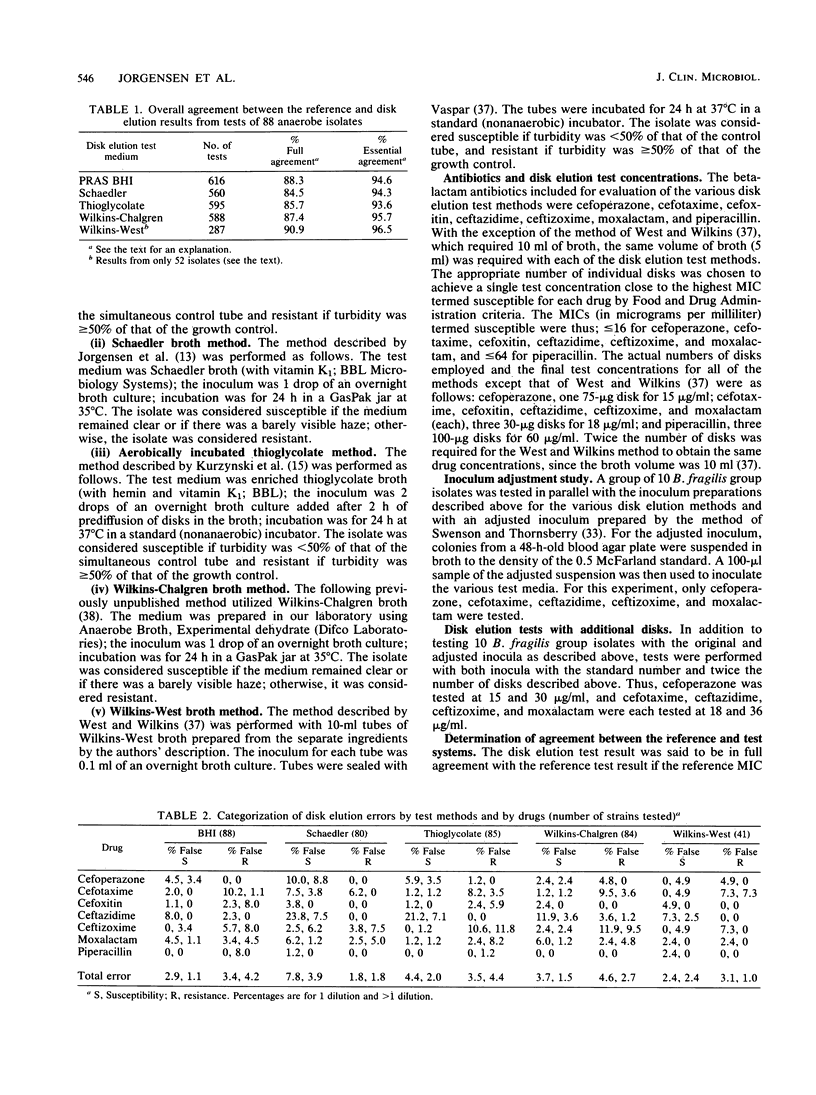
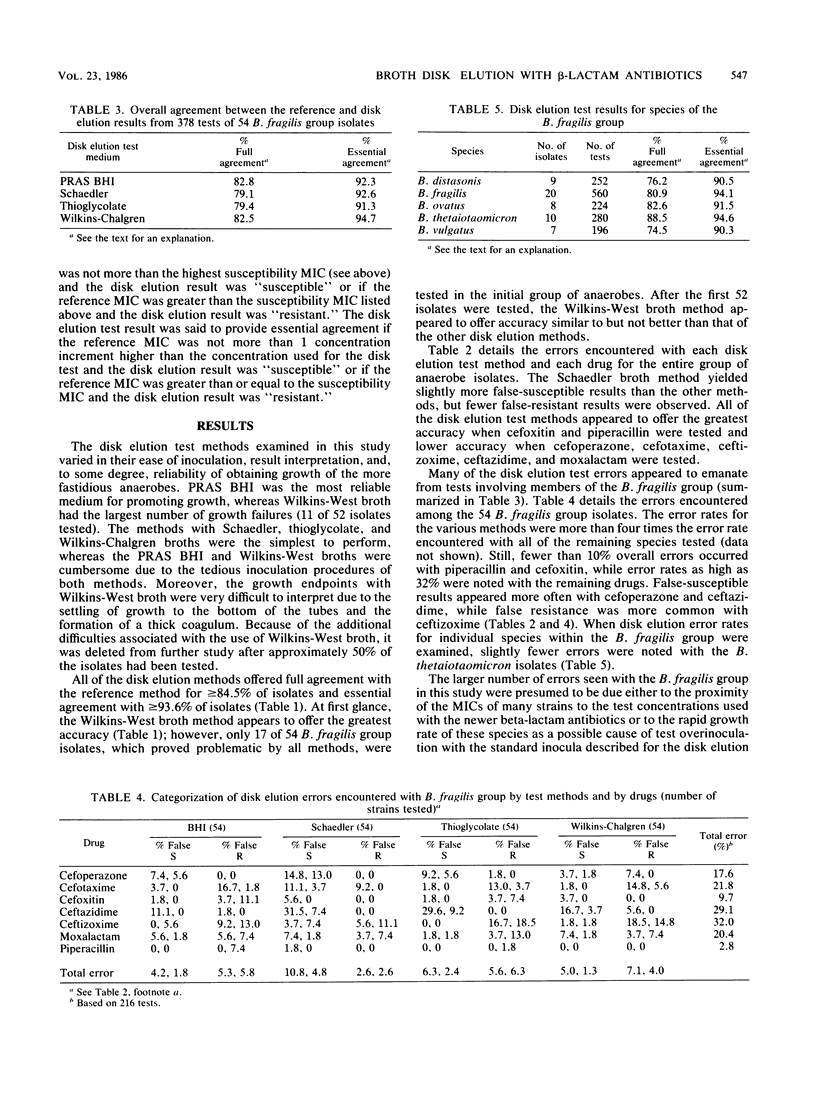
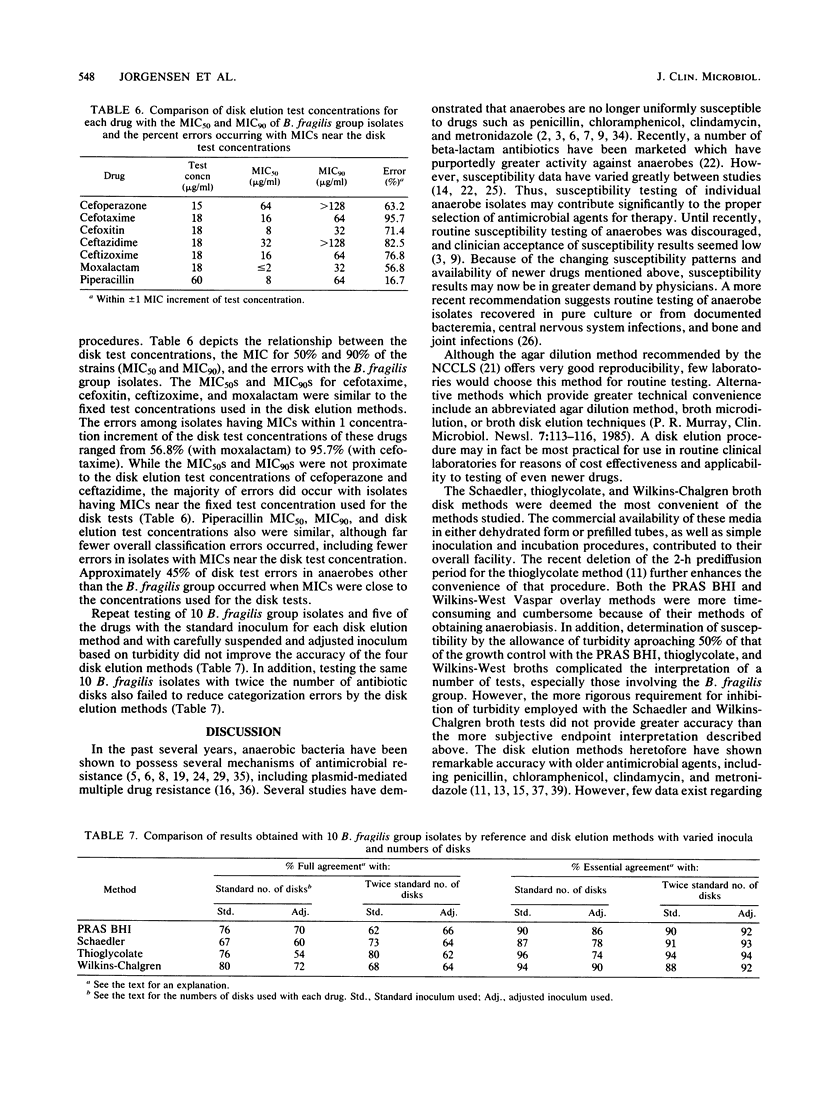
Abstract
Broth disk elution procedures represent one of the most practical means for clinical laboratories to perform routine antibiotic susceptibility tests on anaerobic bacteria. The accuracy of five disk elution test methods and media (including the one to be proposed by the National Committee for Clinical Laboratory Standards) was evaluated for the testing of newer beta-lactam antibiotics, including cefoperazone, cefotaxime, cefoxitin, ceftazidime, ceftizoxime, moxalactam, and piperacillin. Various numbers of antibiotic disks were used to achieve disk elution test concentrations which approximated the highest MIC termed susceptible by the Food and Drug Administration. A group of 88 anaerobes representing many different species was tested in parallel by the five disk elution methods and the National Committee for Clinical Laboratory Standards reference agar dilution procedure. Overall, full agreement between the reference agar dilution MICs and the disk elution category results was 88.3% for PRAS BHI, 84.5% for Schaedler, 85.7% for thioglycolate, and 87.4% for Wilkins-Chalgren broth. Essential agreement (+/- 1 twofold MIC increment from the disk elution concentration) was achieved with 94.6% of PRAS BHI tests, 94.3% of Schaedler tests, 93.6% of thioglycolate tests, and 95.7% of Wilkins-Chalgren tests. Due to growth failures with a number of isolates and difficulties in interpreting results, the use of Wilkins-West broth was discontinued after approximately one-half of the isolates had been tested. The majority of errors with all of the disk elution methods occurred with isolates (most notably members of the Bacteroides fragilis group) having MICs near the single test concentrations used in the disk methods. With the notable exception of tests for the B. fragilis group, the disk elution methods offered acceptable accuracy with the newer beta-lactam antibiotics tested in this study.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Packer R. R. Determination of susceptibility of anaerobic bacteria to cefotetan and cefoxitin by the thioglycolate disk elution method. J Clin Microbiol. 1984 Nov;20(5):912–916. doi: 10.1128/jcm.20.5.912-916.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawdon R. E., Rozmiej E., Palchaudhuri S., Krakowiak J. Variability in the susceptibility pattern of Bacteroides fragilis in four Detroit area hospitals. Antimicrob Agents Chemother. 1979 Nov;16(5):664–666. doi: 10.1128/aac.16.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgault A. M., Harkness J. L., Rosenblatt J. E. Clinical usefulness of susceptibility testing of anaerobes. Arch Intern Med. 1978 Dec;138(12):1825–1827. [PubMed] [Google Scholar]
- Bourgault A. M., Lamothe F. Comparison of anaerobic susceptibility results obtained by two methods of inoculum preparation. J Clin Microbiol. 1984 Dec;20(6):1060–1064. doi: 10.1128/jcm.20.6.1060-1064.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz M. L., Wilkinson R. G. Chloramphenicol acetyltransferase of Bacteroides fragilis. Antimicrob Agents Chemother. 1978 Jul;14(1):105–111. doi: 10.1128/aac.14.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz M. L., Wilkinson R. G. Isolation and properties of metronidazole-resistant mutants of Bacteroides fragilis. Antimicrob Agents Chemother. 1979 Jul;16(1):19–27. doi: 10.1128/aac.16.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P., Jacobus N. V., Gorbach S. L., Aldridge K., Cleary T., Finegold S. M., Hill G., Iannini P., O'Keefe J. P. Antimicrobial susceptibilities of 1,292 isolates of the Bacteroides fragilis group in the United States: comparison of 1981 with 1982. Antimicrob Agents Chemother. 1984 Aug;26(2):145–148. doi: 10.1128/aac.26.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P., Jacobus N. V., Marsh P. K., Mayhew J. W. Cefoxitin inactivation by Bacteroides fragilis. Antimicrob Agents Chemother. 1983 Dec;24(6):936–940. doi: 10.1128/aac.24.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M., Rolfe R. D. Susceptibility testing of anaerobic bacteria. Diagn Microbiol Infect Dis. 1983 Mar;1(1):33–40. doi: 10.1016/0732-8893(83)90030-5. [DOI] [PubMed] [Google Scholar]
- Hauser K. J., Johnston J. A., Zabransky R. J. Economical agar dilution technique for susceptibility testing of anaerobes. Antimicrob Agents Chemother. 1975 May;7(5):712–714. doi: 10.1128/aac.7.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helstad A. G., Hutchinson M. A., Amos W. P., Kurzynski T. A. Evaluation of two broth disk methods for antibiotic susceptibility testing of anaerobes. Antimicrob Agents Chemother. 1984 Oct;26(4):601–603. doi: 10.1128/aac.26.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Cotton J. L., Sutter V. L., Swenson J. M. Collaborative evaluation of the micro-media systems anaerobe susceptibility panel: comparisons with reference methods and test reproducibility. J Clin Microbiol. 1982 Aug;16(2):245–249. doi: 10.1128/jcm.16.2.245-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Alexander G. A., Johnson J. E. Practical anaerobic broth-disk elution susceptibility test. Antimicrob Agents Chemother. 1980 Apr;17(4):740–742. doi: 10.1128/aac.17.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzynski T. A., Yrios J. W., Helstad A. G., Field C. R. Aerobically incubated thioglycolate broth disk method for antibiotic susceptibility testing of anaerobes. Antimicrob Agents Chemother. 1976 Oct;10(4):727–732. doi: 10.1128/aac.10.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy M. H., Tally F. P. Mechanisms of drug-resistance transfer in Bacteroides fragilis. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):59–75. doi: 10.1093/jac/8.suppl_d.59. [DOI] [PubMed] [Google Scholar]
- Mangels J. I., Lindberg L. H. Evaluation of broth microdilution susceptibility results for anaerobic organisms by use of a rapid direct colony inoculum. J Clin Microbiol. 1985 Feb;21(2):269–272. doi: 10.1128/jcm.21.2.269-272.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Niles A. C. Inoculum preparation for anaerobic susceptibility tests. J Clin Microbiol. 1983 Sep;18(3):733–734. doi: 10.1128/jcm.18.3.733-734.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Rosenblatt J. E. Penicillin resistance and penicillinase production in clinical isolates of Bacteroides melaninogenicus. Antimicrob Agents Chemother. 1977 Apr;11(4):605–608. doi: 10.1128/aac.11.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polomski J. C., Bauer S. H., McClatchey K. D. Micro-Media Systems anaerobe panel versus broth disk method in anaerobe antimicrobial testing. J Clin Microbiol. 1983 Jun;17(6):949–952. doi: 10.1128/jcm.17.6.949-952.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Fayolle F., Sebald M. Resistance to tetracycline, erythromycin, and clindamycin in the Bacteroides fragilis group: inducible versus constitutive tetracycline resistance. Antimicrob Agents Chemother. 1981 Sep;20(3):314–320. doi: 10.1128/aac.20.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Comparative in vitro activity of new beta-lactam antibiotics against anaerobic bacteria. Antimicrob Agents Chemother. 1981 Nov;20(5):600–609. doi: 10.1128/aac.20.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E. Antimicrobial susceptibility testing of anaerobic bacteria. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S242–S248. doi: 10.1093/clinids/6.supplement_1.s242. [DOI] [PubMed] [Google Scholar]
- Rotilie C. A., Fass R. J., Prior R. B., Perkins R. L. Microdilution technique for antimicrobial susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1975 Mar;7(3):311–315. doi: 10.1128/aac.7.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Mitsuhashi S. Properties of a new penicillinase type produced by Bacteroides fragilis. Antimicrob Agents Chemother. 1982 Oct;22(4):579–584. doi: 10.1128/aac.22.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu D. L., Weinberg E., Cerami A. T. Evaluation of three broth disk methods for testing the susceptibility of anaerobic bacteria to imipenem. J Clin Microbiol. 1985 Jun;21(6):875–879. doi: 10.1128/jcm.21.6.875-879.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa A., Tally F. P., Jacobus N. V., Gorbach S. L. Bacteroides fragilis resistance to clindamycin in vitro. Antimicrob Agents Chemother. 1982 Nov;22(5):771–774. doi: 10.1128/aac.22.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Emmerman J., Randall E., Zabransky R. J., Birk R. J. Establishment of MICs of moxalactam for control and reference anaerobic organisms in agar dilution and microdilution techniques. Antimicrob Agents Chemother. 1985 Mar;27(3):424–426. doi: 10.1128/aac.27.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Thornsberry C. Preparing inoculum for susceptibility testing of anaerobes. J Clin Microbiol. 1984 Mar;19(3):321–325. doi: 10.1128/jcm.19.3.321-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jacobus N. V., Gorbach S. L., Aldridge K. E., Cleary T. J., Finegold S. M., Hill G. B., Iannini P. B., McCloskey R. V. Susceptibility of the Bacteroides fragilis group in the United States in 1981. Antimicrob Agents Chemother. 1983 Apr;23(4):536–540. doi: 10.1128/aac.23.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., O'Keefe J. P., Sullivan N. M., Gorbach S. L. Inactivation of cephalosporins by Bacteroides. Antimicrob Agents Chemother. 1979 Nov;16(5):565–571. doi: 10.1128/aac.16.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Snydman D. R., Gorbach S. L., Malamy M. H. Plasmid-mediated, transferable resistance to clindamycin and erythromycin in Bacteroides fragilis. J Infect Dis. 1979 Jan;139(1):83–88. doi: 10.1093/infdis/139.1.83. [DOI] [PubMed] [Google Scholar]
- West S. E., Wilkins T. D. Vaspar broth-disk procedure for antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1980 Feb;17(2):288–291. doi: 10.1128/aac.17.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1976 Dec;10(6):926–928. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Thiel T. Modified broth-disk method for testing the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother. 1973 Mar;3(3):350–356. doi: 10.1128/aac.3.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]


