracellular ATP, thus depriving P2X7 of its physiological ligand.
NDK is ubiquitously expressed in most species with similar amino acid sequence; however, the quaternary structure of NDK varies among species. It exists as a hexamer (humans, rats, pigs, bovine, Drosophila) in most species and as a tetramer in some bacteria (Myxococcus xanthus, Escherichia coli) [52]. In 1997, Shankar et al. [53] purified the 18-kDa NDK from Mycobacterium smegmatis. Approximately 70% homology was reported between the N terminus of M. smegmatis NDK and those of P. aeruginosa, E. coli, and Myxococcus xanthus. Mycobacterium bovis BCG is capable of secreting NDK upon stimulation with eukaryotic proteins (bovine serum albumin or ovalbumin) [50]. Later, recombinant M. tuberculosis NDK was crystallized by Chen et al., who showed that it has 45% sequence identity to human NDK with similar secondary and tertiary structures and exists as a hexamer, unlike most other bacteria [54]. Another group purified NDK from M. tuberculosis and demonstrated similar structural results to the recombinant protein [55].
Other ectonucleotidases produced by parasites
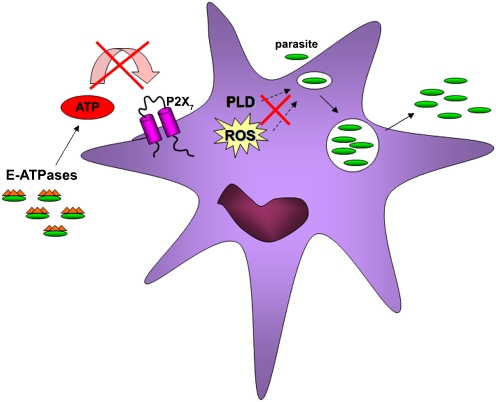
Examples of membrane-bound ecto-ATPases have been described in several parasites. In 1981, a Ca2 + -dependent nucleotidase was localized and characterized in Entamoeba histolytica, whose ability to hydrolyze extracellular ATP increases in the presence of MgCl2 [56, 57] (Fig. 2). In 1987, a nucleoside triphosphate hydrolase was described in T. gondii [58]. This hydrolase is present in the tachyzoite form of T. gondii and was detected as a circulating antigen in the sera of infected mice. Moreover, avirulent T. gondii strains express only nucleoside triphosphatase (NTPase) II, whereas virulent strains express both NTPase I and NTPase II [59]. Another study showed the membrane localization of a nucleotide triphosphate hydrolase in Toxoplasma [60], and its isoform activity was linked with T. gondii virulence [61]. Neospora caninum, also an apicomplexan parasite, expresses a type I nucleoside triphosphate hydrolase [62]. Recently, ecto-ATPases have been identified in diverse trypanosomatids, including T. cruzi [63], T. rangeli [64], and T. brucei brucei [65]. While the physiological significance of some of these ecto-ATPases remains to be shown, virulent Leishmania amazonensis promastigotes can hydrolyze ATP by a Mg-dependent ecto-ATPase more efficiently than avirulent promastigotes [66]. Mg-dependent ecto-ATPase activity was also described in Leishmania tropica [67]. In general, high levels of surface expression of ecto-ATPases correlate with virulence of the pathogens [19].
Fig. 2.
Mechanisms of pathogen evasion. Parasites have several methods to avoid antimicrobial activity of infected cells, including interference of intracellular signaling pathways (in response to ROS) and membrane trafficking (via PLD). Successful pathogens (green) can thus replicate, complete their infection cycle, and escape to the extracellular environment (right side). In addition, some protozoan parasites express ecto-ATPases (green with orange hat), which can contribute to cleavage of extracellular ATP, preventing P2X7R activation (left side). Some intracellular bacteria also protect the infected cell by secreting enzymes that consume extracellular ATP (not shown)
P2X7 receptor polymorphisms and their association with disease susceptibility
Single nucleotide polymorphisms (SNPs) are variations in a DNA sequence that occur when a single nucleotide in the sequence is different from the norm in at least 1% of the population. When SNPs occur inside a gene, they create different variants, or alleles, of that gene. Genetic factors may confer protection or increase susceptibility to infectious disease [68, 69].
P2X7 receptor polymorphisms have been described in several diseases associated with loss- or gain-of-functions of this receptor. Two P2X7 alleles have been associated with human diseases. Recently the Thr283 was found to be a key determinant in P2X7 receptor function [70]. Gu et al. [71] reported that the A1513C polymorphism is associated with normal P2X7 protein expression levels and subcellular localization, but defective pore formation. Functional analysis in transfected HEK293 cells expressing P2X7 confirmed increased ATP-dependent activation of the P2X7 489T mutant, compared to the wild-type receptor. These data identify 489C>T as a gain-of-function polymorphism of P2X7 [71]. The A1513C allele has been correlated with resistance to ATP-induced apoptosis and an increased incidence of familial chronic lymphocytic leukemia [72–74]. In addition, a His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes [75].
P2X7 polymorphisms are also involved in murine and human lupus susceptibility [76] and Crohn’s disease [77]. Several lines of evidence link P2X7 polymorphisms with various diseases such as Alzheimer’s disease [78], bipolar affective disorders [79], multiple sclerosis [80], and diabetes [81].
Skarrat et al. [82] identified a splice site mutation that is an inherited polymorphism in a Caucasian population and gives rise to a P2X7 null allele in 1–2% of the population. Similarly, an Arg307 to Gln change within the ATP-binding site of human P2X7 causes a loss of function of the receptor [83]. This work raises the possibility that low or absent P2X7 receptor function due to inherited polymorphisms may be a genetic susceptibility factor in a range of infections as diverse as tuberculosis, toxoplasmosis, or Chlamydia. Individuals who carry two loss-of-function polymorphisms (compound heterozygotes) in P2X7 may have the highest susceptibility to infections [83].
In the context of infections by intracellular pathogens, heterogeneity of ATP-induced killing of BCG was shown in a small number of patients, suggesting possible genetic differences in P2X7 [47]. A Gambian study showed a weakly protective effect against pulmonary tuberculosis for a polymorphism in the putative promoter [84]. These findings suggest that P2X7 polymorphisms may contribute to host immunity to M. tuberculosis infection in humans [84]. P2X7 is now known to stimulate secretion of the proinflammatory cytokines, interleukin (IL)-1β and IL-18, following inflammasome and caspase-1 activation in macrophages [85, 86]. The Glu-496 to Ala polymorphism was shown to impair ATP-mediated immune responses such as the killing of mycobacteria by human macrophages and the release of IL-1β and IL-18 from human monocytes [87–89]. Three single loss-of-function mutations in human P2X7 cause reduced ATP-induced macrophage apoptosis and killing of mycobacteria [90]. Shemon et al. [91] reported that another single polymorphism decreases the response against mycobacterial infection by the host: a Thr357 to Ser change in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. P2X7 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis was associated with increased susceptibility for M. tuberculosis infection [92]. In conclusion, various polymorphisms in P2X7 abrogate ATP-induced killing of mycobacteria by human macrophages and, thus, may contribute to variability in susceptibility to mycobacterial infections [90].
Infection by T. gondii has also have been studied with respect to P2X7 polymorphisms. Wiley et al. [93] have observed severe illness in two compound heterozygotes whose P2X7 function was totally absent. Moreover Fuller et al. [94] identified three immunocompetent subjects with absent P2X7 function due to single nucleotide polymorphisms and severe disease due to T. gondii infection.
Taken together, the results from studies on patients with tuberculosis or toxoplasmosis suggest an important role for P2X7 polymorphisms in the ability of the immune response to control infection.
Concluding remarks
For many years, P2X7 on macrophages was studied mainly with regards to its pharmacological properties and role in macrophage lysis. A physiological function for P2X7 remained elusive, until more recent studies have shown a role for P2X7 in promoting proinflammatory responses and controlling intracellular infection in vitro. Growing evidence also links P2X7 polymorphisms in humans with susceptibility to infection, further confirming an antimicrobial role for P2X7 in vivo. Several pathogens are now known to protect infected cells against P2X7-dependent apoptosis by producing ATPases that consume extracellular ATP, and it is likely that more intracellular pathogens will be shown to similarly confer resistance against host cell death.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil – CNPq, Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro-Programa de Apoio à Núcleos de Excelência -FAPERJ /PRONEX and the University of California.
Abbreviations
- NDK
nucleoside diphosphate kinase
- NTPase
nucleoside triphosphatase
- PLD
phospholipase D
- ROS
reactive oxygen species
- SNP
single nucleotide polymorphisms
References
- 1.Denkers EY, Butcher BA (2005) Sabotage and exploitation in macrophages parasitized by intracellular protozoans. Trends Parasitol 21(1):35–41 [DOI] [PubMed]
- 2.Alonso A, Garcia-del Portillo F (2004) Hijacking of eukaryotic functions by intracellular bacterial pathogens. Int Microbiol 7(3):181–191 [PubMed]
- 3.Finlay BB, McFadden G (2006) Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124(4):767–782 [DOI] [PubMed]
- 4.Stafford JL, Neumann NF, Belosevic M (2002) Macrophage-mediated innate host defense against protozoan parasites. Crit Rev Microbiol 28(3):187–248 [DOI] [PubMed]
- 5.Sacks D, Sher A (2002) Evasion of innate immunity by parasitic protozoa. Nat Immunol 3(11):1041–1047 [DOI] [PubMed]
- 6.Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449(7164):819–826 [DOI] [PubMed]
- 7.Bhavsar AP, Guttman JA, Finlay BB (2007) Manipulation of host-cell pathways by bacterial pathogens. Nature 449(7164):827–834 [DOI] [PubMed]
- 8.Luzio JP, Pryor PR, Bright NA (2007) Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8(8):622–632 [DOI] [PubMed]
- 9.Brumell JH, Grinstein S (2003) Role of lipid-mediated signal transduction in bacterial internalization. Cell Microbiol 5(5):287–297 [DOI] [PubMed]
- 10.Deretic V, Fratti RA (1999) Mycobacterium tuberculosis phagosome. Mol Microbiol 31(6):1603–1609 [DOI] [PubMed]
- 11.Wyrick PB (2000) Intracellular survival by Chlamydia. Cell Microbiol 2(4):275–282 [DOI] [PubMed]
- 12.Sibley LD (2003) Toxoplasma gondii: perfecting an intracellular life style. Traffic 4(9):581–586 [DOI] [PubMed]
- 13.Mordue DG, Hakansson S, Niesman I, Sibley LD (1999) Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp Parasitol 92(2):87–99 [DOI] [PubMed]
- 14.Lamothe J, Huynh KK, Grinstein S, Valvano MA (2007) Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell Microbiol 9(1):40–53 [DOI] [PubMed]
- 15.Kusner DJ, Adams J (2000) ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol 164(1):379–388 [DOI] [PubMed]
- 16.Stober CB, Lammas DA, Li CM, Kumararatne DS, Lightman SL, McArdle CA (2001) ATP-mediated killing of Mycobacterium bovis bacille Calmette-Guérin within human macrophages Is calcium dependent and associated with the acidification of mycobacteria-containing phagosomes. J Immunol 166(10):6276–6286 [DOI] [PubMed]
- 17.Coutinho-Silva R, Stahl L, Raymond M-N, Jungas T, Verbeke P, Burnstock G, Darville T, Ojcius DM (2003) Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity 19:403–412 [DOI] [PubMed]
- 18.Darville T, Welter-Stahl L, Cruz C, Sater AA, Andrews CW Jr, Ojcius DM (2007) Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol 179(6):3707–3714 [DOI] [PubMed]
- 19.Coutinho-Silva R, da Cruz CM, Persechini PM, Ojcius DM (2007) The role of P2 receptors in controlling infections by intracellular pathogens. Purinergic Signal 3:83–90 [DOI] [PMC free article] [PubMed]
- 20.Gamaley IA, Klyubin IV (1999) Roles of reactive oxygen species: signaling and regulation of cellular functions. Int Rev Cytol 188:203–255 [DOI] [PubMed]
- 21.Rhee SG (1999) Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31(2):53–59 [DOI] [PubMed]
- 22.Dichmann S, Idzko M, Zimpfer U, Hofmann C, Ferrari D, Luttmann W, Virchow C, Di Virgilio F, Norgauer J (2000) Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca(++) mobilization and actin reorganization in human eosinophils. Blood 95(3):973–978 [PubMed]
- 23.Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, Norgauer J, Chiozzi P, Di Virgilio F, Luttmann W (2000) P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett 486(3):217–224 [DOI] [PubMed]
- 24.Suh BC, Kim JS, Namgung U, Ha H, Kim KT (2001) P2X(7) nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol 166(11):6754–6763 [DOI] [PubMed]
- 25.Guerra AN, Gavala ML, Chung HS, Bertics PJ (2007) Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signal 3:39–51 [DOI] [PMC free article] [PubMed]
- 26.Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, Aga M, Hall DJ, Bertics PJ (2007) Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med 42(10):1506–1516 [DOI] [PMC free article] [PubMed]
- 27.Ferrari D, Wesselborg S, Bauer MKA, Schulze-Osthoff K (1997) Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol 139(7):1635–1643 [DOI] [PMC free article] [PubMed]
- 28.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM (2007) ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282(5):2871–2879 [DOI] [PMC free article] [PubMed]
- 29.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H (2008) Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem 283(12):7657–7665 [DOI] [PubMed]
- 30.Heussler VT, Kuenzi P, Rottenberg S (2001) Inhibition of apoptosis by intracellular protozoan parasites. Int J Parasitol 31(11):1166–1176 [DOI] [PubMed]
- 31.Leiriao P, Rodrigues CD, Albuquerque SS, Mota MM (2004) Survival of protozoan intracellular parasites in host cells. EMBO Rep 5(12):1142–1147 [DOI] [PMC free article] [PubMed]
- 32.Luder CG, Gross U, Lopes MF (2001) Intracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite-host interactions. Trends Parasitol 17(10):480–486 [DOI] [PubMed]
- 33.Byrne GI, Ojcius DM (2004) Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol 2(10):802–808 [DOI] [PubMed]
- 34.Ying S, Pettengill M, Ojcius DM, Hacker G (2007) Host-cell survival and death during Chlamydia infection. Curr Immunol Rev 3(1):31–40 [DOI] [PMC free article] [PubMed]
- 35.Osborn JE (1996) Drug use and behavior change. Am J Public Health 86(12):1698–1699 [DOI] [PMC free article] [PubMed]
- 36.Evan G, Littlewood T (1998) A matter of life and cell death. Science 281(5381):1317–1322 [DOI] [PubMed]
- 37.Wyllie A (1997) Apoptosis. Clues in the p53 murder mystery. Nature 389(6648):237–238 [DOI] [PubMed]
- 38.Heussler VT, Machado J Jr., Fernandez PC, Botteron C, Chen CG, Pearse MJ, Dobbelaere DA (1999) The intracellular parasite Theileria parva protects infected T cells from apoptosis. Proc Natl Acad Sci U S A 96(13):7312–7317 [DOI] [PMC free article] [PubMed]
- 39.Heussler VT, Rottenberg S, Schwab R, Kuenzi P, Fernandez PC, McKellar S, Shiels B, Chen ZJ, Orth K, Wallach D, Dobbelaere DA (2002) Hijacking of host cell IKK signalosomes by the transforming parasite Theileria. Science 298(5595):1033–1036 [DOI] [PubMed]
- 40.Kim JY, Ahn MH, Jun HS, Jung JW, Ryu JS, Min DY (2006) Toxoplasma gondii inhibits apoptosis in infected cells by caspase inactivation and NF-kappaB activation. Yonsei Med J 47(6):862–869 [DOI] [PMC free article] [PubMed]
- 41.Clifton DR, Goss RA, Sahni SK, van Antwerp D, Baggs RB, Marder VJ, Silverman DJ, Sporn LA (1998) NF-kappa B-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci U S A 95(8):4646–4651 [DOI] [PMC free article] [PubMed]
- 42.Clark RK, Kuhn RE (1999) Trypanosoma cruzi does not induce apoptosis in murine fibroblasts. Parasitology 118(Pt 2):167–175 [DOI] [PubMed]
- 43.Moore KJ, Matlashewski G (1994) Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol 152(6):2930–2937 [PubMed]
- 44.Leiriao P, Albuquerque SS, Corso S, van Gemert GJ, Sauerwein RW, Rodriguez A, Giordano S, Mota MM (2005) HGF/MET signalling protects Plasmodium-infected host cells from apoptosis. Cell Microbiol 7(4):603–609 [DOI] [PubMed]
- 45.Coutinho-Silva R, Perfettini J-L, Persechini PM, Dautry-Varsat A, Ojcius DM (2001) Modulation of P2Z/P2X7 receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol 280:C81–C89 [DOI] [PubMed]
- 46.Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira SC, Ojcius DM, Burnstock G (2005) P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol 288(5):G1024–G1035 [DOI] [PubMed]
- 47.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS (1997) ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7:433–444 [DOI] [PubMed]
- 48.Molloy A, Laochumroonvorapong P, Kaplan G (1994) Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med 180:1499–1509 [DOI] [PMC free article] [PubMed]
- 49.Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, Lamont RJ, Ojcius DM (2008) ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol 10(4):863–875 [DOI] [PMC free article] [PubMed]
- 50.Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty AM (1999) Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol 31(5):1333–1343 [DOI] [PubMed]
- 51.Kolli BK, Kostal J, Zaborina O, Chakrabarty AM, Chang KP (2008) Leishmania-released nucleoside diphosphate kinase prevents ATP-mediated cytolysis of macrophages. Mol Biochem Parasitol 158(2):163–175 [DOI] [PMC free article] [PubMed]
- 52.Lascu L, Giartosio A, Ransac S, Erent M (2000) Quaternary structure of nucleoside diphosphate kinases. J Bioenerg Biomembr 32(3):227–236 [DOI] [PubMed]
- 53.Shankar S, Hershberger CD, Chakrabarty AM (1997) The nucleoside diphosphate kinase of Mycobacterium smegmatis: identification of proteins that modulate specificity of nucleoside triphosphate synthesis by the enzyme. Mol Microbiol 24(3):477–487 [DOI] [PubMed]
- 54.Chen Y, Morera S, Mocan J, Lascu I, Janin J (2002) X-ray structure of Mycobacterium tuberculosis nucleoside diphosphate kinase. Proteins 47(4):556–557 [DOI] [PubMed]
- 55.Tiwari S, Kishan KV, Chakrabarti T, Chakraborti PK (2004) Amino acid residues involved in autophosphorylation and phosphotransfer activities are distinct in nucleoside diphosphate kinase from Mycobacterium tuberculosis. J Biol Chem 279(42):43595–43603 [DOI] [PubMed]
- 56.McLaughlin J, Muller M (1981) A calcium regulated adenosine triphosphatase in Entamoeba histolytica. Mol Biochem Parasitol 3(6):369–379 [DOI] [PubMed]
- 57.Takeuchi T, Kobayashi S, Masuda M, Tanabe M, Miura S, Fujiwara T (1981) Entamoeba histolytica: localization and characterization of ca2+-dependent nucleotidases. Int J Parasitol 11(3):209–215 [DOI] [PubMed]
- 58.Asai T, Kim TJ, Kobayashi M, Kojima S (1987) Detection of nucleoside triphosphate hydrolase as a circulating antigen in sera of mice infected with Toxoplasma gondii. Infect Immun 55(5):1332–1335 [DOI] [PMC free article] [PubMed]
- 59.Nakaar V, Beckers CJ, Polotsky V, Joiner KA (1998) Basis for substrate specificity of the Toxoplasma gondii nucleoside triphosphate hydrolase. Mol Biochem Parasitol 97(1–2):209–220 [DOI] [PubMed]
- 60.Kikuchi T, Nagata T, Furuta T (2001) Production and characterization of a monoclonal antibody against nucleoside triphosphate hydrolase from Toxoplasma gondii. J Eukaryot Microbiol Suppl:195S-196S [DOI] [PubMed]
- 61.Johnson M, Broady K, Angelici MC, Johnson A (2003) The relationship between nucleoside triphosphate hydrolase (NTPase) isoform and Toxoplasma strain virulence in rat and human toxoplasmosis. Microbes Infect 5(9):797–806 [DOI] [PubMed]
- 62.Asai T, Howe DK, Nakajima K, Nozaki T, Takeuchi T, Sibley LD (1998) Neospora caninum: tachyzoites express a potent type-I nucleoside triphosphate hydrolase. Exp Parasitol 90(3):277–285 [DOI] [PubMed]
- 63.Meyer-Fernandes JR, Saad-Nehme J, Peres-Sampaio CE, Belmont-Firpo R, Bisaggio DF, Do Couto LC, Fonseca De Souza AL, Lopes AH, Souto-Padron T (2004) A Mg-dependent ecto-ATPase is increased in the infective stages of Trypanosoma cruzi. Parasitol Res 93(1):41–50 [DOI] [PubMed]
- 64.Fonseca FV, Fonseca De Souza AL, Mariano AC, Entringer PF, Gondim KC, Meyer-Fernandes JR (2006) Trypanosoma rangeli: characterization of a Mg-dependent ecto ATP-diphosphohydrolase activity. Exp Parasitol 112(2):76–84 [DOI] [PubMed]
- 65.de Souza LM, Thomaz R, Fonseca FV, Panizzutti R, Vercesi AE, Meyer-Fernandes JR (2007) Trypanosoma brucei brucei: biochemical characterization of ecto-nucleoside triphosphate diphosphohydrolase activities. Exp Parasitol 115(4):315–323 [DOI] [PubMed]
- 66.Berredo-Pinho M, Peres-Sampaio CE, Chrispim PP, Belmont-Firpo R, Lemos AP, Martiny A, Vannier-Santos MA, Meyer-Fernandes JR (2001) A Mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch Biochem Biophys 391(1):16–24 [DOI] [PubMed]
- 67.Meyer-Fernandes JR, Dutra PM, Rodrigues CO, Saad-Nehme J, Lopes AH (1997) Mg-dependent ecto-ATPase activity in Leishmania tropica. Arch Biochem Biophys 341(1):40–46 [DOI] [PubMed]
- 68.Fernando SL, Britton WJ (2006) Genetic susceptibility to mycobacterial disease in humans. Immunol Cell Biol 84(2):125–137 [DOI] [PubMed]
- 69.Collins FS, Brooks LD, Chakravarti A (1998) A DNA polymorphism discovery resource for research on human genetic variation. Genome Res 8(12):1229–1231 [DOI] [PubMed]
- 70.Young MT, Pelegrin P, Surprenant A (2006) Identification of Thr283 as a key determinant of P2X7 receptor function. Br J Pharmacol 149(3):261–268 [DOI] [PMC free article] [PubMed]
- 71.Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, Barden JA, Wiley JS (2001) A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem 276(14):11135–11142 [DOI] [PubMed]
- 72.Wiley JS, Dao-Ung LP, Gu BJ, Sluyter R, Shemon AN, Li C, Taper J, Gallo J, Manoharan A (2002) A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: a molecular study. Lancet 359(9312):1114–1119 [DOI] [PubMed]
- 73.Dao-Ung LP, Fuller SJ, Sluyter R, Skarratt KK, Thunberg U, Tobin G, Byth K, Ban M, Rosenquist E, Stewart GJ, Wiley JS (2004) Association of the 1513C polymorphism in the P2X7 gene with familial forms of chronic lymphocytic leukaemia. Br J Haematol 125(6):815–817 [DOI] [PubMed]
- 74.Thunberg U, Tobin G, Johnson A, Soderberg O, Padyukov L, Hultdin M, Klareskog L, Enblad G, Sundstrom C, Roos G, Rosenquist R (2002) Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. Lancet 360(9349):1935–1939 [DOI] [PubMed]
- 75.Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A, Agostini P, Cuneo A, Castoldi G, Baricordi OR, Di Virgilio F (2005) A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol 175(1):82–89 [DOI] [PubMed]
- 76.Elliott JI, McVey JH, Higgins CF (2005) The P2X7 receptor is a candidate product of murine and human lupus susceptibility loci: a hypothesis and comparison of murine allelic products. Arthritis Res Ther 7(3):R468–R475 [DOI] [PMC free article] [PubMed]
- 77.Haas SL, Ruether A, Singer MV, Schreiber S, Bocker U (2007) Functional P2X7 receptor polymorphisms (His155Tyr, Arg307Gln, Glu496Ala) in patients with Crohn’s disease. Scand J Immunol 65(2):166–170 [DOI] [PubMed]
- 78.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R (2003) P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem 278(15):13309–13317 [DOI] [PubMed]
- 79.Barden N, Harvey M, Gagne B, Shink E, Tremblay M, Raymond C, Labbe M, Villeneuve A, Rochette D, Bordeleau L, Stadler H, Holsboer F, Muller-Myhsok B (2006) Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet 141B(4):374–382 [DOI] [PubMed]
- 80.Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P (2006) COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol 6:12 [DOI] [PMC free article] [PubMed]
- 81.Elliott JI, Higgins CF (2004) Major histocompatibility complex class I shedding and programmed cell death stimulated through the proinflammatory P2X7 receptor: a candidate susceptibility gene for NOD diabetes. Diabetes 53(8):2012–2017 [DOI] [PubMed]
- 82.Skarratt KK, Fuller SJ, Sluyter R, Dao-Ung LP, Gu BJ, Wiley JS (2005) A 5′ intronic splice site polymorphism leads to a null allele of the P2X7 gene in 1–2% of the Caucasian population. FEBS Lett 579(12):2675–2678 [DOI] [PubMed]
- 83.Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP, Fuller SJ, Barden JA, Clarke LA, Petrou S, Wiley JS (2004) An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem 279(30):31287–31295 [DOI] [PubMed]
- 84.Li CM, Campbell SJ, Kumararatne DS, Bellamy R, Ruwende C, McAdam KP, Hill AV, Lammas DA (2002) Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis 186(10):1458–1462 [DOI] [PubMed]
- 85.Petrilli V, Dostert C, Muruve DA, Tschopp J (2007) The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 19(6):615–622 [DOI] [PubMed]
- 86.Di Virgilio F (2007) Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci 28(9):465–472 [DOI] [PubMed]
- 87.Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS (2003) A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol 171(10):5442–5446 [DOI] [PubMed]
- 88.Sluyter R, Dalitz JG, Wiley JS (2004) P2X7 receptor polymorphism impairs extracellular adenosine 5′-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun 5(7):588–591 [DOI] [PubMed]
- 89.Sluyter R, Shemon AN, Wiley JS (2004) Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol 172(6):3399–3405 [DOI] [PubMed]
- 90.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS, Britton WJ (2005) Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J Infect Dis 192(1):149–155 [DOI] [PubMed]
- 91.Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, Britton WJ, Petrou S, Wiley JS (2006) A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem 281(4):2079–2086 [DOI] [PubMed]
- 92.Nino-Moreno P, Portales-Perez D, Hernandez-Castro B, Portales-Cervantes L, Flores-Meraz V, Baranda L, Gomez-Gomez A, Acuna-Alonzo V, Granados J, Gonzalez-Amaro R (2007) P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis. Clin Exp Immunol 148(3):469–477 [DOI] [PMC free article] [PubMed]
- 93.Wiley JS, Fernando S, Skarratt K, Saunders B, Sluyter R, Gu B, Fuller S, Shemon A, Dao-Ung P, Georgiou J, Britton W (2006) Polymorphisms in the P2X7 receptor and their clinical associations. Purinergic Signal 2(2):29–30
- 94.Fuller SJ, Lees M, Murray H, Sluyter R, Gu B, Boulter N, Skarratt K, Smith NC, Wiley JS (2006) A role for the P2X7 receptor in control of Toxoplasma gondii infection. Purinergic Signal 2(2):82