Abstract
Calves become infected with Shiga toxin-producing Escherichia coli (STEC) early in life, which frequently results in long-term shedding of the zoonotic pathogen. Little is known about the animals' immunological status at the time of infection. We assessed the quantity and dynamics of maternal and acquired antibodies to Shiga toxins (Stx1 and Stx2), the principal STEC virulence factors, in a cohort of 27 calves. Fecal and serum samples were taken repeatedly from birth until the 24th week of age. Sera, milk, and colostrums of dams were also assessed. STEC shedding was confirmed by detection of stx in fecal cultures. Stx1- and Stx2-specific antibodies were quantified by Vero cell neutralization assay and further analyzed by immunoblotting. By the eighth week of age, 13 and 15 calves had at least one stx1-type and at least one stx2-type positive culture, respectively. Eleven calves had first positive cultures only past that age. Sera and colostrums of all dams and postcolostral sera of all newborn calves contained Stx1-specific antibodies. Calf serum titers decreased rapidly within the first 6 weeks of age. Only five calves showed Stx1-specific seroconversion. Maternal and acquired Stx1-specific antibodies were mainly directed against the StxA1 subunit. Sparse Stx2-specific titers were detectable in sera and colostrums of three dams and in postcolostral sera of their calves. None of the calves developed Stx2-specific seroconversion. The results indicate that under natural conditions of exposure, first STEC infections frequently coincide with an absence of maternal and acquired Stx-specific antibodies in the animals' sera.
Shiga toxin-producing Escherichia coli (STEC), also known as enterohemorrhagic E. coli (EHEC), is a food-borne pathogen which can evoke life-threatening diseases, such as hemorrhagic colitis and hemolytic-uremic syndrome, in humans (26). Cattle and other ruminants are primary reservoirs for STEC serotypes that are typically associated with human disease, e.g., O157:H7. Calves become infected with STEC early in life via horizontal or vertical transmission (55) and do not develop clinical signs of infection but may shed the bacteria for several months and in great quantities (15, 64). Reduction of persistent STEC shedding in cattle would contribute greatly to preventing human STEC infections.
Evidence that vaccination may be a sensible control option has come from studies in which cattle shed E. coli O157 less frequently following immunization with STEC O157:H7 antigens (48). However, several other studies deploying various STEC antigens produced conflicting data regarding the efficacy of vaccines to reduce or prevent STEC shedding by cattle (16, 61). Identification of candidate antigens is hampered by the limited knowledge of the immune responses occurring after bovine STEC infections, their kinetics, and their meaning for the control of STEC shedding. Serological responses against a variety of antigens following E. coli O157 colonization have repeatedly been reported. Infected animals frequently develop antibodies against STEC lipopolysaccharides (LPS), e.g., O157 LPS (25). Such antibodies inhibit STEC O157 adhesion to cells in vitro (45), but shedding is not affected by serum and mucosal O157 titers in vivo (25). Mucosal immune responses are directed mainly against membrane-associated and type III secreted STEC proteins (40). Type III secreted antigens are relatively conserved among non-O157 STEC serotypes and were assumed to be broadly cross-protective (48). Antibodies against Tir (translocated intimin receptor), intimin, and Esps (E. coli secreted proteins) A and B are detectable in calves and adult cattle after natural and experimental STEC infections or after vaccination based on these antigens (9, 16, 48, 60). Nevertheless, they do not limit the magnitude or duration of STEC shedding under field conditions (61), where cattle are confronted with a variety of different STEC strains (19, 55).
Shiga toxins (Stx) are potent protein cytotoxins and represent the principal STEC virulence factors in the pathogenesis of human infections (49). Cumulating evidence shows that Stx act as immunomodulating agents during bovine STEC infections. Stx1 alters the cytokine expression pattern in mucosal macrophages (56) and intraepithelial lymphocytes (38) and suppresses the activation and proliferation of mucosal and peripheral lymphocytes in vitro (36, 37). The development of an adaptive cellular immune response is significantly delayed following experimental infection of calves with Stx2-producing STEC O157:H7 compared to that in animals inoculated with Stx-negative E. coli O157:H7 (22). In vitro and in vivo studies showed that Stx act during the early phases of immune activation rather than downregulating an established immunity (22, 57). Consequently, Stx may principally exhibit their immunomodulating activity upon first STEC infection of hitherto immunologically naïve animals.
Antibodies against Stx may be essential to protect cattle from Stx-mediated immunosuppression, but only when they are present in sufficient amounts at the time of initial STEC infection. Stx-specific antibodies are detectable in sera and colostrums of naturally infected cows (6, 47). In contrast, naturally exposed calves mostly lack Stx-specific antibodies, and antibodies are barely inducible by repeated experimental STEC infections (22, 25). Maternal antibodies were considered to interfere with the development of an acquired anti-Stx immune response in calves (25), but mother-to-offspring transfer of such antibodies has not been confirmed to date. The objectives of this study were to investigate the dynamics of maternal Stx1- and Stx2-specific antibodies in calves held under conditions of natural exposure and to determine the age at the onset of acquired Stx immunity relative to the time of initial STEC infection.
MATERIALS AND METHODS
Animals and sample collection.
Animals were kept at the local dairy farm of the Justus-Liebig-University, Giessen, Germany. Because of herd management practices implemented on the farm, only female calves and their dams were included in the study. Calves (Holstein × German Black Pied) were born between August 2005 and June 2006 and allowed to stay with their dams and suckle colostrum for 1 to 3 days. Calves were then moved to a separate barn, kept in pens in pairs, and fed milk replacer and roughage (hay). From the 12th week of life onwards, calves were housed in groups of 5 to 10 and were fed roughage and grain. Two calves exhibited transient signs of intestinal inflammation early during the observation period. For the preparation of this report, dams (n = 26) were assigned ascending numbers based on their individual Stx1-specific antibody titers in colostrums. Calves (n = 27) were given the same numbers as their dams, supplemented with the letters “a” and “b” in the case of twin calves.
Blood samples were drawn into tubes containing serum clotting activator (Sarstedt GmbH, Nümbrecht, Germany) by venipuncture of the vena jugularis externa before initial colostrum uptake (post natum [p.n.]; precolostral serum samples were not accessible for all calves), after initial colostrum uptake but within the first 24 h after birth, weekly until the 12th week, and every 2 weeks until the 24th week. At the same time points, rectal content was obtained from calves via palpation with a gloved hand and is referred to as a fecal sample throughout the manuscript. Fresh gloves were used for each individual sampling to protect from cross-contamination between animals. Blood and fecal samples were collected from dams either 4 to 8 weeks before or 4 to 8 weeks after parturition; colostrum and feces were also collected within the first 24 h after parturition. Milk samples were obtained once within the first 4 weeks of the lactation period.
Sera were prepared from blood samples (3,290 × g, 10 min, 20°C) and stored at −20°C until further processing. Colostrum and milk samples were centrifuged (3,290 × g, 10 min, 20°C), and the watery phase was transferred to fresh tubes five times to separate it from fat and debris prior to storage (−20°C). Fecal samples were stored for up to 24 weeks at −70°C until further processing.
VCA and VNA.
The Vero cell cytotoxicity assay (VCA) was performed in 96-well microtiter plates (Nunc GmbH, Wiesbaden, Germany) with Vero cells (ATCC CRL 1587; LGC-Promochem GmbH, Wesel, Germany) as previously described (18). The Vero cell neutralization assay (VNA) was applied for quantitation of neutralization activity in serum, colostrum, and milk samples. Sera were treated for 30 min at 56°C before use. Samples were prediluted 1:10 in cell culture medium (RPMI 1640 containing 2 mM stabilized l-glutamine and 2.0 mg/ml NaHCO3 [PAN Biotech GmbH, Aidenbach, Germany], 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum [FCS; PAA Laboratories GmbH, Pasching, Austria]). Within microtiter plates, eight-step log3 titration series of the prediluted samples were generated with medium containing 20% FCS, resulting in a final volume of 50 μl of diluted sample per well. Fifty microliters of a solution of either Stx1 or Stx2 (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) (adjusted to 80 50% cytotoxic doses [CD50] [4 CD50/well] in medium with 10% FCS) was added per well. The following controls were included on each plate: 50 μl of medium (with 20% FCS) plus 50 μl of medium (with 10% FCS) for a negative control; 50 μl of sample (diluted 1:10 in medium with 20% FCS) and 50 μl medium (with 10% FCS) for a serum toxicity control; and 50 μl of Stx solution and 50 μl of medium (with 20% FCS) for a Stx positive control. All samples and controls were done in triplicate. After incubation (1 h at 37°C), 4 × 104 Vero cells suspended in 50 μl medium (with 10% FCS) were added per well. Plates were incubated at 37°C and 5% CO2 for 96 h. Cytotoxic effects in the VCA and VNA were quantified by MTT reduction [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium-bromid; Sigma-Aldrich] as described previously (34). The relative cell activity (%) of a sample dilution was calculated compared to those in the positive and negative controls. Neutralization titers were determined after logit-log transformation of the relative values (Excel 2003; Microsoft Corporation), and the 50% inhibitory dose was calculated as the dilution of the sample causing 50% neutralization of 80 CD50 Stx/ml. The detection limit of the VNA was 30; samples with lower titers were assigned arbitrary titers of 15. Standard deviations (SD) were omitted from the respective figures. Data analysis revealed that SD values of triplicate determinations in single VNA experiments were smaller than 6% of the corresponding mean values.
Preliminary experiments with bovine colostrums and Stx2c-containing bacterial lysates (prepared as described below) of E. coli strain E32511/HSC (kindly provided by H. Karch, University of Münster, Germany) showed that only 1 of 26 colostrums exhibited neutralizing activity against Stx2c, with a titer of 30 (data not shown). We therefore abstained from testing sera for neutralizing activity against Stx2c.
A calf was considered to have undergone seroconversion if two consecutive serum samples harbored StxAb titers that were at least ninefold higher than the lowest titer recorded for the respective animal after maternal antibodies had vanished.
Immunoblotting.
Purified Stx1 (1.9 mg/ml; kindly provided by H. Karch) was separated into its A and B subunits by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 4% stacking and 10% separating gels as described previously (53). Protein bands were either fixed (distilled water, ethanol [50%], and 96% acetic acid [10%]) and stained (distilled water, 96% acetic acid [10%], and Serva Blue G [25 mg/liter] [Serva GmbH, Heidelberg, Germany]) for visualization or transferred to a nitrocellulose membrane (Schleicher & Schuell GmbH, Dassel, Germany) by semidry Western blotting for immunostaining. Membrane strips were blocked for 1 h with blocking solution (10% in distilled water; Roche Diagnostics GmbH, Mannheim, Germany) supplemented with 20% ovine serum. After being washed in phosphate-buffered saline (PBS)-Tween (0.05% Tween 20; Merck KgAa, Darmstadt, Germany), diluted serum samples (1:100 in PBS-Tween for detection of immunoglobulin G1 [IgG1] and IgG2 and 1:10 for detection of IgA) were added for 1 h. After a washing step, peroxidase-conjugated ovine anti-bovine IgG1, anti-bovine IgG2, or anti-bovine IgA (1:500 in PBS-Tween; Bethyl Laboratories, Inc., TX) was added for 1 h. Antibody binding was visualized with a PBS solution containing 20% chloronaphthol in methanol (3 μg/μl) and 0.7 μl/ml H2O2 solution (30%) for 30 min.
Stx ELISA.
For detection of free Stx in fecal samples, 1 g of feces was diluted in 4 ml washing buffer of a commercial enzyme-linked immunosorbent assay (ELISA) kit (Novitec verotoxin ELISA; HISS Diagnostics GmbH, Freiburg, Germany) and mixed for 5 min. One hundred microliters of the suspension was added to the wells, and the ELISA was performed following the manufacturer's instructions for detection of Stx in human stool samples. For detection of Stx-specific antibodies, the ELISA was performed in a modified form. Bacterial lysates that contained Stx1, Stx2, and Stx2c (prepared from E. coli strains 2403, 5720/96, and E32511/HSC, respectively, by ultrasonication of bacteria pelleted from overnight cultures in Luria-Bertani broth [1% tryptone {Becton Dickinson, NJ}, 0.5% yeast extract {Merck KgAa}, and 0.5% NaCl]) were diluted 1:8, 1:2, and 1:2, respectively, in washing buffer. Lysates were incubated (37°C, 30 min) with serum samples (1:10 in cell culture medium, 10% FCS). One hundred microliters of the solution was then tested by ELISA following the instruction manual. The relative optical density was calculated compared to those of the positive (diluted bacterial lysate without serum) and negative (diluted serum sample incubated with lysate from Stx-negative E. coli strain RW2169) controls. Amounts of Stx-specific antibodies in a serum sample were estimated by calculation of the percent reduction of the relative optical density.
Fecal cultures and stx1-type/stx2-type PCR.
Fecal samples (0.2 g) were diluted in 1 ml sterile NaCl solution (0.89%), plated on Gassner agar plates (Oxoid GmbH, Wesel, Germany), and incubated for 18 h at 37°C. Colonies were washed off with 2 ml NaCl solution per plate, diluted in distilled water (250 μl/ml), boiled for 2 min at 100°C, and used as a template. Primers for the stx2 type and the stx1 type (7, 17) were used as described previously (3). stx2-type primers were confirmed to detect stx2 variants stx2, stx2c, stx2vh-a, stx2vh-b (activatable stx2d), stx2-NV206, stx2d (OX-3a; not activatable), and stx2e (3). E. coli strain EDL933 (positive for stx1 and stx2; ATCC 700927D [ATCC, Rockville, MD]) served as a positive control. The detection limits of the method were determined by spiking three stx-negative fecal samples with 10−1 to 108 CFU/g feces of E. coli EDL933 bacteria grown in Luria-Bertani (LB) broth for 18 h and 180 rpm at 37°C. The detection limit was 1 × 101 to 1 × 102 CFU/g feces when samples were spiked with viable bacteria and 106 CFU/g in the case of spiking with heat-inactivated (2 min, 100°C) bacteria.
stx2 subtype-specific PCRs.
Samples positive for the stx2 type were further analyzed in individual PCRs for discrimination of the subtypes stx2, stx2c, stx2d, stx2f, and stx2e as published previously (39). E. coli strains EDL933 (stx1 and stx2), E32511 (stx2c), EH250 (stx2d), 412 (stx2e), and BgVV97/00 (stx2f) served as positive controls (3). The detection limits for the stx2 subtype- and the stx2c subtype-specific PCRs were determined as described above, with EDL933 and E32511/HSC as control strains. The detection limits were 1 × 100 CFU/g feces and 1 × 101 CFU/g feces for stx2 and stx2c, respectively.
Isolation of STEC strains.
Seventy stx-positive fecal samples from nine calves and three dams containing DNAs for different stx subtypes and representing different ages of the sampled animals were chosen. A 0.2-g aliquot of a given sample was diluted with NaCl solution and plated onto Gassner agar plates. After 18 h at 37°C, 10 lactose-fermenting colonies per plate were transferred to individual LB tubes and propagated for 18 h and 180 rpm at 37°C. The purity of cultures was assessed by plating cells on Gassner agar plates. Overall, 700 colonies were obtained and 65 isolates were confirmed to be STEC by the stx1-type/stx2-type multiplex PCR. STEC isolates were derived from samples of nine calves but not from a dam's sample. Heat-inactivated cultures were used as templates for detection of virulence genes (stx, eae, and ehxA), as described elsewhere (17, 41, 44), and the stx subtype was determined as described above. In vitro Stx production was assessed by VCA of lysates of the strains generated with polymyxin B (1 mg/ml in NaCl solution; Pfizer Pharma GmbH, Karlsruhe, Germany), as published previously (65). Macrorestriction patterns of bacterial DNA were investigated by contour-clamped homogeneous electric field pulsed-field gel electrophoresis (PFGE) according to the method of Liebisch and Schwarz (31), as described previously (66). Strains representative of different PFGE patterns were selected, and the eae subtype was determined by PCR-restriction fragment length polymorphism analysis (51). Adhesion characteristics of the strains to HEp2-cells (ATCC CCL23; LGC-Promochem) and primary bovine colonic crypt cells (56) were detected by a modified Giemsa test (14). The ability to induce reorganization of intracellular actin filaments next to the point of bacterial adhesion was determined in the fluorescent-actin staining test (29), comprising an incubation step (3 h at 37°C) of cell monolayers with washed bacterial suspensions from an overnight culture, an intermediate washing step (cell culture medium) to remove unbound bacteria, and another incubation step (3 h at 37°C) to facilitate formation of a specific adherence pattern. Stx secreted upon adhesion of the strains to bovine colonic crypt cells was quantified by VCA of cell culture supernatants obtained from the final incubation step.
RESULTS
Detection of stx-specific DNA in fecal cultures from dams.
DNAs carrying stx genes were detectable in some fecal cultures of dams before and subsequently after parturition (Fig. 1). Both stx1-type and stx2-type specific DNAs could be detected. By advanced analysis of stx2-type-positive cultures, subtypes stx2, stx2c, and stx2d were detectable in two, four, and three cultures, respectively (data not shown). Variants stx2e and stx2f were detectable in none of the samples. Despite a reproducibly positive signal for the stx2 type (stx1-type/stx2-type multiplex PCR), the stx2 subtype in one culture could not be determined.
FIG. 1.
Individual and age-related patterns of stx-positive fecal cultures from calves between birth and the 24th week of age and from their dams before or after parturition. White boxes depict fecal cultures analyzed by stx1-type/stx2-type multiplex PCR. Detection of the stx1 type and the stx2 type is marked in gray and black, respectively. Cultures testing stx2-type positive were further analyzed for the presence of stx2 subtypes: stx2, stx2c, and stx2 plus stx2c were detected in individual cultures, but the results were omitted from the figure for ease of reading. p.n., first sample after birth; 24 h, second sample, taken within first 24 h after birth and after colostrum uptake; a.p., before parturition; p.p., sample taken subsequent to parturition. Arrowhead, dam 15 is listed twice because this dam delivered twin calves (15a and 15b).
Detection of stx in fecal cultures from the calf cohort.
Of 493 cultured fecal samples from calves, obtained between birth and the 24th week of age, stx-specific DNAs were detected in 184 (37.3%) cultures (Fig. 1). Cultures of 29 (15.8%), 71 (38.6%), and 84 (45.7%) samples contained the stx1 type, stx2 type, and both, respectively. The stx2 and stx2c variants were detected in 29 and 15 fecal cultures, respectively; 89 cultures were positive for stx2 plus stx2c. DNAs for stx2d, stx2e, and stx2f were not found in either culture. In 22 samples, the stx2 variant could not be classified (data not shown).
Individual and age-related patterns of stx-positive fecal cultures in the calf cohort.
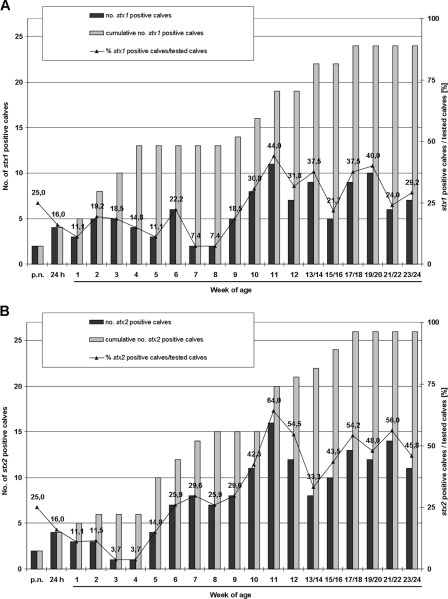
Twenty-six of the 27 calves had at least one stx-positive culture during the observation period (Fig. 1). One calf (no. 12) had no positive culture within the first 9 weeks and was not accessible for further sampling thereafter. The remaining 26 calves had at least one stx2-type-positive culture, and 24 calves had at least one stx1-type-positive culture. Until the 24th week, individual calves had up to 11 and 14 cultures positive for the stx1 type and stx2 type, respectively.
Fecal cultures from two calves (no. 2 and 15b), obtained within the first 24 h of life, were stx1 type plus stx2 type positive (Fig. 1). In subsequent weeks, the cumulative number of calves with positive cultures constantly increased (Fig. 2). While the cumulative number of calves with stx1-type-positive cultures increased stepwise, from 5 to 13, until the 4th week of age, only 6 calves had stx2-type-positive cultures by that time. By the eighth week of age, 13 and 15 calves had at least one stx1-type- and at least one stx2-type-positive culture, respectively. Eleven calves had first positive cultures only past that age.
FIG. 2.
Percentages and absolute and cumulative numbers of calves with stx1-type (A)- and stx2-type (B)-positive fecal cultures, shown relative to age. Because not all calves were sampled at all ages, absolute numbers of positive calves (black bars) and percentages of positive calves among all calves sampled (line with triangles) may vary.
Percentages of calves with stx1-type-positive cultures ranged from 11.1 to 22.2% between the first and sixth weeks, followed by a drop to 7.4% in weeks 7 and 8. Thereafter, the percentage of stx1-type-positive cultures increased, with a maximum value of 44.0% in week 11. In contrast, the percentage of calves with stx2-type-positive cultures reached its lowest values in the third and fourth weeks of age (3.7%) and rapidly increased thereafter, exceeding 64.0% by week 11.
Detection of Stx protein in fecal matter from calves.
Of 71 fecal samples that were positive for stx by PCR and tested by ELISA, Stx protein could be detected in 3 samples (from calf no. 2; samples taken at first, second, and third weeks of age).
Genotypic and phenotypic characterization of STEC isolates.
Sixty-five STEC isolates were obtained from fecal samples of nine calves and were further characterized. With the exception of one isolate, all STEC strains produced Stx in vitro (data not shown). STEC strains were assigned to 10 groups according to their virulence gene and DNA restriction patterns (Table 1). Five isolates could not be assigned to one of the groups because of their unique restriction patterns or extensive autodigestion of DNA. Isolates representative of the predominant groups were able to adhere to the human cell line HEp2 and to primary bovine colonic epithelial cells. The adhesion intensity and pattern varied between isolates and cell type. Representative isolates of groups II and IV caused actin accumulation beneath the bacterial attachment site upon binding to HEp2 and bovine epithelial cells, respectively. All isolates tested released Vero cell cytotoxic activity into the supernatant while adhering to bovine epithelial cells (data not shown).
TABLE 1.
Genotypic and phenotypic properties of STEC isolates from the calf cohort
| PFGE patterna | No. of isolates (n = 65) | Virulence gene pattern (PCR)
|
Adherence pattern and intensityb,c
|
|||
|---|---|---|---|---|---|---|
| stx type(s)/subtype(s) | eae subtypeb | Presence of ehxA | HEp2 | Bovine colonic epithelial | ||
| I | 14 | stx1, stx2 | eaezeta | + | LA, ++ | LA, + |
| II | 12 | stx1 | eaebeta1 | + | LA, +++ | LA, + |
| III | 10 | stx2, stx2c | eaebeta1 | + | LA, ++ | AA, + |
| IV | 4 | stx1 | eaeepsilon1 | + | LA, +++ | LA-AA, +++ |
| V | 4 | stx1 | eaezeta | + | LA, + | LA-AA, ++ |
| VI | 5 | stx2c | NT | NT | ||
| VII | 7 | stx2c | + | AA, +++ | NT | |
| VIII | 2 | stx2c | LA, + | LA-AA, + | ||
| IX | 1 | stx1 | eaeepsilon1 | + | NT | NT |
| X | 1 | stx1 | eaeepsilon1 | + | NT | NT |
| NA | 1 | stx2c | + | NT | NT | |
| NA | 1 | stx2c | NT | NT | ||
| NA | 1 | stx2, stx2c | NT | NT | ||
| NA | 1 | stx2c | + | NT | NT | |
| NA | 1 | stx2d | NT | NT | ||
NA, single isolates could not be assigned to a given PFGE pattern but were unique in their virulence gene pattern and therefore are listed as independent isolates.
One representative strain per PFGE pattern was used to determine the intimin serotype and the adherence pattern.
AA, aggregative; LA, local; LA-AA, mixed pattern; +, weak; ++, intermediate; +++, strong; NT, not tested.
Not subtypeable.
Stx1Ab titers in dam sera, colostrums, and milk.
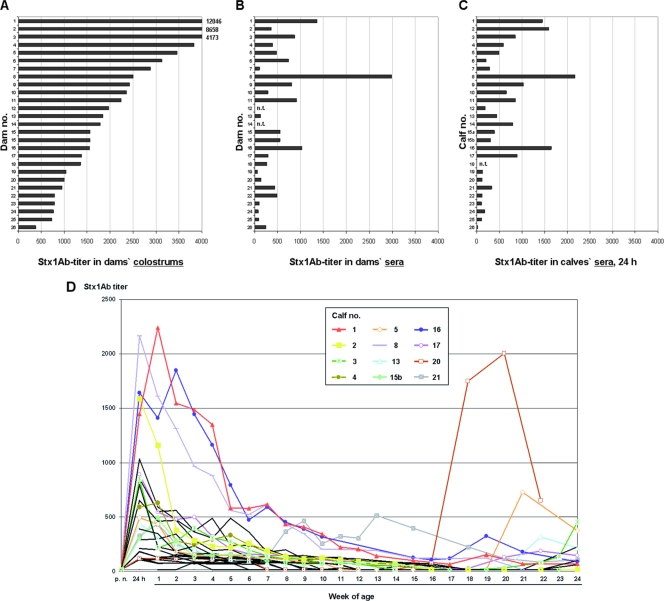
Stx1-specific antibodies (Stx1Ab) were detectable in all 24 sera tested and in all colostrums of dams, but individual titers differed dramatically (Fig. 3A and B). Stx1Ab were detectable in milk samples from only three dams (dam no. 1, 8, and 26) (data not shown), with considerably low titers (30 in all positive samples).
FIG. 3.
Stx1Ab titers. Titers in dam sera (A) and colostrums (B) and in calf sera taken after colostrum uptake, within the first 24 h of age (C), were quantified by VNA. n.t., sample not tested. Dam 15 is listed twice because this dam delivered twin calves (15a and 15b). (D) Dynamics of Stx1Ab titers in calf sera until the 24th week of age. Charts for selected individual calves are depicted in color. Calves 3, 5, 13, 17, and 20 fulfilled the criteria for Stx1-specific seroconversion, as defined in Materials and Methods. Values represent relative mean values from triplicate determinations in single VNA experiments; SD were smaller than 6% of the corresponding mean values and were omitted from the figure.
Dynamics of Stx1Ab titers in calf sera.
Stx1Ab were not detectable in precolostral sera of newborn calves but were detected in all sera tested after colostrum uptake (Fig. 3C). Postcolostral Stx1Ab titers varied individually, independent of colostrum titers of the respective dams. Most calf Stx1Ab titers peaked by 24 h after birth, but some calves (no. 1, 4, 16, and 21) had maximum titers by 1 to 2 weeks of age (Fig. 3D). Stx1Ab titers declined rapidly within the first 6 weeks. With the exception of two calves (no. 21 and 16) that continued to have detectable titers throughout the study, Stx1Ab titers of all other calves fell below the detection limit for at least two consecutive samplings. By applying the criteria described in Materials and Methods, only 5 of 27 calves (no. 3, 5, 13, 17, and 20) were found to exhibit Stx1-specific seroconversion during the observation period. The dynamics of Stx1Ab in serum samples of two representative calves (no. 1 and 17) were confirmed by competitive ELISA in that the relative reduction in the ELISA value corresponded with the titers of neutralizing Stx1Ab detected by VNA (data not shown).
Stx2Ab titers in dams and calves.
Neutralizing Stx2Ab were exclusively detectable, at a low titer (titer = 30), in 3/24, 8/26, and 0/24 dam sera, colostrums, and milk samples, respectively (data not shown). Only postcolostral sera of the three calves (no. 2, 17, and 21) whose dams possessed Stx2Ab serum titers had Stx2Ab in detectable amounts. Stx2Ab titers were low (30 in all cases) and were detectable only until 24 h (calf no. 2) or 7 (calf no. 21) or 9 (calf no. 17) weeks. None of the calves showed a Stx2-specific seroconversion. To confirm the results of the VNA, nine serum samples with Stx2-neutralizing antibodies (titer = 30) and 31 Stx2Ab-negative sera from calves with stx2-positive fecal cultures were reexamined by competitive ELISA. While no reduction of the relative ELISA value was induced by negative sera, a reduction was observed with all sera classified as containing neutralizing Stx2Ab by VNA (data not shown). Forty VNA-negative serum samples from calves with stx2c-positive fecal cultures were also negative by the competitive ELISA when tested against a Stx2c-containing bacterial lysate. The nine sera reacting with Stx2 in the ELISA did not react with Stx2c.
Isotypes and subunit specificities of Stx1Ab in sera of dams and calves.
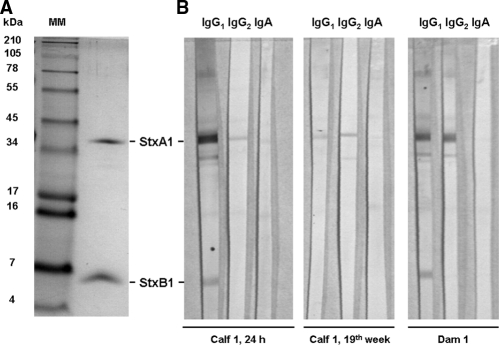
Selected sera tested against purified Stx1 by immunoblotting reacted with one or two bands, at approximately 35 and 6.5 kDa (Fig. 4; Table 2). Occasionally, a signal at 27 kDa could also be observed. Signal intensities did not strictly reflect VNA titers (Table 2). Stx1Ab were detectable by immunoblotting in the sera of all seven dams tested. Four dams had antibodies against the Stx1 A subunit (StxA1), and three dams had antibodies against StxA1 and StxB1. None of the dams' sera harbored StxB1-specific antibodies exclusively. Antibodies were of the IgG1 and IgG2 isotypes in all sera. Trace amounts of Stx1-specific IgA were detectable in the sera of four dams and were directed against StxA1. Stx1-specific antibodies were not detectable in the one precolostral serum sample tested (from calf no. 26; obtained p.n.). In contrast, StxA1-specific antibodies were found in the postcolostral sera of all seven calves tested and were mainly IgG1. StxB1-specific IgG1 was detected in the sera of the three calves that descended from the three dams with StxB1-specific IgG1. IgG2 and IgA were also present in postcolostral sera, but they were almost exclusively against StxA1. Sera obtained from calves aged several months, i.e., after the disappearance of maternal antibodies (compare to Fig. 3D), were predominantly StxA1 specific and of the IgG1 and IgG2 isotypes.
FIG. 4.
Subunit specificity of Stx1Ab in representative bovine sera. (A) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis of Stx1, separating the toxin into the StxA1 and StxB1 subunits. MM, molecular size standard. (B) Blots were immunostained with serum of calf 1, taken after 24 h and colostrum uptake, with serum taken at the 19th week of age, and with serum taken from the corresponding dam.
TABLE 2.
Stx1-specific antibodies in sera of dams and calves, detected by immunoblotting
| Calf/dam no. | Time of sampling | Stx1Ab titer (VNA)a | Isotype/subunit specificity of antibodies (immunoblotting)b
|
||
|---|---|---|---|---|---|
| IgG1 | IgG2 | IgA | |||
| Calf 21 | 1st wk | 433 | A++, B++ | A++ | A+ |
| 52nd wk | 1,706 | A+++ | A++, B+ | − | |
| Dam 21 | 594 | A++, B+ | A++, B+ | A+ | |
| Calf 17 | 24 h | 891 | A+++ | A++ | A++, B++ |
| 48th wk | 73 | A+++ | A++ | A+ | |
| Dam 17 | 293 | A++ | A++ | − | |
| Calf 2 | 24 h | 1,590 | A+++, B+ | A+ | A+++, B+ |
| 24th wk | − | A++ | A++ | A+ | |
| Dam 2 | 367 | A++, B+ | A+++ | A+ | |
| Calf 1 | 1st wk | 2,240 | A+++, B++ | A++ | A+ |
| 19th wk | 154 | A++ | A++ | − | |
| Dam 1 | 1,362 | A+++, B++ | A++ | − | |
| Calf 26 | p.n. | − | − | − | − |
| 24 h | − | A+ | A+ | A+ | |
| 40th wk | − | A++ | A++ | A+ | |
| Dam 26 | 150 | A++ | A++ | A+ | |
| Calf 20 | 24 h | 118 | A++ | A++ | A++ |
| 20th wk | 2,004 | A++ | A++ | A+ | |
| Dam 20 | 137 | A++ | A++ | A+ | |
| Calf 8 | 24 h | 2,163 | A++ | A++ | A++ |
| 24th wk | − | A++ | A++ | − | |
| Dam 8 | 2,986 | A++ | A++ | − | |
Titers below the detection limit of the VNA (titers of <30) are indicated as negative.
A, StxA1; B, StxB1; −, nil; +, weak; ++, intermediate; +++, strong.
DISCUSSION
Calves infected with STEC early in life subsequently shed these zoonotic pathogens for several months (15). Stx are putative colonization factors that prolong the duration of STEC shedding by interfering with the onset of an acquired immune response in hitherto naïve animals (37, 57). For the establishment of effective STEC eradication strategies, a better understanding of the immunological status of calves at the time of first contact with STEC antigens is indispensable, particularly with regard to anti-Stx immunity.
All dams in the herd investigated had Stx1-neutralizing antibodies in serum and colostrum, and antibodies were efficiently transferred to calves. Calves had detectable serum Stx1Ab after the first colostrum uptake, but titers reached maximum values at different times p.n. Most calves showed the highest titers at 24 h p.n., whereas some animals did so after 1 week. Colostral immunoglobulins (Ig) are rapidly resorbed by calves, resulting in a rapid increase of serum concentrations within the first 24 h p.n. (33). Individual variations in the titer dynamics may result from variations in the time of onset of gut closure. Calves lose the ability to resorb luminal IgG, IgM, and IgA from the intestine within 21 to 48 h, 16 to 23 h, and 23 to 25 h after birth, respectively (11, 27, 58), but gut closure may not be complete before 32 h after birth, in particular when colostrum uptake is delayed (58).
Stx1Ab titers rapidly declined, such that Stx1Ab were barely detectable in the sera of the majority of calves at 3 months of age. Only animals with high initial titers had detectable amounts of Stx1Ab for four more weeks. IgG1 accounts for the largest fraction of Ig in bovine colostrum (81%), while IgA and IgM accounts for only 7% and IgG2 accounts for 5% of colostral Ig (10). IgG1 also represents the predominant Ig in postcolostral sera of calves (8). The serum half-life of bovine IgG is 16 to 32 days (5, 24) and is therefore considerably longer than those for IgM (4 days) (24) and IgA (2 to 3 days) (2). The titer kinetics observed in our study thus likely reflect the half-life of maternal IgG. Calves were fed milk replacer from the first week onwards. The observed vast absence of Stx1Ab in postcolostral milk samples suggests that colostral IgG is the principal source of maternal protection against Stx, both systemically and in the intestinal lumen. This notion also applies to calves that are allowed to suckle from their dams for longer periods, as on farms with different herd management practices from those for the farm investigated herein.
Sera of calves contained low titers of Stx2Ab only in cases where the animals received colostral antibodies from their dams. None of the calves showed Stx2-specific seroconversion. Only five calves transiently developed indigenous Stx1Ab within the first 6 months of age and several weeks after the disappearance of maternal antibodies. To determine the age of calves at the onset of acquired Stx1 immunity, we sampled heifers and young cows and discovered that indigenous Stx1Ab were first detectable at up to 30 months of age in some animals (data not shown). These findings are in sharp contrast to the general ability of calves to mount specific immune responses against various antigens early in life (20, 28). Maternal antibodies have been suspected to interfere with seroconversion against Stx in experimentally infected calves (25). This study shows for the first time that Stx1-specific seroconversion, eventually resulting in high Stx1Ab prevalence like that found in dams, occurs with a considerable delay after maternal antibodies have disappeared, implying that other modes of action account for the absence of Stx1Ab in calves.
The VNA, initially introduced by Gentry and Dalrymple (18), has been used by several authors to quantify Stx-specific antibodies in bovine sera and colostrums (22, 25, 47). The highly sensitive assay (one 50% verocytotoxic dose corresponds to 0.4 to 0.8 pg/ml Shiga toxin [42]) particularly detects antibodies with neutralizing activity (46, 59). Although sera and colostrums may contain other components that ameliorate the verocytotoxic activity of Stx (63), Pirro et al. showed that the Stx-neutralizing activity of bovine sera and colostrums is attributable to Ig of the IgG1 isotype (47). We substantiated the VNA titration curves and confirmed negative results from the VNA by an alternate method, namely, competitive ELISA. Some VNA-negative sera gave rise to a positive signal in the immunoblot. This apparent discrepancy can be explained by the higher sensitivity of the immunoblot than that of the VNA (52). The cumulative findings by VNA, ELISA, and immunoblotting led us to conclude that the neutralizing titers determined in this study can be ascribed to the effects of Ig and that VNA-negative samples are truly negative in the sense that they do not sufficiently protect calves from the immunosuppressing effects of Stx.
The cytotoxicity of Stx for Vero cells principally relies on receptor-mediated binding via the B subunit and on translocation of the active A subunit into the cytosol, where the latter exhibits its cytotoxic effect (32). Monoclonal antibodies recognizing the B subunit and those recognizing the A subunit both are able to protect cells from the toxins (46, 59). The sera investigated in the present study contained Ig directed either against the A subunit or against both the A and B subunits. None of the sera exclusively contained B-subunit-specific antibodies. Sera with StxB1-specific antibodies as well as sera exclusively containing StxA1-specific antibodies had high neutralizing titers in the VNA, indicating that bovine StxA1-specific antibodies also mediate protection against Stx.
Cultures of small amounts of meconium taken from four calves within the first 24 h of life were stx positive. Although STEC strains have been detected in fecal samples taken from calves hours after birth (13), we cannot rule out that in the case of animals sampled that young, contamination had occurred, e.g., by saliva from the dams cleaning the neonates by extensive licking. Detection of stx-specific DNA in primary bacterial cultures of fecal samples obtained from the first week of age onwards was considered indicative of STEC shedding by the respective animal at the time of sampling. All calves studied for more than 9 weeks of life shed STEC on at least one occasion. The portion of STEC-shedding calves constantly rose until weaning, in week 12, and remained constantly high thereafter. At any age, the portion of calves shedding STEC exceeded the portion of STEC-shedding dams beyond the parturition period. An increased prevalence of STEC shedding in weaned calves compared to that for both younger calves and adult animals has been found in several studies (13, 68). Similar to our results, previous reports detected differences in the stx type of STEC shed at different ages. STEC strains possessing stx1 are more frequently isolated from suckling calves, while stx2-possessing strains dominate in weaned calves and adult cattle (12, 55). Subtypes stx2 and stx2c are found in STEC isolates from calves more often than stx2d is found (55, 62), whereas detection of stx2e (43), stx2-NV206 (4), and stx2g (30, 62) is rare.
STEC shedding was monitored by a highly sensitive stx1-type/stx2-type multiplex PCR. We abstained from applying the method to DNAs extracted directly from fecal samples and introduced a single culture step. This resulted in a detection limit of 100 CFU per gram of feces, while the PCR provided a positive signal only when a negative sample was spiked with 1 × 105 to 1 × 106 heat-inactivated STEC CFU per gram. Our data therefore mainly reflect shedding of viable bacteria rather than intestinal passage of genetic material. To substantiate this perception, 65 STEC isolates were further characterized and assigned to 15 clones. Some possessed stx, eae, and ehxA, characteristics of potential human pathogens but also typical for bovine STEC (12, 19, 55). Selected strains were capable of binding to bovine colonic epithelial cells in vitro, in a typical adherence pattern (29, 67). Shedding dynamics and genotypic and functional traits of isolated strains are considered sufficient evidence that at least 26 of the 27 calves of the study became infected with STEC during the observation period.
A low seroprevalence of Stx2Ab has been described for naturally infected cattle (6, 47). The present study revealed for the first time that naturally infected calves also have a low seroprevalence of Stx1Ab, which was not noted in previous studies focusing on adult cattle (6, 47). There is no obvious explanation for the apparent discrepancy between frequent STEC shedding and a lack of Stx-specific serum antibodies. In experimental STEC infections, antibodies against Stx1 and Stx2 were barely inducible, even by repeated inoculations (22, 25). The poor immunogenicity of the toxins in cattle may be a result of (i) an insufficient amount of Stx protein in the bovine intestinal tract, (ii) an insufficient translocation of the toxin to the inductive sites of the humoral immune response, and (iii) the immunosuppressive effect of Stx in cattle. (i) Representative strains in our study produced Stx1 and Stx2 when they were propagated in bacterial broth and, more importantly, when cocultured with bovine intestinal epithelial cells in vitro. Stx protein could be detected by ELISA in fecal samples. Given the prolonged or repeated colonization of calves with STEC producing Stx1 and/or Stx2/Stx2c, the extensive lack of indigenous Stx-specific antibodies is unlikely to originate from a lack of luminal antigen(s). (ii) Bovine colonic epithelial cells reportedly possess Stx1 binding sites of the Gb3/CD77 type (21) but do not bind Stx2 (54). Different efficacies of translocation of Stx1 and Stx2, as observed with Gb3/CD77-positive human colonic carcinoma cell lines (23), would be an intriguing explanation for the particular lack of Stx2 antibodies in cattle. However, recent work from our group substantiated findings by others (50) that the few Gb3/CD77 molecules of bovine colonic epithelial cells are located almost exclusively intracellularly and are not accessible for luminal Stx (56). Consequently, Stx permeate the bovine intestinal barrier by Gb3/CD77-independent mechanisms, such as transcellular pathways, that are less toxin type selective (1, 54). Indeed, oral infections of 6- to 8-week-old calves showed that even the amount of translocated Stx2 is sufficient to suppress the onset of a systemic cellular immune response (22). (iii) Bovine B cells are susceptible to Stx in vitro (37), but Stx do not seem to repress B cells in vivo. Upon infection with STEC O157:H7, calves developed O157 LPS-specific antibodies with the same dynamics as a control group infected with Stx-negative E. coli O157:H7 (22). LPS is a T-cell-independent antigen, whereas induction of a humoral immune response to proteinaceous Stx involves activation of CD4+ helper T cells. Bovine CD4+ cells are only slightly sensitive to Stx1 in vitro (37) and possess only a few Gb3/CD77 receptors ex vivo (35). Nonetheless, Gb3/CD77 receptor expression by CD4+ cells can be induced, e.g., by mitogens (37), and it cannot be excluded that Stx suppresses helper T cells locally at the inductive sites of the intestinal immune system, thereby selectively inhibiting the onset of a humoral immune response against protein antigens like Stx.
For the current study, we focused on the quantitation of serum antibodies. Mucosal antibodies against membrane-associated and secreted STEC proteins, predominantly those implicated in bacterial adhesion to the bovine intestinal mucosa, are readily induced upon experimental STEC infection of calves (40) and may contribute to the reduction in STEC shedding by vaccinated animals (48). Most STEC strains possess adherence traits, but the Stx secreted do not immediately act on bovine epithelial cells (56). Bovine Stx target cells of the adaptive immune system are situated beyond the epithelial barrier (34, 37, 57). Consequently, protection against Stx is likely conferred by humoral antibodies, as quantified in the present study, rather than by mucosal antibodies, which were not investigated herein. By assessing the quantities and dynamics of maternal and acquired antibodies in calves under conditions of natural exposure, we found that first STEC infections coincide with a lack of Stx-specific antibodies. It is tempting to speculate that this immunological gap renders a considerable portion of calves fully susceptible to the immunomodulating effects of the toxins and prevents the prompt induction of an efficient immune response upon first contact with the STEC strains prevalent to the herd. Additional investigations are needed to reveal whether this phenomenon paves the way for the establishment of persistent infections in single animals and for increased STEC prevalence on the herd level.
Acknowledgments
We thank Gabriele Köpf and Ursula Leidner for excellent technical assistance and Stefanie Barth for helpful discussions. We acknowledge Helge Karch, University of Münster, Germany, for provision of purified Stx1 and E. coli reference strains and for valuable suggestions. We thank Amir Abdulmawjood, Institute of Food Science, Giessen, Germany, for intimin typing.
This work was supported by grants from the German Research Foundation to J.F. (Molecular Veterinary Medicine Research Training Group grant 455) and C.M. (Collaborative Research Centre grant 535).
Footnotes
Published ahead of print on 10 April 2009.
REFERENCES
- 1.Acheson, D. W., R. Moore, S. De Breucker, L. Lincicome, M. Jacewicz, E. Skutelsky, and G. T. Keusch. 1996. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect. Immun. 64:3294-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks, K. L. 1982. Host defense in the newborn animal. J. Am. Vet. Med. Assoc. 181:1053-1056. [PubMed] [Google Scholar]
- 3.Barth, S., A. Tscholshiew, G. Vallejo, and R. Bauerfeind. 2003. Characterization of Shiga toxin-encoding Escherichia coli (STEC) isolates from pigs in Germany. Int. J. Med. Microbiol. 293(Suppl. 36):421.14760973 [Google Scholar]
- 4.Bertin, Y., K. Boukhors, N. Pradel, V. Livrelli, and C. Martin. 2001. Stx2 subtyping of Shiga toxin-producing Escherichia coli isolated from cattle in France: detection of a new Stx2 subtype and correlation with additional virulence factors. J. Clin. Microbiol. 39:3060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besser, T. E., T. C. McGuire, C. C. Gay, and L. C. Pritchett. 1988. Transfer of functional immunoglobulin G (IgG) antibody into the gastrointestinal tract accounts for IgG clearance in calves. J. Virol. 62:2234-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borman-Eby, H. C., S. A. McEwen, R. C. Clarke, W. B. McNab, K. Rahn, and A. Valdivieso-Garcia. 1993. The seroprevalence of verocytotoxin-producing Escherichia coli in Ontario dairy cows and associations with production and management. Prev. Vet. Med. 15:261-274. [Google Scholar]
- 7.Bosworth, B. T., and T. A. Casey. 1997. Abstr. 97th Gen. Meet. Am. Soc. Microbiol., 4 to 8 May 1997, Miami Beach, FL, abstr. B-509, p. 116.
- 8.Brandon, M. R., D. L. Watson, and A. K. Lascelles. 1971. The mechanism of transfer of immunoglobulin into mammary secretion of cows. Aust. J. Exp. Biol. Med. Sci. 49:613-623. [DOI] [PubMed] [Google Scholar]
- 9.Bretschneider, G., E. M. Berberov, and R. A. Moxley. 2007. Isotype-specific antibody responses against Escherichia coli O157:H7 locus of enterocyte effacement proteins in adult beef cattle following experimental infection. Vet. Immunol. Immunopathol. 118:229-238. [DOI] [PubMed] [Google Scholar]
- 10.Butler, J. E. 1983. Bovine immunoglobulins: an augmented review. Vet. Immunol. Immunopathol. 4:43-152. [DOI] [PubMed] [Google Scholar]
- 11.Clover, C. K., and A. Zarkower. 1980. Immunologic responses in colostrum-fed and colostrum-deprived calves. Am. J. Vet. Res. 41:1002-1007. [PubMed] [Google Scholar]
- 12.Cobbold, R., and P. Desmarchelier. 2001. Characterisation and clonal relationships of Shiga-toxigenic Escherichia coli (STEC) isolated from Australian dairy cattle. Vet. Microbiol. 79:323-335. [DOI] [PubMed] [Google Scholar]
- 13.Cobbold, R., and P. Desmarchelier. 2000. A longitudinal study of Shiga-toxigenic Escherichia coli (STEC) prevalence in three Australian diary herds. Vet. Microbiol. 71:125-137. [DOI] [PubMed] [Google Scholar]
- 14.Cravioto, A., R. J. Gross, S. M. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr. Microbiol. 13:95-99. [Google Scholar]
- 15.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dziva, F., I. Vlisidou, V. F. Crepin, T. S. Wallis, G. Frankel, and M. P. Stevens. 2007. Vaccination of calves with EspA, a key colonisation factor of Escherichia coli O157:H7, induces antigen-specific humoral responses but does not confer protection against intestinal colonisation. Vet. Microbiol. 123:254-261. [DOI] [PubMed] [Google Scholar]
- 17.Franck, S. M., B. T. Bosworth, and H. W. Moon. 1998. Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. J. Clin. Microbiol. 36:1795-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentry, M. K., and J. M. Dalrymple. 1980. Quantitative microtiter cytotoxicity assay for Shigella toxin. J. Clin. Microbiol. 12:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geue, L., M. Segura-Alvarez, F. J. Conraths, T. Kuczius, J. Bockemühl, H. Karch, and P. Gallien. 2002. A long-term study on the prevalence of Shiga toxin-producing Escherichia coli (STEC) on four German cattle farms. Epidemiol. Infect. 129:173-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgins, D. C., and P. E. Shewen. 2000. Vaccination of neonatal colostrum-deprived calves against Pasteurella haemolytica A1. Can. J. Vet. Res. 64:3-8. [PMC free article] [PubMed] [Google Scholar]
- 21.Hoey, D. E., C. Currie, R. W. Else, A. Nutikka, C. A. Lingwood, D. L. Gally, and D. G. Smith. 2002. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J. Med. Microbiol. 51:143-149. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman, M. A., C. Menge, T. A. Casey, W. Laegreid, B. T. Bosworth, and E. A. Dean-Nystrom. 2006. Bovine immune response to Shiga-toxigenic Escherichia coli O157:H7. Clin. Vaccine Immunol. 13:1322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurley, B. P., M. Jacewicz, C. M. Thorpe, L. L. Lincicome, A. J. King, G. T. Keusch, and D. W. Acheson. 1999. Shiga toxins 1 and 2 translocate differently across polarized intestinal epithelial cells. Infect. Immun. 67:6670-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husband, A. J., M. R. Brandon, and A. K. Lascelles. 1972. Absorption and endogenous production of immunoglobulins in calves. Aust. J. Exp. Biol. Med. Sci. 50:491-498. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, R. P., W. C. Cray, Jr., and S. T. Johnson. 1996. Serum antibody responses of cattle following experimental infection with Escherichia coli O157:H7. Infect. Immun. 64:1879-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, J. W., and F. W. Schmidt. 1983. Absorption of colostral immunoglobulins by the calf. Dtsch. Tierarztl. Wochenschr. 90:283-286. [PubMed] [Google Scholar]
- 28.Kimman, T. G., F. Westenbrink, B. E. Schreuder, and P. J. Straver. 1987. Local and systemic antibody response to bovine respiratory syncytial virus infection and reinfection in calves with and without maternal antibodies. J. Clin. Microbiol. 25:1097-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung, P. H., J. S. Peiris, W. W. Ng, R. M. Robins-Browne, K. A. Bettelheim, and W. C. Yam. 2003. A newly discovered verotoxin variant, VT2g, produced by bovine verocytotoxigenic Escherichia coli. Appl. Environ. Microbiol. 69:7549-7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liebisch, B., and S. Schwarz. 1996. Evaluation and comparison of molecular techniques for epidemiological typing of Salmonella enterica subsp. enterica serovar Dublin. J. Clin. Microbiol. 34:641-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lingwood, C. A. 1996. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 4:147-153. [DOI] [PubMed] [Google Scholar]
- 33.Logan, E. F., D. G. McBeath, and B. G. Lowman. 1974. Quantitative studies on serum immunoglobulin levels in suckled calves from birth to five weeks. Vet. Rec. 94:367-370. [DOI] [PubMed] [Google Scholar]
- 34.Menge, C. 2003. Protocols to study effects of Shiga toxin on mononuclear leukocytes. Methods Mol. Med. 73:275-289. [DOI] [PubMed] [Google Scholar]
- 35.Menge, C., M. Blessenohl, T. Eisenberg, I. Stamm, and G. Baljer. 2004. Bovine ileal intraepithelial lymphocytes represent target cells for Shiga toxin 1 from Escherichia coli. Infect. Immun. 72:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menge, C., I. Stamm, P. M. Van Diemen, P. Sopp, G. Baljer, T. S. Wallis, and M. P. Stevens. 2004. Phenotypic and functional characterization of intraepithelial lymphocytes in a bovine ligated intestinal loop model of enterohaemorrhagic Escherichia coli infection. J. Med. Microbiol. 53:573-579. [DOI] [PubMed] [Google Scholar]
- 37.Menge, C., L. H. Wieler, T. Schlapp, and G. Baljer. 1999. Shiga toxin 1 from Escherichia coli blocks activation and proliferation of bovine lymphocyte subpopulations in vitro. Infect. Immun. 67:2209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moussay, E., I. Stamm, A. Taubert, G. Baljer, and C. Menge. 2006. Escherichia coli Shiga toxin 1 enhances IL-4 transcripts in bovine ileal intraepithelial lymphocytes. Vet. Immunol. Immunopathol. 113:367-382. [DOI] [PubMed] [Google Scholar]
- 39.Nakao, H., K. Kimura, H. Murakami, T. Maruyama, and T. Takeda. 2002. Subtyping of Shiga toxin 2 variants in human-derived Shiga toxin-producing Escherichia coli strains isolated in Japan. FEMS Immunol. Med. Microbiol. 34:289-297. [DOI] [PubMed] [Google Scholar]
- 40.Nart, P., N. Holden, S. P. McAteer, D. Wang, A. F. Flockhart, S. W. Naylor, J. C. Low, D. L. Gally, and J. F. Huntley. 2008. Mucosal antibody responses of colonized cattle to Escherichia coli O157-secreted proteins, flagellin, outer membrane proteins and lipopolysaccharide. FEMS Immunol. Med. Microbiol. 52:59-68. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen, T. V., P. Le Van, C. Le Huy, K. N. Gia, and A. Weintraub. 2005. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J. Clin. Microbiol. 43:755-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsnes, S., R. Reisbig, and K. Eiklid. 1981. Subunit structure of Shigella cytotoxin. J. Biol. Chem. 256:8732-8738. [PubMed] [Google Scholar]
- 43.Osek, J., P. Gallien, and D. Protz. 2000. Characterization of Shiga toxin-producing Escherichia coli strains isolated from calves in Poland. Comp. Immunol. Microbiol. Infect. Dis. 23:267-276. [DOI] [PubMed] [Google Scholar]
- 44.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paton, A. W., E. Voss, P. A. Manning, and J. C. Paton. 1998. Antibodies to lipopolysaccharide block adherence of Shiga toxin-producing Escherichia coli to human intestinal epithelial (Henle 407) cells. Microb. Pathog. 24:57-63. [DOI] [PubMed] [Google Scholar]
- 46.Perera, L. P., L. R. Marques, and A. D. O'Brien. 1988. Isolation and characterization of monoclonal antibodies to Shiga-like toxin II of enterohemorrhagic Escherichia coli and use of the monoclonal antibodies in a colony enzyme-linked immunosorbent assay. J. Clin. Microbiol. 26:2127-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pirro, F., L. H. Wieler, K. Failing, R. Bauerfeind, and G. Baljer. 1995. Neutralizing antibodies against Shiga-like toxins from Escherichia coli in colostra and sera of cattle. Vet. Microbiol. 43:131-141. [DOI] [PubMed] [Google Scholar]
- 48.Potter, A. A., S. Klashinsky, Y. Li, E. Frey, H. Townsend, D. Rogan, G. Erickson, S. Hinkley, T. Klopfenstein, R. A. Moxley, D. R. Smith, and B. B. Finlay. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362-369. [DOI] [PubMed] [Google Scholar]
- 49.Proulx, F., E. G. Seidman, and D. Karpman. 2001. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 50:163-171. [DOI] [PubMed] [Google Scholar]
- 50.Pruimboom-Brees, I. M., T. W. Morgan, M. R. Ackermann, E. D. Nystrom, J. E. Samuel, N. A. Cornick, and H. W. Moon. 2000. Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc. Natl. Acad. Sci. USA 97:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramachandran, V., K. Brett, M. A. Hornitzky, M. Dowton, K. A. Bettelheim, M. J. Walker, and S. P. Djordjevic. 2003. Distribution of intimin subtypes among Escherichia coli isolates from ruminant and human sources. J. Clin. Microbiol. 41:5022-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reymond, D., M. A. Karmali, I. Clarke, M. Winkler, and M. Petric. 1997. Comparison of the Western blot assay with the neutralizing-antibody and enzyme-linked immunosorbent assays for measuring antibody to verocytotoxin 1. J. Clin. Microbiol. 35:609-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 54.Schüller, S., G. Frankel, and A. D. Phillips. 2004. Interaction of Shiga toxin from Escherichia coli with human intestinal epithelial cell lines and explants: Stx2 induces epithelial damage in organ culture. Cell. Microbiol. 6:289-301. [DOI] [PubMed] [Google Scholar]
- 55.Shaw, D. J., C. Jenkins, M. C. Pearce, T. Cheasty, G. J. Gunn, G. Dougan, H. R. Smith, M. E. Woolhouse, and G. Frankel. 2004. Shedding patterns of verocytotoxin-producing Escherichia coli strains in a cohort of calves and their dams on a Scottish beef farm. Appl. Environ. Microbiol. 70:7456-7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stamm, I., M. Mohr, P. S. Bridger, E. Schröpfer, M. König, W. C. Stoffregen, E. A. Dean-Nystrom, G. Baljer, and C. Menge. 2008. Epithelial and mesenchymal cells in the bovine colonic mucosa differ in their responsiveness to Escherichia coli Shiga toxin 1. Infect. Immun. 76:5381-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stamm, I., M. Wuhrer, R. Geyer, G. Baljer, and C. Menge. 2002. Bovine lymphocytes express functional receptors for Escherichia coli Shiga toxin 1. Microb. Pathog. 33:251-264. [DOI] [PubMed] [Google Scholar]
- 58.Stott, G. H., D. B. Marx, B. E. Menefee, and G. T. Nightengale. 1979. Colostral immunoglobulin transfer in calves. I. Period of absorption. J. Dairy Sci. 62:1632-1638. [DOI] [PubMed] [Google Scholar]
- 59.Strockbine, N. A., L. R. Marques, R. K. Holmes, and A. D. O'Brien. 1985. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect. Immun. 50:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Diemen, P. M., F. Dziva, A. Abu-Median, T. S. Wallis, H. van den Bosch, G. Dougan, N. Chanter, G. Frankel, and M. P. Stevens. 2007. Subunit vaccines based on intimin and Efa-1 polypeptides induce humoral immunity in cattle but do not protect against intestinal colonisation by enterohaemorrhagic Escherichia coli O157:H7 or O26:H. Vet. Immunol. Immunopathol. 116:47-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Donkersgoed, J., D. Hancock, D. Rogan, and A. A. Potter. 2005. Escherichia coli O157:H7 vaccine field trial in 9 feedlots in Alberta and Saskatchewan. Can. Vet. J. 46:724-728. [PMC free article] [PubMed] [Google Scholar]
- 62.Vu-Khac, H., and N. A. Cornick. 2007. Prevalence and genetic profiles of Shiga toxin-producing Escherichia coli strains isolated from buffaloes, cattle, and goats in central Vietnam. Vet. Microbiol. 126:356-363. [DOI] [PubMed] [Google Scholar]
- 63.Watarai, S., Tana, K. Inoue, Y. Kushi, E. Isogai, K. Yokota, K. Naka, K. Oguma, and H. Kodama. 2001. Inhibition of Vero cell cytotoxic activity in Escherichia coli O157:H7 lysates by globotriaosylceramide, Gb3, from bovine milk. Biosci. Biotechnol. Biochem. 65:414-419. [DOI] [PubMed] [Google Scholar]
- 64.Widiasih, D. A., N. Ido, K. Omoe, S. Sugii, and K. Shinagawa. 2004. Duration and magnitude of faecal shedding of Shiga toxin-producing Escherichia coli from naturally infected cattle. Epidemiol. Infect. 132:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wieler, L. H., R. Bauerfeind, and G. Baljer. 1992. Characterization of Shiga-like toxin producing Escherichia coli (SLTEC) isolated from calves with and without diarrhoea. Zentralbl. Bakteriol. 276:243-253. [DOI] [PubMed] [Google Scholar]
- 66.Wieler, L. H., B. Busse, H. Steinrück, L. Beutin, A. Weber, H. Karch, and G. Baljer. 2000. Enterohemorrhagic Escherichia coli (EHEC) strains of serogroup O118 display three distinctive clonal groups of EHEC pathogens. J. Clin. Microbiol. 38:2162-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wieler, L. H., A. Schwanitz, E. Vieler, B. Busse, H. Steinrück, J. B. Kaper, and G. Baljer. 1998. Virulence properties of Shiga toxin-producing Escherichia coli (STEC) strains of serogroup O118, a major group of STEC pathogens in calves. J. Clin. Microbiol. 36:1604-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson, J. B., S. A. McEwen, R. C. Clarke, K. E. Leslie, R. A. Wilson, D. Waltner-Toews, and C. L. Gyles. 1992. Distribution and characteristics of verocytotoxigenic Escherichia coli isolated from Ontario dairy cattle. Epidemiol. Infect. 108:423-439. [DOI] [PMC free article] [PubMed] [Google Scholar]