Abstract
The ability of Staphylococcus aureus to colonize the human nares is a crucial prerequisite for disease. IsdA is a major S. aureus surface protein that is expressed during human infection and required for nasal colonization and survival on human skin. In this work, we show that IsdA binds to involucrin, loricrin, and cytokeratin K10, proteins that are present in the cornified envelope of human desquamated epithelial cells. To measure the forces and dynamics of the interaction between IsdA and loricrin (the most abundant protein of the cornified envelope), single-molecule force spectroscopy was used, demonstrating high-specificity binding. IsdA acts as a cellular adhesin to the human ligands, promoting whole-cell binding to immobilized proteins, even in the absence of other S. aureus components (as shown by heterologous expression in Lactococcus lactis). Inhibition experiments revealed the binding of the human ligands to the same IsdA region. This region was mapped to the NEAT domain of IsdA. The NEAT domain also was found to be required for S. aureus whole-cell binding to the ligands as well as to human nasal cells. Thus, IsdA is an important adhesin to human ligands, which predominate in its primary ecological niche.
Staphylococcus aureus frequently occurs as a commensal of humans. It can cause a variety of life-threatening diseases that often are difficult to treat, owing to the organism's commonly found resistance to clinically used antibiotics (15, 29, 44, 53). A crucial facet of infection is the ability of the pathogen to adhere to host tissues. S. aureus possesses an extensive array of surface protein adhesins with a broad substrate repertoire, and many of them are able to bind multiple ligands. Significant functional overlap also exists, with several adhesins being able to bind the same host proteins (reviewed in references 9 and 17). The purpose of such overlap is not well understood, but the possession of a wide-ranging adhesive capability could allow the production of specific components under a broad range of environmental conditions. This seems particularly likely given that S. aureus can infect a spectrum of different host tissues and is the etiological agent of a wide range of pathologies. However, the human nares are the main niche for S. aureus, and the nasal colonization of patients and health care workers is a crucial prerequisite for disease, which occurs when the bacterium spreads to normally sterile parts of the body (30, 52, 55, 56, 60). Thus, understanding the molecular basis of nasal colonization and, consequently, the development of novel prevention strategies are key goals in reducing nosocomial infections.
S. aureus colonizes the moist squamous epithelium of the anterior nares. This is due in part to the ability of the bacterium to adhere to desquamated epithelial cells (corneocytes) of the stratum corneum. Two ubiquitous surface proteins, clumping factor B (ClfB) and iron-regulated surface determinant protein A (IsdA), promote adhesion to nasal cells in vitro and the colonization of the nares of rodents (8, 37, 42) and, in the case of ClfB, the nares of humans (57). In fact, both proteins can be used in vaccination experiments to protect against nasal carriage and thus have the potential to be useful in the hospital setting to reduce the prevalence of S. aureus and, consequently, the burden of disease (8, 42). IsdA is an S. aureus surface protein that is negatively regulated by environmental iron (7, 31, 32, 34, 49, 59). It is able to bind a broad spectrum of host extracellular matrix and serum proteins (7, 20, 32, 49, 51), as well as promoting nasal colonization and binding to desquamated nasal epithelial cells (8). However, even though IsdA can bind fibronectin, fibrinogen, heme, etc., it is unknown, and in many cases unlikely, that these interactions are important in nasal cell adhesion. The ability of IsdA to bind several purified human ligands in vitro has been mapped to a segment know as the near iron transporters (NEAT) domain (1, 7, 20). However, here again the relevance of this to nasal cell binding and colonization has not been determined. This is made more intriguing by the recent finding that the C-terminal domain of IsdA has an important function in survival on human skin (10). This is mediated by the hydrophilic nature of the C domain, thus making the cells more resistant to hydrophobic compounds such as human skin fatty acids, which are potent antistaphylococcal agents. This creates an apparent paradox, whereby a single protein can mediate both cellular adhesion and resistance to human innate defenses. To resolve this problem, it is important to identify relevant human ligands in the natural niche for S. aureus, the nose, and also to determine the relative roles of the IsdA domains in this interaction.
The upper moist layer of squamous epithelium consists of 10 to 15 layers of anucleated, keratin-rich corneocytes that are surrounded by a proteinaceous structure called the cornified envelope. Protein precursors of the cornified envelope include loricrin (4, 23, 46) and involucrin (27). Loricrin is the major component of the cornified envelope, ca. 65 to 70% (wt/wt) (23), while involucrin is ca. 5% (wt/wt) (45). The proteins are cross-linked by transglutamination. Ceramides and fatty acids are covalently linked to the proteins on the outer face of the envelope (28). Cytokeratin 10 also is uniquely expressed in corneocytes and is exposed on their surface (37). It has been shown previously that S. aureus ClfB binds strongly to cytokeratin 10 in vitro, which implicates keratin as a ligand used by ClfB-expressing bacteria to adhere to corneocytes (37).
In this study, the role of the major S. aureus surface protein IsdA in the interaction with human nasal cornified envelope protein ligands (loricrin, involucrin, and cytokeratin 10) was determined. Specific single-molecule interactions between IsdA and loricrin were established using atomic force microscopy (AFM) (22). Also, the IsdA domain necessary for ligand binding and whole-cell adhesion was mapped. The important implications for the role of IsdA in the adhesion of S. aureus to nasal ligands and its ability to promote bacterial survival in such a harsh environment are discussed.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used in this work are listed in Table 1. Escherichia coli was grown in Luria-Bertani medium, using selection with kanamycin (50 μg/ml) where appropriate. Staphylococcus aureus strains were grown in brain heart infusion medium (Oxoid) or chemically defined CL medium (24). When included, antibiotics were added at the following concentrations: erythromycin, 5 μg/ml; lincomycin, 25 μg/ml; and tetracycline, 2.5 μg/ml. All E. coli and S. aureus cultures were grown at 37°C. Lactococcus lactis was grown in M17 medium (Oxoid) supplemented with 10 μg/ml 0.5% (wt/vol) glucose at 30°C, where appropriate.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Staphylococcus aureus | ||
| SH1000 | Wild type | 25 |
| SRC005 | isdA::Tn917 Emr in SH1000 | 7 |
| SRC008 | isdAΔNEAT in SH1000 | 11 |
| SRC009 | isdAΔC in SH1000 | 11 |
| Escherichia coli | ||
| BL21(DE3) | F−ompT gal [dcm] [lon] hsdSB (rB− mB−) | Novagen |
| TOPP3 | Rifr [F′ proAB lacIqZΔM15 Tn10(Tetr)] Kanr | Stratagene |
| Lactococcus lactis | ||
| NZ9800 | Host for expression plasmids | 13 |
| Plasmids | ||
| pIsdA | IsdA overexpression vector | 7 |
| pMK4 | E. coli-S. aureus shuttle vector | 47 |
| pSRC001 | rIsdA complementation vector | 7 |
| pNZ8148 | L. lactis expression plasmid | 26 |
| pLIsdA | IsdA expression vector | 10 |
| pLΔNEAT | IsdAΔNEAT expression vector | 10 |
| pLΔC | IsdAΔC expression vector | 10 |
| pQE30 | Overexpression plasmid | Qiagen |
| pHuman K10 | pQE30 human cytokeratin 10 expression | 54 |
| pCMC-SPORT6-INV | Involucrin cDNA clone 4749952 | IMAGE consortium, MRC Geneservice |
| pQE30-INV | 6× His-tagged involucrin | This study |
| pET11a-LOR | Loricrin cDNA clone | Gift from D. Roop and P. Koch |
Cloning the involucrin gene.
The coding sequence for involucrin was amplified from pCMV-SPORT6-INV by PCR employing Pfu polymerase and the forward primer CGCGGATCCATGTCCCAGCAACACACAC, incorporating a BamHI site (underlined), and the reverse primer CCCAAGCTTTATTTATGTTTGGGTGGCCAC, incorporating a HindIII site (underlined). The fragment was cloned into pQE30 cut with BamHI and HindIII, forming pQE30-INV.
Expression and purification of recombinant proteins.
The expression of hexahistidine-tagged recombinant IsdA (rIsdA), cytokeratin 10, and involucrin was induced by the addition of 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG) to growing cells. Purification was achieved by using nickel chelate chromatography using the Hi-Trap system (Amersham Biosciences) as described previously (7, 54). Recombinant loricrin is not affinity tagged. It was purified from lysed E. coli T4037.
ELISA analysis of ligand binding.
An enzyme-linked immunosorbent assay (ELISA) was used to analyze the ability of rIsdA to bind ligands. The method used was based on that described previously (6). Briefly, 100 μl of appropriate ligand or bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (5 μg/ml) was added to wells of a 96-well microtiter plate (Nunc) overnight at 4°C. Plates were washed three times with PBST (PBS containing 0.05% [vol/vol] Tween 20), and the remaining protein-binding sites were blocked with 5% (wt/vol) BSA in PBS for 2 h at room temperature. The plates were again washed three times in PBST, and different concentrations of purified rIsdA (0 to 15 μM) diluted in PBS-0.1% (wt/vol) BSA were added. The plates were incubated for a further 1 h before being washed three times, and anti-IsdA (diluted 1:1,000) was added in PBS-0.1% (wt/vol) BSA, followed by 1 h of incubation. Mouse antibodies used for the detection of bound IsdA were raised in a previous study (7). The plates were washed an additional three times, and alkaline phosphatase-conjugated anti-mouse antibodies diluted 1:30,000 in PBS-0.1% (wt/vol) BSA were added and the plates incubated for 1 h. Finally, bound antibodies were detected by using the Sigma Fast p-nitrophenyl phosphate system (Sigma). Plates were read at 405 nm in a Victor microtiter plate reader (Wallac).
Studies of the inhibition of binding were performed by the incubation of rIsdA with various concentrations of inhibitor or BSA (0 to 10 μM) for 1 h at room temperature in PBS-0.1% (wt/vol) BSA as described previously (54). The reaction mixtures then were added to ligand-coated wells, and bound protein was detected as described above.
AFM.
AFM tips and supports were functionalized with IsdA and loricrin molecules as follows. AFM cantilevers (Microlevers; Veeco Metrology Group, Santa Barbara, CA) and silicon wafers (Siltronix, France) were coated using electron beam thermal evaporation with a 5-nm-thick Cr layer followed by a 30-nm-thick Au layer. Before use, the gold-coated surfaces were rinsed with ethanol, dried with a gentle nitrogen flow, and cleaned for 15 min by UV/ozone treatment (Jelight Co., Irvine, CA). Gold tips and gold supports were immersed overnight in ethanol solutions containing 0.05 mM nitrilotriacetate-terminated (5%) (Prochimia) and tri(ethylene glycol) [tri(EG)]-terminated (95%) alkanethiols (kindly supplied by N. L. Abbott) and rinsed with ethanol. Sonication was briefly applied to remove alkanethiol aggregates that can be adsorbed. The SAM (self-assembled monolayer)-coated surfaces then were immersed in a 40 mM aqueous solution of NiSO4 (pH 7.2) for 1 h and rinsed with PBS. Finally, the samples were incubated in PBS with 2 μM His-tagged IsdA for 2 h and further rinsed several times with PBS. For loricrin and BSA, gold supports were immersed for 36 h in an ethanol solution containing a 20 μM mixture (95:5, mol/mol) of alkanethiols terminated with oligo(EG) (OEG) and OEG propionic acid (kindly supplied by H. J. Gruber). Following a rinse with ethanol, sonication was briefly applied to remove alkanethiol aggregates that may be adsorbed. The supports were immersed for 30 min into MilliQ water (Millipore) containing 20 g/liter N-hydroxysuccinimide (NHS) (Aldrich) and 50 g/liter 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) (Sigma), rinsed with MilliQ water, incubated with 10 μg/liter loricrin or BSA (sigma) in PBS for 3 h, further rinsed, and then used immediately.
AFM images and force-distance curves were obtained in PBS at room temperature using a Picoforce Multimode AFM (Veeco Metrology Group, Santa Barbara, CA). The supports were immobilized on a steel sample puck using a small piece of adhesive tape. The mounted samples were immediately transferred into the AFM liquid cell while avoiding dewetting. All force curves were recorded with a maximum applied force of ∼450 pN. The spring constants of the cantilevers were measured using the thermal noise method (Picoforce; Veeco Metrology Group), yielding values (0.011 N/m) that were slightly larger than those announced by the manufacturer (0.01 N/m). To account for the flexibility of the biomolecules, loading rates (in piconewtons per second) were estimated by multiplying the tip retraction velocity (in nanometers per second) by the slope of the rupture peaks (in piconewtons per nanometer).
Bacterial interactions with immobilized ligands.
The binding of mid-log-growth-phase cells to immobilized ligands was measured using a previously described method (36).
Bacterial adherence to squamous nasal epithelial cells.
Squamous cells were harvested from the anterior nares of healthy donors, and the binding (37) of S. aureus grown in iron-free medium (7) was assayed. Comparisons between strains were performed using Student's t test.
RESULTS AND DISCUSSION
rIsdA binds cytokeratin K10, loricrin, and involucrin.
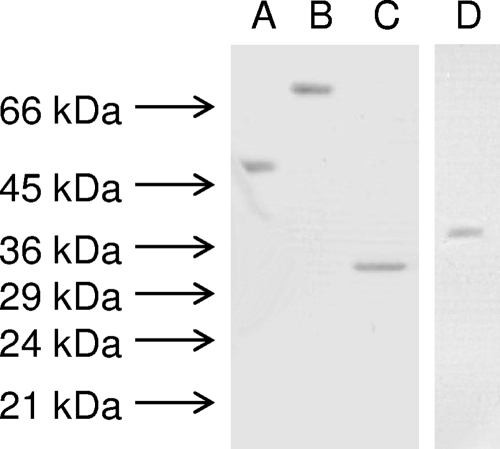
IsdA previously has been shown to be an adhesin, mediating binding between S. aureus and human nasal cells (8). However, the nasal cell ligands with which IsdA interacts are unknown. To address this, three important human corneocyte proteins and IsdA were overexpressed in E. coli and purified. The purified, recombinant proteins had apparent molecular masses of approximately 54, 69, 30, and 36 kDa, as expected for cytokeratin K10, involucrin, loricrin, and IsdA, respectively (Fig. 1). Also, each protein appeared as a single well-defined band.
FIG. 1.
Coomassie blue-stained sodium dodecyl sulfate-12% (wt/vol) polyacrylamide gel electrophoresis of the recombinant proteins cytokeratin K-10 (A), involucrin (B), loricrin (C), and rIsdA (D). All lanes contain ca. 0.5 μg of recombinant protein. The sizes of molecular mass markers also are shown.
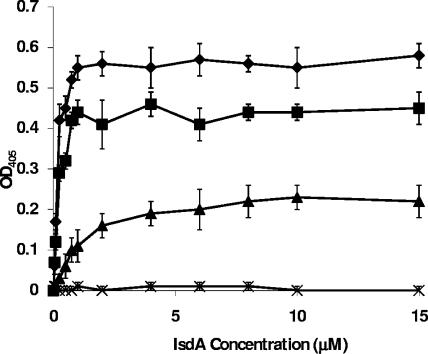
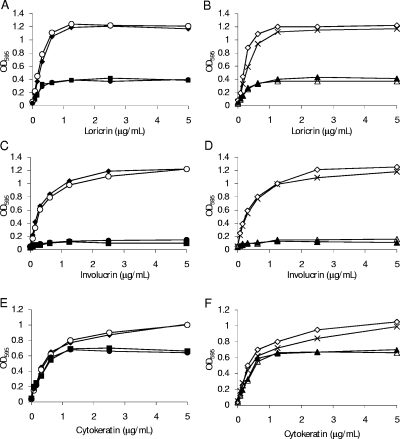
The ability of rIsdA to bind to immobilized recombinant cytokeratin K10, loricrin, and involucrin was assayed by ELISA (Fig. 2). Binding to all three ligands occurred in a dose-dependent and saturable manner (Fig. 2). For cytokeratin K10, saturation occurred at ca. 8 μM and an apparent, approximate dissociation constant (Kd) of 1 μM was calculated from the concentration, giving half-maximum binding. The saturation of binding to loricrin occurred at around 1 μM, and the apparent, approximate Kd of 0.15 μM was calculated. Binding to involucrin was saturable at approximately 1 μM, and the apparent, approximate Kd was estimated to be 0.25 μM. These apparent Kds are in the same range as that observed for IsdA binding to fibrinogen, fibronectin, and other human ligands according to ELISA and surface plasmon resonance (7). IsdA is produced under conditions of iron limitation, which is a marker for host association (7). This is reinforced by the roles of IsdA in nasal colonization, human skin survival, and its expression during human infection (8). Thus, IsdA is present in a wide range of host niches where different protein ligands are likely to predominate.
FIG. 2.
Binding of rIsdA to microtiter plate wells coated with loricrin (⧫), involucrin (▪), cytokeratin K10 (▴), or BSA (X). Increasing concentrations of rIsdA were incubated in wells for 1 h at room temperature. Bound protein was detected with mouse anti-IsdA antibodies and anti-mouse alkaline phosphatase-conjugated antibodies. Results are the means of triplicate wells from three independent experiments. OD405, optical density at 405 nm.
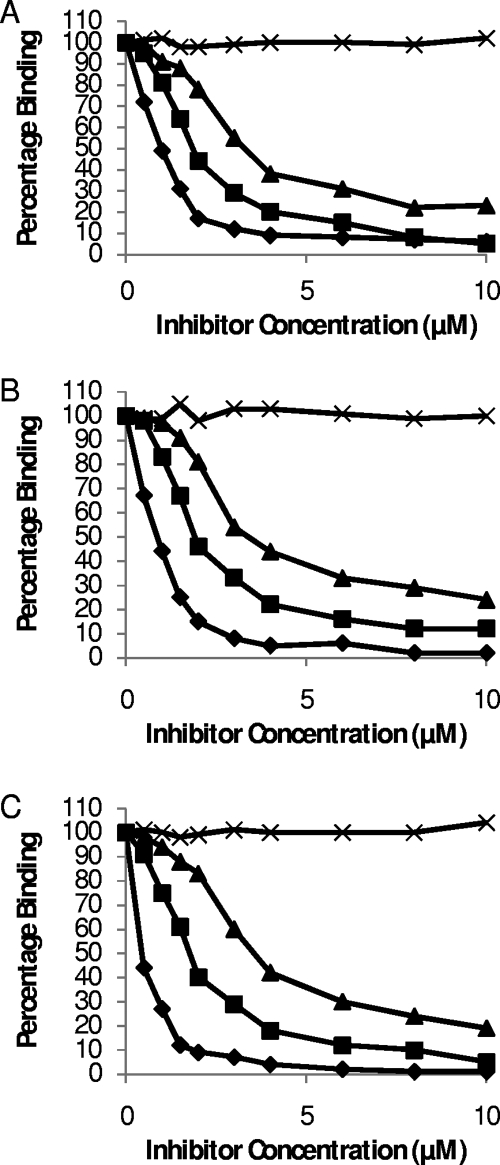
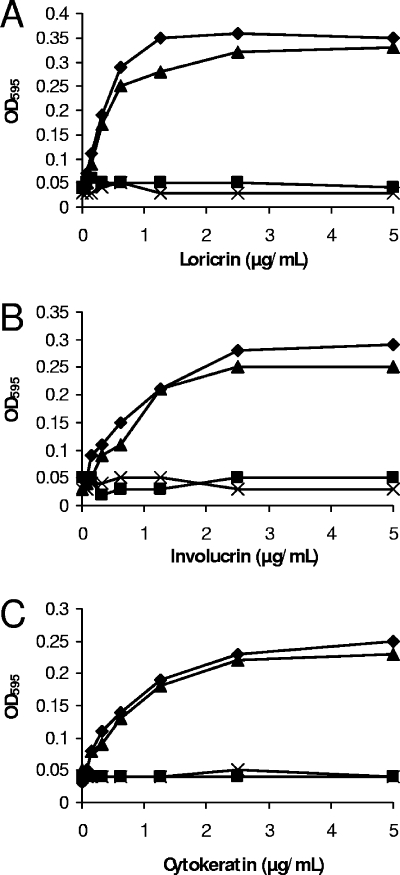
IsdA, although able to bind many ligands, seemingly cannot do so simultaneously. This was shown by the fact that cytokeratin 10, involucrin, or loricrin in solution could inhibit the binding of rIsdA to each of the ligands when immobilized (Fig. 3). In each case, the soluble ligand caused inhibition, whereas BSA had no effect. This suggests that the ligands bound specifically to the same region of IsdA. Interestingly, other S. aureus cell surface proteins have been shown to bind multiple ligands through a single domain (9). Protein A binds the Fc region of immunoglobulin G and von Willebrand factor via a common domain, with the binding of either ligand being competitive (38). A highly expressed, broad-spectrum, host ligand binding protein will give S. aureus adhesive capacity in the wide range of niches that this pathogen is able to inhabit. The ligand(s) to which IsdA is bound in vivo likely is determined by the local prevalence, and the many IsdA molecules on an individual bacterial cell may be bound to different ligands.
FIG. 3.
Inhibition of rIsdA binding to immobilized ligands loricrin (A), involucrin (B), and cytokeratin (C). rIsdA was preincubated for 1 h at room temperature with increasing concentrations of loricrin (⧫), involucrin (▪), cytokeratin K10 (▴), or BSA (X) before incubation in protein-coated wells. Bound protein was detected with mouse anti-IsdA antibodies and anti-mouse alkaline phosphatase-conjugated antibodies. Values represent the means of triplicate wells taken from three independent experiments.
Single-molecule force spectroscopy of the IsdA-loricrin interaction.
During recent years, AFM has been used increasingly to measure the forces between receptors and ligands down to the single-molecule level (2, 3, 21, 22). In microbiology, the interaction forces of single adhesin molecules have been explored, both on model surfaces and on live bacteria (14, 50).
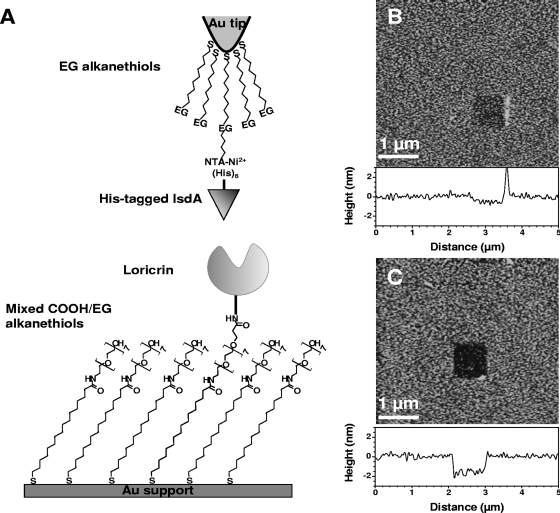
As loricrin is the most abundant protein of the cornified envelope, we measured the forces and the dynamics of single IsdA-loricrin interactions using AFM. To this end, loricrin was covalently immobilized onto flat supports modified with mixed SAMs terminated with OEG and OEG propionic acid (Fig. 4A). Histidine-tagged rIsdA proteins were attached, via their C-terminal end, to gold-coated AFM tips modified with Ni2+-nitrilotriacetate and tri(EG)-terminated alkanethiols. These two methods allow proteins to be immobilized at low density, thus ensuring single-molecule recognition.
FIG. 4.
Strategy for measuring the IsdA-loricrin interaction forces using AFM. (A) Schematics of the surface chemistry used to functionalize AFM tips and supports with IsdA and loricrin (see the text for details). (B and C) AFM images of the biologically modified supports in PBS confirming the presence of smooth, homogeneous IsdA (B) and loricrin (C) layers. To determine the thickness of the layers, small square areas first were scanned at large forces (>25 nN), followed by imaging 5- by 5-μm images of the same areas under smaller forces.
The quality of functionalized, flat surfaces was assessed by using AFM topographic imaging in aqueous solution. Figures 4B and C show that the IsdA and loricrin surfaces were smooth and stable upon repeated scanning. To confirm the presence of peptide layers, a small area was first recorded at large forces (>25 nN) for short time periods, followed by imaging a larger image of the same area under normal load. Figures 4B and C show that imaging at high forces resulted in pushing the grafted material aside, thereby revealing the underlying support. The thickness of the removed films was found to be 0.7 ± 0.1 nm and 1.8 ± 0.2 nm, confirming the presence of protein layers on both surfaces.
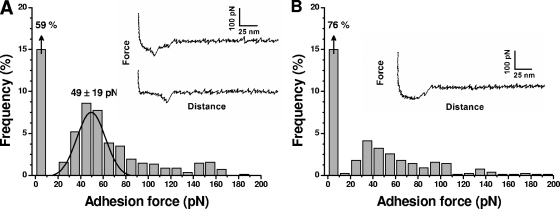
Force-distance curves were recorded first between IsdA and loricrin surfaces, using a pulling speed of 1,000 nm·s−1 (Fig. 5A). A significant fraction of the curves (41%) displayed single adhesion forces at short rupture distances, with the remaining measurements exhibiting no adhesion. The corresponding adhesion force histogram displayed a well-defined maximum at 49 ± 19 pN that we attribute to the rupture of single IsdA-loricrin complexes. This value is comparable to that reported for other cell adhesion proteins (14, 50). Adhesion forces in the 100- to 200-pN range also were observed and attributed to the simultaneous detection of two or three interactions. The specificity of the measured forces was demonstrated by showing a reduction of adhesion frequency (from 41 to 24%) when the curves were recorded on a BSA-terminated support instead of a loricrin support (Fig. 5B).
FIG. 5.
Single-molecule force spectroscopy of the IsdA-loricrin interaction. (A) Representative force curves and an adhesion force histogram (n = 853) obtained in PBS between an IsdA tip and a loricrin support, using approach and retraction speeds of 1,000 nm/s and an interaction time of 500 ms. Similar data were obtained with more than 10 different tips and independent supports. (B) The same experiment as that described for panel A was performed, except that a BSA-terminated support was used instead of a loricrin support.
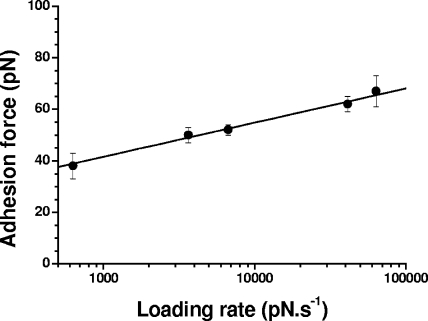
We also explored the dynamics of the interaction by measuring the binding force as a function of the loading rate (dynamic force spectroscopy) (16, 18, 33, 35). Figure 6 shows that the mean adhesion force (F) increased linearly with the logarithm of the loading rate (r), as observed for other receptor-ligand systems (16, 18, 19, 33). From this relationship, the length scale of the energy barrier (xβ) was assessed from the slope of the F versus ln(r) plot, xβ = 0.31 nm, while extrapolation to zero forces yielded the kinetic off-rate constant of dissociation at zero force, koff = (rF = 0)(xβ/kBT) = 0.12 s−1, where kB is the Boltzmann constant and T is the absolute temperature. Taken together, the above-described single-molecule data indicate that IsdA binds to loricrin with high specificity. As loricrin also is a predominant protein of the cornified envelope, it may well be a relevant ligand for the adhesion of S. aureus in this niche.
FIG. 6.
Dynamics of the IsdA-loricrin interaction. Shown is a plot of the adhesion force as a function of the logarithm of the loading rate applied during retraction, while keeping constant the interaction time (500 ms) and the approach speed (1,000 nm/s). Data represent the means of 100 measurements, and the bars indicate standard errors from the means.
Adhesion of S. aureus to immobilized cytokeratin K10, loricrin, and involucrin is enhanced by IsdA.
The binding of recombinant protein to a ligand in vitro does not necessarily equate to any physiological relevance to the living cell. It was important to determine if IsdA on the S. aureus cell surface could promote adhesion to the corneocyte proteins. Adhesion experiments were performed with wild-type S. aureus SH1000 and a mutant lacking IsdA (SRC005). S. aureus SH1000 adhered in a dose-dependent and saturable fashion to loricrin and involucrin, whereas SRC005 (isdA) adhered poorly (Fig. 7). Interestingly for the major cornified envelope proteins loricrin and involucrin, IsdA is the major S. aureus adhesin, as SRC005 (isdA) shows only limited binding compared to that of SH1000, which is not seen for cytokeratin K10. To verify that cellular adhesion to ligands was mediated by IsdA alone and was not dependent on the context of other surface components, isdA was expressed in L. lactis. This heterologous host has been used as an expression system for gram-positive surface proteins (10, 37). The binding of L. lactis to loricrin, involucrin, and cytokeratin K10 was greatly enhanced by the production of IsdA, thus demonstrating that IsdA itself has the capacity to be an effective adhesin to human nasal cell ligands (Fig. 8A to C).
FIG. 7.
S. aureus adherence to immobilized ligands. Wells in ELISA plates were coated with different concentrations of human loricrin (A and B), involucrin (C and D), or cytokeratin (D and E). After being bound and fixed, S. aureus cells were stained with crystal violet and the optical density was measured at 595 nm (OD595). Graphs A, C, and E show wild-type S. aureus SH1000 compared to isdA mutants. Graphs B, D, and F show data derived using strains with an isdAΔNEAT or isdAΔC mutation. In all experiments, the following S. aureus strains were used: ⧫, SH1000 (wild type); ▪, SRC005 (isdA); ▴, SRC008 (isdAΔNEAT); X, SRC009 (isdAΔC); ○, SRC005 [pSRC001]; •, SRC005 [pMK4]; ⋄, SRC008 [pSRC001]; and ▵, SRC008 [pMK4]. Note that the symbols ○/⧫, •/▪, ⋄/×, and ▴/▵ overlap extensively. Values represent the means of triplicate wells taken from three independent experiments.
FIG. 8.
L. lactis adherence to immobilized ligands. Wells in ELISA plates were coated with different concentrations of human loricrin (A), involucrin (B), or cytokeratin (C). After being bound and fixed, L. lactis cells were stained with crystal violet and the optical density was measured at 595 nm (OD595). L. lactis was used in all experiments, carrying the following plasmids: ⧫, pLIsdA; ▪, pLΔNEAT; ▴, pLΔC; and X, pNZ8148.
To our knowledge, this is the first report of a bacterial interaction with either loricrin or involucrin. These are very prevalent proteins in the cornified envelope (23, 45) and may constitute important host ligands for interaction with many bacterial species able to inhabit such an environment, including several important human pathogens. Bacterial interactions with mammalian keratins are well documented. Cytokeratin K10 is the predominant type I keratin in squamous epithelial cells and, despite keratins being regarded as intracellular molecules, is exposed on the surface of desquamated nasal epithelial cells and keratinocytes (37). Adherence to it by S. aureus is a common feature of laboratory and clinical strains that may be important for the establishment of nasal colonization (37). Adherence to mammalian cells, mediated by keratins, has been reported in other bacterial species. Streptococcus agalactiae, Streptococcus pyogenes, and Enterococcus faecalis all bind to cytokeratin 8 (48), while S. agalactiae also has been reported to bind cytokeratin K4, promoting adhesion to epithelial cells (41). Porphyromonas gingivalis fimbriae bind to surface epithelial cytokeratin in the colonization of the oral mucus membranes (43), Burkholderia cepacia adheres to cytokeratin K13 in the airway epithelia (40), and the entry of Salmonella enterica into eukaryotic cells is mediated by an interaction between SipC, a secreted invasion protein, and cytokeratin 18 (5).
Role of IsdA domains in ligand binding and interaction with human squamous nasal epithelial cells.
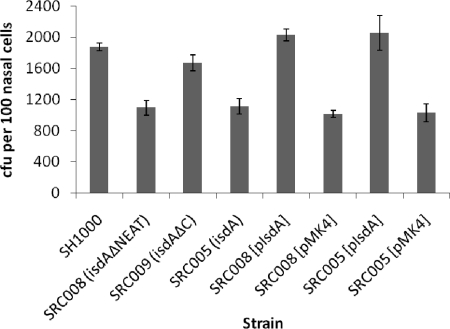
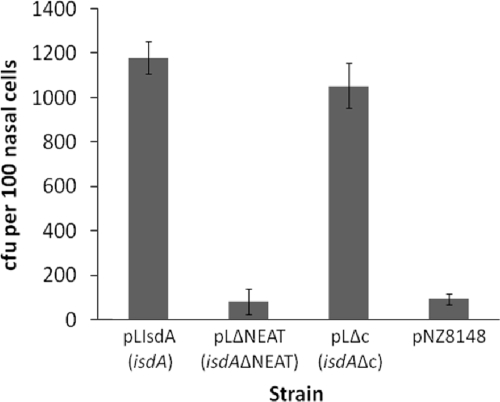
The ligand binding inhibition experiments (Fig. 3) suggested that cytokeratin K10, loricrin, and involucrin all bind to the same IsdA region. Previous studies have shown that many protein ligands bind to the N-terminal NEAT domain of IsdA (7, 11), whereas the C-terminal domain is involved in resistance to human skin fatty acids and antimicrobial peptides (10). Using the whole-cell adhesion assay, the NEAT domain was found to be responsible for the IsdA-mediated binding of S. aureus to loricrin, involucrin, and cytokeratin K10, as a mutant lacking the NEAT domain (SRC008) demonstrated reduced binding, whereas SRC009 (isdAΔC) did not (Fig. 7). This was verified in the heterologous host L. lactis, where whole-cell ligand binding was enhanced by the production of IsdAΔC but not by IsdAΔNEAT (Fig. 8). This strongly suggested that it is the NEAT domain that is responsible for the IsdA-mediated binding of S. aureus to human nasal cells, and thus it likely has a important role in colonization. Binding to human squamous nasal epithelial cells by S. aureus and L. lactis expressing various forms of IsdA was examined. As we reported previously (8), there was a significant increase in the binding of the wild-type strain to nasal cells when the bacteria expressed IsdA (P < 0.01). Here, strain SRC009 (isdAΔC), which expresses a mutant form of IsdA containing the ligand-binding NEAT domain, was significantly more able to bind nasal epithelial cells than SRC005 (isdA) or SRC008 (isdAΔNEAT) (P < 0.01 in both cases) (Fig. 9). There was no difference in the binding activity of SRC008 (isdAΔNEAT) compared to that of SRC005 (isdA) (P > 0.5). Furthermore, using L. lactis, significantly higher levels of binding for cells expressing wild-type or ΔC domain versions of the protein were observed compared to the levels of cells that expressed either the ΔNEAT domain (P < 0.004) or lacked IsdA altogether (P < 0.003) (Fig. 10). Thus, it is the NEAT domain of IsdA that exhibits the capacity to bind whole cells of S. aureus to human nasal cells. Overall binding to nasal cells requires the cumulative activities of at least the surface proteins IsdA, ClfB, and SdrCD and thus is multifactorial (8, 12, 37). However, even given this apparent redundancy, individual proteins are important, as an isdA or clfB mutation leads to attenuated nasal carriage (8, 42, 57).
FIG. 9.
Bacterial adherence to human desquamated epithelial cells. Staphylococcus aureus strain SH1000 (wild type [wt]) and isogenic isdA, isdAΔNEAT, and isdAΔC mutants, complemented where appropriate, were grown in CL broth. The washed cells then were incubated with human nasal epithelial cells before bacterial cell enumeration by microscopy. Results are expressed as the means from three independent experiments.
FIG. 10.
Bacterial adherence to human desquamated epithelial cells. Lactococcus lactis displaying wild-type IsdA (pLIsdA), IsdAΔNEAT (pLΔNEAT), IsdAΔC (pLΔC), or no extra proteins (pNZ8148) was used. The washed cells then were incubated with human nasal epithelial cells before bacterial cell enumeration by microscopy. Results are expressed as the means from three independent experiments.
IsdA is a multifunctional S. aureus surface protein that is required for nasal carriage and presents ubiquitously in a wide range of strains isolated in the clinic and from human nasal carriers (8). It acts both as an adhesin for several human ligands and as a resistance factor against human innate defense mechanisms. These distinct roles are accounted for by the two-domain nature of IsdA. The mature protein is present in the cell wall and is linked by a C-terminal sorting signal to the peptidoglycan (7, 20, 34, 49). The N-terminal NEAT domain apparently is responsible for all of the ligand binding capacity of IsdA, including the three human corneocyte envelope proteins analyzed here. Such binding likely makes a contribution to human nasal carriage, allowing the bacterium to adhere to its host. Conversely, the C-terminal domain of IsdA is required for survival on live human skin via its ability to alter cellular biophysical properties, resulting in more hydrophilic cells with increased resistance to skin fatty acids (11). A question remains as to why an adhesin able to bind loricrin might also be involved in fatty acid resistance. It is extremely interesting that such fatty acids can be found in the cornified envelope (39, 58) attached to serine residues of exposed loricrin (28). It therefore is tempting to speculate that IsdA has evolved to mediate adhesion to an important host cell protein, loricrin, which itself is covalently attached to an otherwise bactericidal fatty acid. The decreased cellular hydrophobicity conferred by IsdA then may be able to abrogate the ability of the fatty acid to form lethal interactions with the bacterium.
Acknowledgments
This work was funded by Biosynexus Inc. (S.J.F.), the Medical Research Council (S.J.F.), The Wellcome Trust (T.J.F.), Science Foundation Ireland (T.J.F.), the National Foundation for Scientific Research (FNRS) (Y.F.D.), the Foundation for Training in Industrial and Agricultural Research (Y.F.D.), the Université Catholique de Louvain (Fonds Spéciaux de Recherche) (Y.F.D.), the Federal Office for Scientific, Technical and Cultural Affairs (Interuniversity Poles of Attraction Programme) (Y.F.D.), and the Research Department of the Communauté Française de Belgique (Concerted Research Action) (Y.F.D.). Y.F.D. is a Research Associate of the FNRS.
We thank Dennis Roop and Peter Koch, University of Colorado, Denver, for their generous gift of pET11b-LOR and for helpful discussions.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Andrade, M. A., F. D. Ciccarelli, C. Perez-Iratxeta, and P. Bork. 2002. NEAT: a domain duplicated in genes near the components of a putative Fe3+ siderophore transporter from gram-positive bacteria. Genome Biol. 347.1-47.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner, W., P. Hinterdorfer, W. Ness, A. Raab, D Vestweber, H. Schindler, and D. Drenckhahn. 2000. Cadherin interaction probed by atomic force microscopy. Proc. Natl. Acad. Sci. USA 974005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit, M., D. Gabriel, G. Gerisch, and H. E. Gaub. 2000. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat. Cell Biol. 2313-317. [DOI] [PubMed] [Google Scholar]
- 4.Candi, E., G. Melino, G. Mei, E. Tarcsa, S.-I. Chung, L. N. Marekov, and P. M. Steinert. 1995. Biochemical, structural, and transglutaminase substrate properties of human loricrin, the major epidermal cornified cell envelope protein. J. Biol. Chem. 27026382-26390. [DOI] [PubMed] [Google Scholar]
- 5.Carlson, S. A., M. B. Omary, and B. D. Jones. 2002. Identification of cytokeratins as accessory mediators of Salmonella entry into eukaryotic cells. Life Sci. 701415-1426. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, S. R., L. G. Harris, R. G. Richards, and S. J. Foster. 2002. Analysis of Ebh, a 1.1-megadalton cell wall-associated fibronectin-binding protein of Staphylococcus aureus. Infect. Immun. 706680-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke, S. R., M. D. Wiltshire, and S. J. Foster. 2004. IsdA of Staphylococcus aureus is a broad spectrum, iron-regulated adhesin. Mol. Microbiol. 511509-1519. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, S. R., K. J. Brummell, M. J. Horsburgh, P. W. McDowell, S. A. Syed Mohamad, M. R. Stapleton, J. Acevedo, R. C. Read, N. P. J. Day, S. J. Peacock, J. J. Mond, J. F. Kokai-Kun, and S. J. Foster. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J. Infect. Dis. 1931098-1108. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, S. R., and S. J. Foster. 2006. Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 51187-224. [DOI] [PubMed] [Google Scholar]
- 10.Clarke, S. R., R. Mohamed, L. Bian, A. F. Routh, J. F. Kokai-Kun, J. J. Mond, A. Tarkowski, and S. J. Foster. 2007. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe 1199-212. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, S. R., and S. J. Foster. 2008. IsdA protects Staphylococcus aureus against the bactericidal protease activity of apolactoferrin. Infect. Immun. 761518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan, R. M., H. Miajlovic, and T. J. Foster. 2009. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Ruyter, P. G., O. P. Kuipers, M. M. Beerthuyzen, I. van Alen-Boerrigter, and W. M. de Vos. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J. Bacteriol. 1783434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupres, V., F. D. Menozzi, C Locht, B. H. Clare, N. L. Abbott, S. Cuenot, C. Bompard, D Raze, and Y. F. Dufrêne. 2005. Nanoscale mapping and functional analysis of individual adhesins on living bacteria. Nat. Methods 2515-520. [DOI] [PubMed] [Google Scholar]
- 15.Emori, T. G., and R. P. Gaynes. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans, E., and K. Ritchie. 1997. Dynamic strength of molecular adhesion bonds. Biophys. J. 721541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster, T. J. 2002. Surface protein adhesins of staphylococci, p. 3-26. In M. Wilson (ed.), Bacterial adhesion to host tissues: mechanisms and consequences. Cambridge University Press, Cambridge, United Kingdom.
- 18.Fritz, J., A. G. Katopodis, F. Kolbinger, and D. Anselmetti. 1998. Force-mediated kinetics of single P-selectin/ligand complexes observed by atomic force microscopy. Proc. Natl. Acad. Sci. USA 9512283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert, Y., M Deghorain, L. Wang, B. Xu, P. D. Pollheimer, H. J. Gruber, J. Errington, J. B. Hallet, X. Haulot, C. Verbelen, P. Hols, and Y. F. Dufrêne. 2007. Single-molecule force spectroscopy and imaging of the vancomycin/D-Ala-D-Ala interaction. Nano Lett. 7796-801. [DOI] [PubMed] [Google Scholar]
- 20.Grigg, J. C., C. L. Vermeiren, D. E. Heinrichs, and M. E. P. Murphy. 2007. Haem recognition by a Staphylococcus aureus NEAT domain. Mol. Microbiol. 63139-149. [DOI] [PubMed] [Google Scholar]
- 21.Hinterdorfer, P., W. Baumgartner, H. J. Gruber, K. Schilcher, and H. Schindler. 1996. Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc. Natl. Acad. Sci. USA 933477-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinterdorfer, P., and Y. F. Dufrêne. 2006. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 3347-355. [DOI] [PubMed] [Google Scholar]
- 23.Hohl, D., T. Mehrel, U. Lichti, M. L. Turner, D. R. Roop, and P. M. Steinert. 1991. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J. Biol. Chem. 2666626-6636. [PubMed] [Google Scholar]
- 24.Horsburgh, M. J., E. Ingham, and S. J. Foster. 2001. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 183468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 1845457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuipers, O. P., P. G. G. A. de Ruyter, M. Kleerebezem, and W. M. de Vos. 1998. Controlled overproduction of proteins by lactic acid bacteria. J. Biotechnol. 6415-21. [DOI] [PubMed] [Google Scholar]
- 27.Lazo, N. D., and D. T. Downing. 1999. A mixture of alpha-helical and 3(10)-helical conformations for involucrin in the human epidermal corneocyte envelope provides a scaffold for the attachment of both lipids and proteins. J. Biol. Chem. 27437340-37344. [DOI] [PubMed] [Google Scholar]
- 28.López, O., M. Cócera, P. W. Wertz, C. López-Iglesias, and A. de la Maza. 2007. New arrangement of proteins and lipids in the stratum corneum cornified envelope. Biochim. Biophys. Acta 1768521-529. [DOI] [PubMed] [Google Scholar]
- 29.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 30.Luzar, M. A., G. A. Coles, B. Faller, A. Slingeneyer, G. D. Dah, C. Briat, C. Wone, Y. Knefati, M. Kessler, and F. Peluso. 1990. Staphylococcus aureus nasal carriage and infection in patients on continuous ambulatory peritoneal dialysis. N. Engl. J. Med. 322505-509. [DOI] [PubMed] [Google Scholar]
- 31.Mazmanian, S. K., H. Ton-That, K. Su, and O. Schneewind. 2002. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA 992293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazmanian, S. K., E. P. Skaar, A. H. Gasper, M. Humayan, P. Gorniki, J. Jelenska, A. Joachmiak, D. M. Missiakas, and O. Schneewind. 2003. Passage of heme iron across the envelope of Staphylococcus aureus. Science 299906-909. [DOI] [PubMed] [Google Scholar]
- 33.Merkel, R., P. Nassoy, A. Leung, K. Ritchie, and E. Evans. 1999. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 39750-53. [DOI] [PubMed] [Google Scholar]
- 34.Morrissey, J. A., A. Cockayne, J. Hammacott, K. Bishop, A. Denman-Johnson, P. J. Hill, and P. Williams. 2002. Conservation, surface exposure, and in vivo expression of the Frp family of iron-regulated cell wall proteins in Staphylococcus aureus. Infect. Immun. 732399-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nevo, R., C. Stroh, F. Kienberger, D. Kaftan, V. Brumfeld, M. Elbaum, Z. Reich, and P. Hinterdorfer. 2003. A molecular switch between alternative conformational states in the complex of Ran and importin beta1. Nat. Struct. Biol. 10553-557. [DOI] [PubMed] [Google Scholar]
- 36.Ní Eidhin, D., S. Perkins, P. François, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30245-257. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien, L. M., E. J. Walsh, R. C. Massey, S. J. Peacock, and T. J. Foster. 2002. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell. Microbiol. 4759-770. [DOI] [PubMed] [Google Scholar]
- 38.O'Seaghdha, M., C. J. van Schooten, S. W. Kerrigan, J. Emsley, G. J. Silverman, D. Cox, P. J. Lenting, and T. J. Foster. 2006. Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions. FEBS J. 2734831-4841. [DOI] [PubMed] [Google Scholar]
- 39.Ponec, M., E. Boelsma, and A. Weerheim. 2000. Covalently bound lipids in reconstructed human epithelia. Acta Derm. Venereol. 8089-93. [PubMed] [Google Scholar]
- 40.Sajjan, U., C. Ackerley, and J. Forstner. 2002. Interaction of cblA/adhesin-positive Burkholderia cepacia with squamous epithelium. Cell. Microbiol. 473-86. [DOI] [PubMed] [Google Scholar]
- 41.Samen, U., B. J. Eikmanns, D. J. Reinscheid, and F. Borges. 2007. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect. Immun. 755405-5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaffer, A. C., R. M. Solinga, J. Cocchiaro, M. Portoles, K. B. Kiser, A. Risley, S. M. Randall, V. Valtulina, P. Speziale, E. Walsh, T. Foster, and J. C. Lee. 2006. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect. Immun. 742145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sojar, H. T., A. Sharma, and R. J. Genco. 2002. Porphyromonas gingivalis fimbriae bind to cytokeratin of epithelial cells. Infect. Immun. 7096-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinberg, J. P., C. C. Clark, and B. O. Hackman. 1996. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin. Infect. Dis. 23255-259. [DOI] [PubMed] [Google Scholar]
- 45.Steinert, P. M., and L. N. Marekov. 1997. Direct evidence that involucrin is a major early isopeptide cross-linked component of the keratinocyte cornified cell envelope. J. Biol. Chem. 2722021-2030. [DOI] [PubMed] [Google Scholar]
- 46.Steven, A. C., and P. M. Steinert. 1994. Protein composition of cornified cell envelopes of epidermal keratinocytes. J. Cell Sci. 107693-700. [PubMed] [Google Scholar]
- 47.Sullivan, M. A., R. E. Yasbin, and F. E. Young. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 2921-26. [DOI] [PubMed] [Google Scholar]
- 48.Tamura, G. S., and A. Nittayajarn. 2000. Group B streptococci and other gram-positive cocci bind to cytokeratin 8. Infect. Immun. 682129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor, J. M., and D. E. Heinrichs. 2002. Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein. Mol. Microbiol. 431603-1614. [DOI] [PubMed] [Google Scholar]
- 50.Verbelen, C., D. Raze, F. Dewitte, C. Locht, and Y. F. Dufrêne. 2007. Single-molecule force spectroscopy of mycobacterial adhesin-adhesin interactions. J. Bacteriol. 1898801-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vermeiren, C. L., M. Pluym, J. Mack, D. E. Heinrichs, and M. J. Stillman. 2006. Characterization of the heme binding properties of Staphylococcus aureus IsdA. Biochemistry 4512867-12875. [DOI] [PubMed] [Google Scholar]
- 52.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344505-509. [DOI] [PubMed] [Google Scholar]
- 53.Waldvogel, F. A. 1995. Staphylococcus aureus (including toxic shock syndrome), p. 1754-1777. In G. L. Mandell, J. E. Bennett, and R. Dolio (ed.), Principles and practice of infectious diseases. Churchill Livingstone, New York, NY.
- 54.Walsh, E. J., L. M. O'Brien, X. Liang, M. Höök, and T. J. Foster. 2004. Clumping factor B, a fibrinogen binding MSCRAMM (microbial surface component recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus also binds the tail region of type I cytokeratin 10. J. Biol. Chem. 27950691-50699. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein, H. J. 1959. The relationship between nasal staphylococcal carriage state and the incidence of post-operative complications. N. Engl. J. Med. 2601303-1308. [DOI] [PubMed] [Google Scholar]
- 56.Wertheim, H. F., M. C. Vos, A. Ott, A. van Belkum, A. Voss, J. A. Kluytmans, P. H. van Keulen, C. M. Vandenbroucke-Grauls, M. H. Meester, and H. A. Verbrugh. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364703-705. [DOI] [PubMed] [Google Scholar]
- 57.Wertheim, H. F. L., E. Walsh, R. Choudhurry, D. C. Melles, H. A. M. Boelens, H. Miajlovic, H. A. Verbrugh, T. Foster, and A. van Belkum. 2008. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 5e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wertz, P. W., and D. T. Downing. 1986. Covalent attachment of omega-hydroxyacid derivatives to epidermal macromolecules: a preliminary characterization. Biochem. Biophys. Res. Commun. 137992-997. [DOI] [PubMed] [Google Scholar]
- 59.Wiltshire, M. D., and S. J. Foster. 2001. Identification and analysis of Staphylococcus aureus components expressed by a model system of growth in serum. Infect. Immun. 695198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu, V. L., A. Goetz, M. Wagener, P. B. Smith, J. D. Rihs, J. Hanchett, and J. J. Zuravleff. 1986. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N. Engl. J. Med. 31591-96. [DOI] [PubMed] [Google Scholar]