Abstract
Enterocytozoon bieneusi is the most common cause of chronic diarrhea in individuals with human immunodeficiency virus infection or AIDS, and there is no effective therapy. The inhibitory activities of polyamine analogues (PG-11157, PG-11158, and PG-11302) against E. bieneusi infection were evaluated in SCID mice preconditioned with anti-gamma interferon monoclonal antibody intraperitoneally (i.p.). Mice were challenged orally with 104 E. bieneusi spores, and groups of mice were treated orally or i.p. 14 days later for 7 days. The inhibitory activities of the drugs against infection were determined by enumerating the E. bieneusi spores in feces three times a week by an immunofluorescence assay. Immunohistochemistry staining confirmed the infection within enterocytes. Oral administration of the analogues PG-11157 (at 150 or 75 mg/kg of body weight/day) and PG-11302 (at 250 mg/kg/day) had significant inhibitory activity (96.2 to 99.6%) that was slightly better than that of fumagillin (1 mg/kg/day; 93.7%). The inhibitory activity with i.p. injection was significant only with PG-11302 at 20 mg/kg/day. While the treatments considerably reduced the levels of spore excretion, neither polyamine analogues nor fumagillin was able to completely eliminate E. bieneusi, as excretion reappeared within 7 days after the end of treatment. Drug toxicity was apparent during treatment, but it disappeared at the end of treatment. These results warrant further examination of the analogues PG-11157 and PG-11302.
While it has been recognized as a disease-causing agent in humans since 1985, little progress was made in the diagnosis or treatment of Enterocytozoon bieneusi over the last two decades because of a lack of laboratory tools and reagents, as well as the inability to propagate the parasite in cell culture or in animals. The lack of immune reagents and a spore size similar to that of most bacteria have made identification, concentration, and purification of the organism difficult. Consequently, many investigators have used clinically less significant, surrogate species. Current therapies for microsporidia, like albendazole, are ineffective against E. bieneusi. Fumagillin is not curative and is known to cause thrombocytopenia in AIDS patients; it is also toxic when it is administered systemically. There is a clear need for therapy for E. bieneusi infections.
Our laboratories have recently made significant discoveries in several pivotal areas, which have contributed to the progress on E. bieneusi research. These discoveries are as follows. (i) We have developed molecular diagnostic tools that are of help with investigation of the role of E. bieneusi in children (29, 30). (ii) By using the macaque model, the relationship between immune dysfunction in the gastrointestinal tract due to simian immunodeficiency virus infection or simian AIDS and the persistence of E. bieneusi infection in the immunodeficient host were defined (25, 26). (iii) Methods for the purification of E. bieneusi spores from the stools of infected patients were developed, and these methods allowed the generation of sufficient quantities of highly purified microbial antigen (23). (iv) With purified spores, polyclonal antibodies and monoclonal antibodies (MAbs) against E. bieneusi were generated and characterized (24, 33). (v) The purified spores and the antibodies were used to generate DNA to perform the first survey of the E. bieneusi genome sequence (5). (vi) Finally, the successful infection and continuous propagation of E. bieneusi in rodents (10) made the studies described in this report feasible.
Polyamines are indispensable cellular components implicated in many physiological functions, such as DNA replication and repair, transcription, protein synthesis, and posttranslational protein modifications (28). Polyamine analogues which interfere with polyamine function and metabolism when they are transported into cells by the polyamine transport system have been developed. They are used as probes in an effort to clarify the functions of natural polyamines (11, 12, 13), and they are also potential cancer chemotherapeutic agents (3, 14, 15, 17, 31) and agents that may be used to treat several parasitic diseases. Polyamines showed activity in vitro against microsporidia and are effective agents for the treatment of several causes of microsporidiosis (22).
In the study described here, have evaluated three polyamine analogs (PG-11157, PG-11158, and PG-11302) for their efficacies against E. bieneusi infection and compared their activities with the activity of fumagillin in the newly developed preconditioned SCID mouse model.
MATERIALS AND METHODS
Parasite.
E. bieneusi spores from infected rats (the spores were originally isolated from human patients) (29) were purified from fresh stools, as described previously (23). Briefly, fecal samples were washed with phosphate-buffered saline (PBS), passed through a sieve to remove large particulate material, and concentrated by centrifugation. The spores were enriched by a salt flotation step, followed by a Percoll gradient centrifugation step, which separated the spores from the fecal material, as described previously (23). The purified spores were counted by an indirect immunofluorescence (IF) assay with specific antibodies against E. bieneusi (23, 24).
Mouse model.
B6.CB17-Prkdc SCID/beige mice (age, 4 weeks) were purchased from Charles River Laboratories. For each set of experiments, the mice were injected intraperitoneally (i.p.) with 1 mg/mouse of anti-gamma interferon (anti-IFN-γ) MAb (MAb XMG1.2) 2 h before they were randomly assigned to seven groups containing seven mice each. The mice were orally challenged with 104 E. bieneusi spores per mouse or placebo (PBS). All groups were given subsequent injections of 0.5 mg of anti-IFN-γ MAb i.p. every other week thereafter until the end of the experiment. The impact of drug toxicity on the mice was determined for a group of five C.B-17 SCID mice which received the larger drug dose but no E. bieneusi spores (Tables 1 to 3). To determine the potential drug toxicity in the treated mice, the body weights were recorded weekly; the status of the fur (which is ruffled when the mice are not well) and the amount of movement were recorded, and a hunched back and lethargy were also recorded when they were observed.
TABLE 1.
Summary of therapeutic evaluation of PG-11157 and fumagillin in SCID mice challenged with E. bieneusi spores
| Mouse group | No. of mice | E. bieneusi spore challenge dose | Drug (treatment [mg/kg/day]) | % Inhibitiona
|
|
|---|---|---|---|---|---|
| At end of treatment with PG-11157 | On the week following anti-IFN-γ antibody injection | ||||
| 1 | 5 | 0 | PG-11157 (150)b | 100 | 100 |
| 2 | 6 | 104 | Placebo | 0 | 0 |
| 3 | 7 | 104 | Fumagillin (1)c | 93.7 | −6.1 |
| 4 | 7 | 104 | PG-11157 (150) | 99.6 | 75.8 |
| 5 | 7 | 104 | PG-11157 (75)d | 99.5 | 43.1 |
| 6 | 7 | 104 | PG-11157 (10)e | −20 | −59.8 |
Percent inhibition is the percent reduction in the average level of spore excretion in feces at the end of drug treatment and the week after the end of the experiment compared with the level of spore excretion in feces in the placebo-treated group.
PG-11157 was given orally twice daily (150 mg/kg/day) for 7 days.
Fumagillin was given orally once daily (1 mg/kg/day) for 7 days.
PG-11157 was given orally once daily (75 mg/kg/day) for 7 days.
PG-11157 was given i.p. twice over a week (10 mg/kg/injection).
TABLE 3.
Summary of therapeutic evaluation of PG-11158 and fumagillin in SCID mice challenged with E. bieneusi spores
| Mouse group | No. of mice | E. bieneusi spore challenge dose | Drug (dose [mg/kg/day]) | % Inhibitiona
|
|
|---|---|---|---|---|---|
| At end of treatment with PG-11158 | On the week following anti-IFN-γ antibody injection | ||||
| 1 | 6 | 0 | PG-11158 (150)b | 100 | 100 |
| 2 | 6 | 104 | Placebo | 0 | 0 |
| 3 | 7 | 104 | Fumagillin (1)c | 71.6 | 49.9 |
| 4 | 7 | 104 | PG-11158 (150) | 69.8 | 82.7 |
| 5 | 6 | 104 | PG-11158 (75)d | 25.9 | 19.0 |
| 6 | 7 | 104 | PG-11158 (10)e | 45.4 | 27.2 |
Percent inhibition is the percent reduction in the average level of spore excretion in feces at the end of drug treatment and the week after the end of the experiment compared with the level of spore excretion in feces in the placebo-treated group.
PG-11158 was given orally daily (150 mg/kg/day) for 7 days.
Fumagillin was given orally once daily (1 mg/kg/day) for 7 days.
PG-11158 was given orally daily (75 mg/kg/day) for 7 days.
PG-11158 was given i.p. daily over a week (10 mg/kg/injection).
Synthetic polyamine analogues.
PG-11157, PG-11158, and PG-11302 were provided by Medigen Biosciences LLC under agreement with Progen Pharmaceuticals. The doses of the three drugs given to the mice, either orally or i.p. daily over 7 days, are summarized in Tables 1 to 3. Fumagillin was used as a positive control drug and was given at 1 mg/kg of body weight/day for 7 days (22 μg/mouse/day for 7 day) (Tables 1 to 3). To determine the inhibitory activity of the drug, the following formula by Su et al. (27) was used: [(number of spores in the placebo group − number of spores in the drug-treated group)/(number of spores in the placebo-treated control group)] × 100.
Evaluation of activities of polyamine analogues against E. bieneusi in mice.
The presence of E. bieneusi spores in fecal samples was monitored three times per week by the IF assay (10). Briefly, spores were detected by using a rabbit anti-E. bieneusi polyclonal antibody (1:1,000 dilution) and goat anti-rabbit immunoglobulin G conjugated with Alexa 488 secondary antibody (1:800 dilution; Molecular Probes, Eugene, OR). The slides were examined by fluorescence microscopy (BX40; Olympus Optical Pvt. Ltd.) at ×400 magnification, and the number of spores per 30 high-power fields was counted. The IF assay was also used to enumerate the spores for the oral inoculation described above. The mean body weight was measured once per week; and the symptoms (ruffled fur, lethargy, hunched back, reluctance to move), if any, were recorded.
IHC.
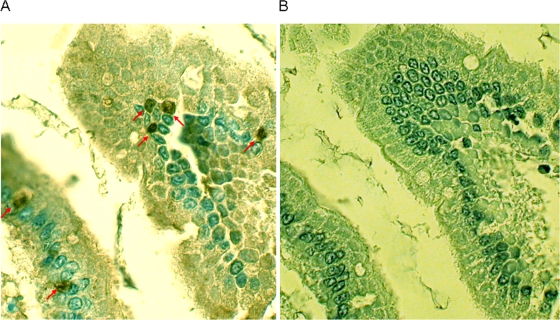
Immunohistochemistry (IHC) was performed on gut sections taken from the small intestine (duodenum, jejunum, ileum), the large intestine (cecum, colon), and all visceral organs by using MAbs specific for E. bieneusi (10). Tissue sections were cut at 5 μm and immunostained by using an avidin-biotin-horseradish peroxidase complex technique with diaminobenzidine chromogen. The sections were identified as E. bieneusi positive by staining with an MAb. An irrelevant immunoglobulin G antibody reactive against a common antigen of three microsporidia (E. cuniculi, E. intestinalis, and E. hellem) but negative for E. bieneusi was included in the assay as a negative control (see Fig. 3). Briefly, sections were deparaffinized and rehydrated. The sections were blocked with normal horse serum for 30 min at room temperature and were then incubated overnight at 4°C with E. bieneusi-specific antibody, followed by a biotinylated horse anti-mouse immunoglobulin antibody (dilution, 1:1,000; Vector Laboratories, Burlingame, CA) and VECTASTAIN Elite ABC immuno-histology stain (dilution, 1:50; Vector Laboratories) for 30 min each at room temperature. The slides were developed by using the diaminobenzidine substrate kit (Vector Laboratories) and were then counterstained with Mayer's hematoxylin.
FIG. 3.
IHC was performed on a small section of intestine from a control SCID mouse. (A) E. bieneusi forms within infected cells labeled with E. bieneusi-specific antibody (arrows); (B) an adjacent section from the same block of panel A but stained with antibody that reacted with three other microsporidia of human origin but not with E. bieneusi (B), showing the specificity of the antibody and the infection. The signal was also absent when E. bieneusi-specific antibody was reacted with a tissue section from an uninfected mouse (picture not shown here).
Statistical analysis.
As the experiment was conducted with groups of seven animals, the results were analyzed by the analysis of variance test.
RESULTS
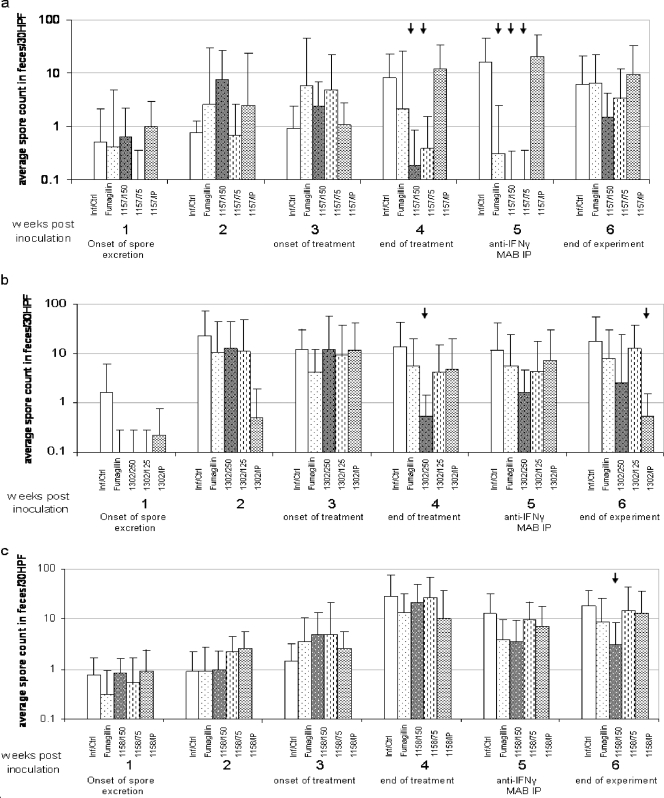
The inhibitory activities of synthetic polyamines (PG-11157, PG-11158, and PG-11302) against E. bieneusi were evaluated and compared with the activity of fumagillin. SCID mice orally challenged with 104 spores each began to excrete spores in their feces, and the level of excretion peaked after the second i.p. injection of 0.5 mg/mouse of IFN-γ MAb. The infection became apparent on week 2 and reached a statistically equivalent amount of spore excretion among all infected groups, which allowed for the onset of drug treatment on week 3. At this point the mice were treated either orally or i.p. daily for 7 days. One infected group of mice received placebo, and another uninfected control group was treated with the higher drug concentration for the evaluation of drug toxicity (Tables 1 to 3). After the completion of the 7-day treatment course, all groups were given another i.p. injection of anti IFN-γ MAb to boost spore excretion. They were monitored for another week after the end of treatment to determine whether spore excretion would increase at the end of drug treatment. The same design was applied to the evaluation of all three drugs (Tables 1 to 3). The spores were counted and the results are expressed as the mean number of spores excreted per week. Drug activity was expressed as the percent inhibition of spore excretion compared with the level of excretion by the respective placebo-treated group (Tables 1 to 3 and Fig. 1).
FIG. 1.
Effects of PG-11157 (1157), PG-11302 (1302), PG-11158 (1158), and fumagillin on the excretion of spores by E. bieneusi-infected mice. B6.CB17-Prkdc SCID/beige mice were inoculated orally with 104 spores and 3 weeks later were treated orally or i.p. with one of three test compounds or fumagillin; the dose and the timeline are reflected on the horizontal bar, and the percent inhibition is indicated on the vertical bar. The arrows show that the level of spore shedding was significantly reduced by drug treatment compared with that which occurred in the placebo-treated mice (P < 0.05 or 0.001). Inf, infected; Ctrl, control; numbers after the slashes, doses in mg/kg/day; HPF, high-power field.
Effect of PG-11157.
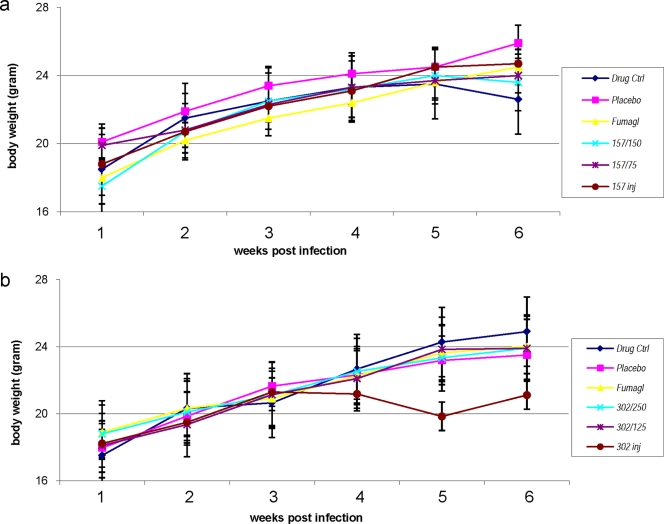
While PG-11157 and, to a lesser extent, fumagillin had significant inhibitory effects on spore excretion in mice infected with E. bieneusi, the mice were incapable of eliminating the infection after 7 days of treatment with this regimen, as was evident from the reemergence of spore excretion once the treatment was ceased. The i.p. injection of PG-11157 at a 10-mg/kg dose given twice per week had no effect at all. Apparent signs of toxicity, that is, reduced body weight gain (Fig. 2) and hypersensitivity, precluded a dose increase to try to eliminate the infection. The two drugs used orally, PG-11157 and fumagillin, showed similar patterns, with maximal rates of inhibition of >99% for PG-11157 at 150 and 75 mg/kg/day for 7 days of treatment (P < 0.05) and 93.7% for fumagillin at 1 mg/kg/day for 7 days. However, after the cessation of treatment, spore excretion emerged more rapidly in the fumagillin group, indicating the inferior inhibitory action of fumagillin compared with that of PG-11157 (Table 1 and Fig. 1).
FIG. 2.
Impact of treatment with either PG-11157 (a) or PG-11302 (b) on body weight in mice. Drug treatment commenced on week 3 and was completed on week 4; an anti-IFNγ MAb boost was given on week 5, and the experiment was terminated on week 6. Drug Ctrl, uninfected group treated with the drug at the high dose; placebo, infected, PBS-treated mice; Fumagl, mice infected and treated with Fumagillin orally; 157/150, mice infected and treated with PG-11157 at 150 mg/kg/day orally; 157/75, mice infected and treated with PG-11157 at 75 mg/kg/day orally; 157 inj, mice infected and treated with PG-11157 at 10 mg/kg/week i.p.; 302/250, mice infected and treated with PG-11302 at 250 mg/kg/day orally; 302/125, mice infected and treated with PG-11302 at 125 mg/kg/day orally; 302 inj, mice infected and treated with PG-11302 at 20 mg/kg/day i.p. The group standard deviations are shown.
Effect of PG-11302.
Spore shedding was significantly decreased after treatment with PG-11302. The difference in spore shedding in feces after a week of treatment between the placebo (PBS)-treated group and the group treated orally with the high dose of PG-11302 was significant (96.2% inhibition; P < 0.05) by the analysis of variance test. However, spore shedding resumed during the second week after treatment, following the i.p. injection of anti-IFN-γ MAb (Fig. 1 and Table 2); the exception was the group treated with the drug i.p. Seven days after the end of treatment, the group treated i.p. showed the continuous suppression of spore excretion; the difference between the control group and the group treated i.p. was significant (97.1% inhibition; P < 0.05), and the loss of body weight was significant (14.37% lower; P < 0.05).
TABLE 2.
Summary of therapeutic evaluation of PG-11302 and fumagillin in SCID mice challenged with E. bieneusi spores
| Mouse groupa | E. bieneusi spore challenge dose | Drug (dose [mg/kg/day]) | % Inhibitionb
|
|
|---|---|---|---|---|
| At end of treatment with PG-11302 | On the week following anti-IFN-γ antibody injection | |||
| 1 | 0 | PG-11302 (250)c | 100 | 100 |
| 2 | 104 | Placebo | 0 | 0 |
| 3 | 104 | Fumagillin (1)d | 64.2 | 56.1 |
| 4 | 104 | PG-11302 (250) | 96.2 | 85.7 |
| 5 | 104 | PG-11302 (125)e | 69.4 | 28.7 |
| 6 | 104 | PG-11302 (20)f | 65.1 | 97.1 |
There were six mice in each group.
Percent inhibition is the percent reduction in the average level of spore excretion in feces at the end of drug treatment and the week after the end of the experiment compared with the level of spore excretion in feces in the placebo-treated group.
PG-11302 was given orally once daily (250 mg/kg/day) for 7 days.
Fumagillin was given orally once daily (1 mg/kg/day) for 7 days.
PG-11302 was given once orally daily (125 mg/kg/day) for 7 days.
PG-11302 was given i.p. daily over a week (20 mg/kg/injection).
Effect of PG-11158.
Spore shedding was decreased only slightly after treatment with PG-11158. A statistically significant difference among the groups was detected for the group which received the higher dose of PG-22258 compared to the results for all other groups during the week posttreatment (82.7% inhibition; P < 0.05) (Fig. 1 and Table 3). PG-11158 was, in general, less effective than the other two polyamines, although in contrast, it caused minimal toxicity.
Body weight loss.
PG-11157 and PG-11158 caused a slight drop in body weight after the 7-day drug treatment (body weight, 6.5% lower than that for the placebo-treated group; Fig. 2a), while oral PG-11302 caused no significant changes (<2%) in body weight. However, there was a 14.37% decrease in body weight in the group treated with PG-11302 i.p. (Fig. 2b). The groups treated orally with fumagillin showed signs of hypersensitivity to touch. The other groups treated orally showed signs of slow movement compared with the untreated group and the group treated i.p. All symptoms, however, disappeared within 5 days after the end of treatment. The infected placebo-treated group was not affected. Another sign of some toxicity was the apparent loss of fur texture and shine among mice treated orally with the higher dose compared with the condition of the fur of the group treated with placebo. IHC was performed to demonstrate evidence of infection in the gastrointestinal tract (Fig. 3).
DISCUSSION
Current therapies against microsporidiosis are inconsistent in their efficacies. Albendazole is effective against several microsporidia, including Encephalitozoon species, but is less effective against E. bieneusi (2, 4, 8, 9). Fumagillin is effective against Encephalitozoon spp. and, to a lesser extent, E. bieneusi infections, but there have been reports of relapse and toxicity associated with the systemic administration of fumagillin (4, 16, 21). Polyamine analogs have recently been applied experimentally as chemotherapeutic agents against opportunistic pathogens, including Pneumocystis jirovecii (18), Cryptosporidium parvum (32), and E. cuniculi (1). E. bieneusi is clinically the most common microsporidian species associated with a serious disease in humans, yet so little is known about the metabolism of the parasite. The limited long-term growth in cell culture and the lack of animal models have limited progress on drug screening and discovery (6, 7, 10). The ability to propagate E. bieneusi in rodents and the development of the SCID mouse preconditioned with regular i.p. injections with 0.5 to 1 mg/mouse of anti-IFN-γ MAb are accomplishments which were exploited in the present study to evaluate polyamine analogues as potential inhibitors of this infection. However, the model is far from perfect. The infection rate is moderate at best; it fluctuates and is often intermittent. It induces no gastrointestinal or any other clinical symptoms, which to a large extent mimics infections in all other mammals, including healthy humans. The mouse model of E. bieneusi did, however, provide useful information on the efficacies of fumagillin and the polyamine analogs PG-11157 and PG-11302, which were given orally and, in the case of PG-11302, i.p. While PG-11157, PG-11302, and, to a lesser extent, fumagillin had significant inhibitory effects on spore excretion in mice infected with E. bieneusi, they were incapable of eliminating the infection after daily treatment for 7 days, as was evident from the reemergence of spore excretion within 7 days after the treatment was ceased. The i.p. injection of PG-11302 had a strong inhibitory effect (97.1%) on intestinal infection. Mild signs of toxicity would, however, preclude any dose increase in order to eliminate the infection. It is possible, however, that a longer course of treatment with a reduced dose given twice or even three times per day may accomplish this. The two analogs used (PG-11157 and PG-11302) and the positive control drug (fumagillin) given orally showed similar patterns of efficacy; the maximal inhibitory rates were 99.6% for PG-11157 at 150 mg/kg/day, 96.2% for PG-11302, and 93.7% for fumagillin at 1 mg/kg/day for 7 days. The efficacy of the analogue PG-11157 was superior to that of fumagillin, with reduced spore excretion occurring between weeks 4 and 5 (Fig. 1), while treatment with fumagillin resulted in reduced spore excretion during week 4 only.
The efficacy of the third analogue, PG-11158, was considerably less than the efficacies of the other two analogues and fumagillin, but there was an unexpected prolonged inhibitory action into the final week. What is important to note is that while the polyamine analogues resulted in a reduction in spore excretion of over 96.2% after 7 days of treatment, it bounced back only moderately a week after the end of treatment compared with the rebound achieved with fumagillin, indicating that the polyamine analogues are more potent drugs against E. bieneusi infection than fumagillin, which has been evaluated as a treatment for human disease and which was found to be effective in controlling diarrhea and eliminating spores (19, 20). As these experiments were specifically designed to explore the effects of polyamines on E. bieneusi infection, treatment was limited to 1 week to avoid drug toxicity at this preliminary phase of the experiment. In future, treatment for a longer period will also include longer periods of dosing with fumagillin. In addition, the polyamine analogue compounds were effective when they were given orally.
In conclusion, we have tested three polyamine analogues in the newly developed animal model for E. bieneusi infection. While the model is not ideal, it is sensitive enough to determine the relative inhibitory activities of the three targeted drugs compared with the activity of fumagillin, the only drug used therapeutically against microsporidial infection in humans. The mouse model has shown that, as in humans, fumagillin is not very effective in rapidly eliminating the infection before toxicity develops. In contrast, these experiments demonstrated that at least two of the three tested drugs were more effective and that further studies are required to optimize the treatment regimen. While the current regimen of 7 days of treatment did not eliminate the infection entirely, we strongly believe that changing the formulation by dividing the daily dose further from two to three in the case of PG-11157 and PG-11302 to reduce toxicity and allow treatment for longer periods will lead to the elimination of the infection by treatment with at least one of the three drugs, if not two of the three drugs. Furthermore, if toxicity remains a concern, particularly with a longer course of treatment, combination therapy may be a solution.
Acknowledgments
This work was supported by SBIR grant 1R34 AIO65231.
Footnotes
Published ahead of print on 16 March 2009.
REFERENCES
- 1.Bacchi, C. J., D. Orozco, L. M. Weiss, B. Frydman, A. Valasinas, N. Yarlett, L. J. Marton, and M. Wittner. 2001. SL-11158, a synthetic oligoamine, inhibits polyamine metabolism of Encephalitozoon cuniculi. J. Eukaryot. Microbiol. Suppl.:92S-94S. [DOI] [PubMed]
- 2.Blanshard, C., D. S. Ellis, D. G. Tovey, S. Dowell, and B. G. Gazzard. 1992. Treatment of intestinal microsporidiosis with albendazole in patients with AIDS. AIDS 6:311-313. [DOI] [PubMed] [Google Scholar]
- 3.Casero, R. A., Jr., and L. J. Marton. 2007. Polyamine metabolism and function: a target rich environment in the battle against cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 6:373-390. [DOI] [PubMed] [Google Scholar]
- 4.Conteas, C. N., O. G. Berlin, L. R. Ash, and J. S. Pruthi. 2000. Therapy for human gastrointestinal microsporidiosis. Am. J. Trop. Med. Hyg. 63:121-127. [DOI] [PubMed] [Google Scholar]
- 5.Corradi, N., D. E. Akiyoshi, H. G. Morrison, X. Feng, L. M. Weiss, S. Tzipori, and P. J. Keeling. 2007. Patterns of genome evolution among the microsporidian parasites Encephalitozoon cuniculi, Antonospora locustae and Enterocytozoon bieneusi. PLoS One 2:e1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Didier, E. S. 1997. Effects of albendazole, fumagillin, and TNP-470 on microsporidial replication in vitro. Antimicrob. Agents Chemother. 41:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didier, E. S., P. J. Didier, K. F. Snowden, and J. A. Shadduck. 2000. Microsporidiosis in mammals. Microbes Infect. 2:709-720. [DOI] [PubMed] [Google Scholar]
- 8.Didier, E. S., J. A. Maddry, P. J. Brindley, M. E. Stovall, and P. J. Didier. 2005. Therapeutic strategies for human microsporidia infections. Expert Rev. Anti-Infect. Ther. 3:419-434. [DOI] [PubMed] [Google Scholar]
- 9.Dieterich, D. T., E. A. Lew, D. P. Kotler, M. A. Poles, and J. M. Orenstein. 1994. Treatment with albendazole for intestinal disease due to Enterocytozoon bieneusi in patients with AIDS. J. Infect. Dis. 169:178-183. [DOI] [PubMed] [Google Scholar]
- 10.Feng, X., D. E. Akiyoshi, A. Sheoran, I. Singh, J. Hanawalt, Q. Zhang, G. Widmer, and S. Tzipori. 2006. Serial propagation of the microsporidian Enterocytozoon bieneusi of human origin in immunocompromised rodents. Infect. Immun. 74:4424-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fogel-Petrovic, M., D. L. Kramer, S. Vujcic, J. Miller, J. S. Mcmanis, R.J. Bergeron, and C. W. Porter. 1997. Structural basis for differential induction of spermidine/spermine N1-acetyltransferase activity by novel spermine analogs. Mol. Pharmacol. 52:69-74. [DOI] [PubMed] [Google Scholar]
- 12.Ha, H. C., P. M. Woster, J. D. Yager, and R. A. Casero, Jr. 1997. The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc. Natl. Acad. Sci. USA 94:11557-11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashi, K., K. Yoshida, K. Nishimura, E. Momiyama, K. Kashiwagi, S. Matsufuji, A. Shirahata, and K. Igarashi. 2004. Structural and functional relationship among diamines in terms of inhibition of cell growth. J. Biochem. 136:533-539. [DOI] [PubMed] [Google Scholar]
- 14.Holst, C. M., B. Frydman, L. J. Marton, and S. M. Oredsson. 2006. Differential polyamine analogues effects in four human breast cancer cell lines. Toxicology 223:71-81. [DOI] [PubMed] [Google Scholar]
- 15.Huang, Y., J. C. Keen, A. Pledgie, L. J. Marton, T. Zhu, S. Sukumar, B. H. Park, B. Blair, K. Brenner, R. A. Cassero, and N. E. Davidson. 2006. Polyamine analogues down-regulate estrogen receptor α expression in human breast cancer cells. J. Biol. Chem. 281:19055-19063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingber, D., T. Fujita, S. Kishimoto, K. Sudo, T. Kanamaru, H. Brem, and J. Folkman. 1990. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 348:555-557. [DOI] [PubMed] [Google Scholar]
- 17.Marton, L. J., and A. E. Pegg. 1995. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 35:55-91. [DOI] [PubMed] [Google Scholar]
- 18.Merali, S., M. Saric, K. Chin, and A. B. J. Clarkson. 2000. Effect of a bis-benzyl polyamine analogue on Pneumocystis carinii. Antimicrob. Agents Chemother. 44:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina, J. M., J. Goguel, C. Sarpati, J. F. Michiels, I. Desportes-Livage, C. L. Chastang, and L. Cotte. 1998. Efficacy and safety of intermittent oral fumagillin for the treatment of Enterocytozoon bieneusi infections in patients with AIDS, abstr. ANRS 054, p.308. Abstr. 12th Int. Conf AIDS.
- 20.Molina, J. M., J. Goguel, C. Sarfati, J. F. Michiels, I. Desportes-Livage, S. Balkan, C. Chastang, L. Cotte, C. Maslo, A. Struxiano, F. Derouin, and J. M. Decazes. 2000. Trial of oral fumagillin for the treatment of intestinal microsporidiosis in patients with HIV infection. AIDS 14:1341-1348. [DOI] [PubMed] [Google Scholar]
- 21.Molina, J. M., M. Tourneur, C. Sarfati, S. Chevret, A. de Gouvello, J. G. Gobert, S. Balkan, and F. Derouin. 2002. Fumagillin treatment of intestinal microsporidiosis. N. Engl. J. Med. 346:1963-1969. [DOI] [PubMed] [Google Scholar]
- 22.Reguera, R. M., B. L. Tekwani, and R. Balana-Fouce. 2005. Polyamine transport in parasites: a potential target for new antiparasitic drug development. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 140:151-164. [DOI] [PubMed] [Google Scholar]
- 23.Sheoran, A. S., X. Feng, S. Kitaka, L. Green, C. Pearson, E. S. Didier, S. Chapman, J. K. Tumwine, and S. Tzipori. 2005. Purification of Enterocytozoon bieneusi from stools and production of specific antibodies. J. Clin. Microbiol. 43:387-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheoran, A. S., X. Feng, I. Singh, S. Chapman-Bonofiglio, S. Kitaka, J. Hanawalt, J. Nunnari, K. Mansfield, J. K. Tumwine, and S. Tzipori. 2005. Monoclonal antibodies against Enterocytozoon bieneusi of human origin. Clin. Diagn. Lab. Immunol. 12:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh, I., A. S. Sheoran, Q. Zhang, A. Carville, and S. Tzipori. 2005. Sensitivity and specificity of a monoclonal antibody-based fluorescence assay for detecting Enterocytozoon bieneusi spores in feces of simian immunodeficiency virus-infected macaques. Clin. Diagn. Lab. Immunol. 12:1141-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh, I., W. Li, M. Woods, A. Carville, and S. Tzipori. 2006. Factors contributing to spontaneous Enterocytozoon bieneusi infection in simian immunodeficiency virus-infected macaques. J. Med. Primatol. 35:352-360. [DOI] [PubMed] [Google Scholar]
- 27.Su, R. B., X. L. Wei, Y. Liu, and J. Li. 2003. Antimalarial effect of agmantine on Plasmodium berghei K173 strain. Acta Pharmacol. Sin. 24:918-922. [PubMed] [Google Scholar]
- 28.Tabor, C. W., and H. Tabor. 1985. Polyamines in microorganisms. Microbiol. Rev. 49:81-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumwine, J. K., A. Kekitiinwa, N. Nabukeera, D. E. Akiyoshi, M. A. Buckholt, and S. Tzipori. 2002. Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. Am. J. Trop. Med. Hyg. 67:299-303. [DOI] [PubMed] [Google Scholar]
- 30.Tumwine, J. K., A. Kekitiinwa, S. Bakeera-Kitaka, G. Ndeezi, R. Downing, X. Feng, D. E. Akiyoshi, and S. Tzipori. 2005. Cryptosporidiosis and microsporidiosis in Ugandan children with persistent diarrhea with and without concurrent infection with the human immunodeficiency virus. Am. J. Trop. Med. Hyg. 73:921-925. [PubMed] [Google Scholar]
- 31.Wallace, H. M., A. V. Fraser, and A. Hughes. 2003. A perspective of polyamine metabolism. Biochem. J. 376:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters, W. R., B. Frydman, L. J. Marton, A. Valasinas, V. K. Reddy, J. A. Harp, M. J. Wannemuehler, and N. Yarlett. 2000. [(1)N,(12)N]bis(ethyl)-cis-6,7-dehydrospermine: a new drug for treatment and prevention of Cryptosporidium parvum infection of mice deficient in T-cell receptor alpha. Antimicrob. Agents Chemother. 44:2891-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, Q., I. Singh, A. Sheoran, X. Feng, J. Nunnari, A. Carville, and S. Tzipori. 2005. Production and characterization of monoclonal antibodies against Enterocytozoon bieneusi purified from rhesus macaques. Infect. Immun. 73:5166-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]