Summary
Consistent left-right (LR) patterning is a clinically important embryonic process. However, key questions remain about the origin of asymmetry and its amplification across cell fields. Planar cell polarity (PCP) solves a similar morphogenetic problem, and although core PCP proteins have yet to be implicated in embryonic LR asymmetry, studies of mutations affecting planar polarity, together with exciting new data in cell and developmental biology, provide a new perspective on LR patterning. Here we propose testable models for the hypothesis that LR asymmetry propagates as a type of PCP that imposes coherent orientation onto cell fields, and that the cue that orients this polarization is a chiral intracellular structure.
Introduction
Internal organs of bilaterally symmetric organisms adopt a consistent left-right (LR) asymmetry. Errors in this process are of clinical importance because they cause serious birth defects (Ramsdell, 2005). LR patterning encompasses many of the key themes that fascinate developmental biologists: the mapping of the same morphogenetic problem and similar molecular mechanisms upon different embryonic architectures; a patterning event that cuts across scales of organization; and the linking of epigenetic, biophysical and transcriptionally mediated mechanisms. This is a particularly appropriate time for a fresh synthesis of the data on LR patterning because of diverse recent studies that suggest new ways to understand asymmetry (Aw et al., 2008; Danilchik et al., 2006; Takano et al., 2007; Xu et al., 2007).
LR patterning accomplishes several logical steps (Brown and Wolpert, 1990) (Fig. 1). A midline must be established, and one side made different from the other. Defects in this process result in the loss of asymmetry and in changes in organ number and placement (such as bilateral spleens). LR asymmetry must also become oriented consistently with respect to the anteroposterior (AP) and dorsoventral (DV) axes, so that all individuals are consistently asymmetric. The information needs to be transmitted to multiple organ systems, but midline structures must perform a restriction function to keep left-sided signals from affecting the right side, and vice versa. Ultimately, individual primordia carry out distinct left and right organogenesis programs. Defects in these processes cause a multitude of LR phenotypes.
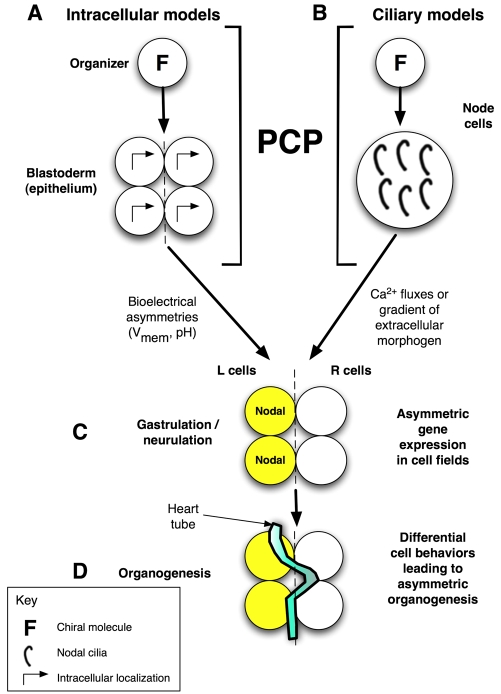
Fig. 1.
Overview of LR patterning phases and proposed role for PCP. Consistent LR asymmetry includes sequential phases of symmetry breaking (providing cells with a consistent orientation along the LR axis), asymmetric gene expression, and organogenesis. (A) In our model, the initial orientation occurs via a cytoskeletal structure (F) in very early blastomeres that we term the LR `organizer'. This involves the coordination of the apical-basal, LR and dorsoventral (DV) axes and is imposed across the embryonic epithelial field by a PCP mechanism (Aw et al., 2008). (B) An alternative cilia-based model is also compatible with this hypothesis as PCP is required to orient the cilia, the chiral flow of which has been proposed to initiate asymmetry (Brueckner, 2001). In the intracellular model (A), the directional information is converted to LR position relative to the midline by the establishment of physiological asymmetries in membrane voltage (Vmem) that redistribute intracellular LR morphogens (Fukumoto et al., 2005). In the ciliary models (B), LR position is dictated to cells by the redistribution of an extracellular signal (e.g. Ca2+). In both models, these steps are followed by (C) cascades of asymmetric gene expression that (D) drive organogenesis. L, left; R, right.
The positioning of organs is determined by cascades of asymmetrically expressed genes (Whitman and Mercola, 2001). Despite exciting recent work on the molecular mechanisms that lie upstream of asymmetric transcription, the field still faces some fundamental questions (Aw and Levin, 2008). What is the first event that computes LR orientation? How are direction and position with respect to the midline communicated from putative LR-organizing centers and imposed over distant cell fields? How conserved are the molecular mechanisms involved throughout phyla and do they reuse modules employed in other morphogenetic events?
The early processes of LR patterning are especially controversial (Levin and Palmer, 2007; Tabin, 2005). One view, motivated by LR randomization observed in mutants of ciliary proteins, holds that LR asymmetry is initiated by motile cilia beating in the extracellular space at the node (Fig. 1B) (Marszalek et al., 1999; Nonaka et al., 1998; Supp et al., 1997). Technologically elegant studies that examined the movement of such cilia showed that their beating generates a leftward fluid flow (Kramer-Zucker et al., 2005; Nonaka et al., 2002; Schweickert et al., 2007) that could set up an extracellular morphogen gradient or differential activation of immotile, mechanosensory cilia (McGrath et al., 2003). These models are attractive because they demonstrate how organismal asymmetry could initiate from the molecular structure of cilia, although there are no data to show that cilia definitively initiate asymmetry rather than being a downstream step in LR patterning. A key feature of this class of models is that asymmetry is first determined at gastrulation; another is that the origin of LR asymmetry is extracellular, as the first detectable molecular difference between the left and right sides occurs in extracellular space. Consistent with this hypothesis, motile cilia are necessary for proper LR patterning in fish, mice and Xenopus (Bisgrove et al., 2005; McGrath et al., 2003; Nonaka et al., 1998; Okada et al., 1999; Schweickert et al., 2007). However, because organisms such as frog, chick, snail, plants and nematodes develop LR asymmetry prior to, or entirely without, motile cilia (Spéder et al., 2007; Levin and Palmer, 2007), this cannot be the source of LR patterning information in all organisms.
By contrast, a second model, which is motivated by data derived first in the chick and frog (Levin, 2006), focuses on intracellular events and physiological mechanisms (Fig. 1A). In Xenopus, the asymmetric transport machinery within early blastomeres (Aw et al., 2008; Qiu et al., 2005) localizes differential ion channels to the prospective left and right sides of the embryo (Adams et al., 2006; Levin et al., 2002; Morokuma et al., 2008). These ion transporters then set up physiological gradients that drive the LR accumulation of morphogens (Esser et al., 2006; Fukumoto et al., 2005). The hallmarks of these models are that LR asymmetry is derived extremely early (long before nodal cilia appear), that asymmetry originates in the orientation of a cytoplasmic structure, that components of this system might be very widely conserved, and that intracellular physiological signals and cell-cell interactions are important in transmitting LR information across cell fields.
The relative merits of these two models have been discussed in detail (Levin and Palmer, 2007; Spéder et al., 2007). Here, we synthesize several recent findings in cell and developmental biology and propose a new model based on two independent but compatible hypotheses: (1) that LR patterning is a kind of planar cell polarity (PCP) that spreads LR information throughout the embryo; and (2) that the origin of the signal that orients the coordinated LR planar array is intracellular, operating in single cells or small groups of cells in which the microtubule cytoskeleton performs the initial computation to orient polarity. Although many important details remain to be clarified regarding the mechanisms of LR and PCP patterning, and although core PCP proteins have yet to be implicated in LR patterning, our proposal is consistent with recent findings and provides an alternative mechanism for how symmetry-breaking and orientation steps that first occur in a small LR coordinator region can be amplified over large cell fields. As our model awaits direct molecular testing, we also outline several specific predictions to guide future experimental tests of these ideas.
PCP solves the same morphogenetic problems as LR patterning
PCP and patterning signal amplification
Tissue function often requires coordinated cell behavior, whether to achieve directed movement in a plane, as in convergent extension during gastrulation, or to produce an oriented field of polarized structures, such as the bristles of the Drosophila wing (Wang and Nathans, 2007). This requires directional information to be present in the plane of a cell field that lies orthogonal to apical-basal polarity. This polarized coordination of cells in a plane is known as PCP (Fig. 2). The existence of a conserved `core' group of PCP genes that are crucial in patterning planar-polarized structures in different organisms, which includes frizzled (fz), dishevelled (dsh), flamingo (fmi; starry night - FlyBase) and prickle (pk) in Drosophila (Axelrod and McNeill, 2002), suggests that a common mechanism exists for the establishment of PCP (Seifert and Mlodzik, 2007).
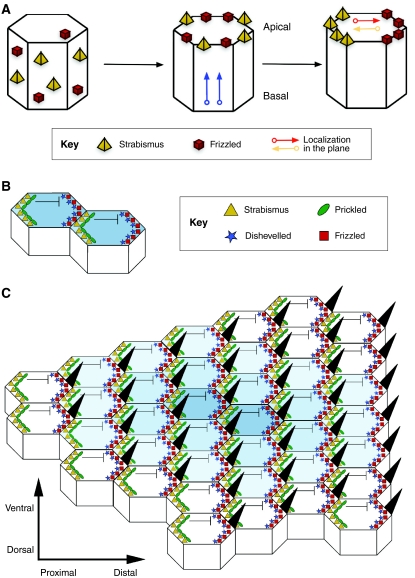
Fig. 2.
PCP establishment in the Drosophila wing. (A) Polarity establishment in the Drosophila wing epithelium as a classic model of PCP. PCP establishment first requires key proteins [e.g. Strabismus (Stbm) and Frizzled (Fz)] to be apically distributed and then localized to the proximal and distal sides of the apical surface, respectively, in the plane that lies orthogonal to the apical-basal axis. See Zallen (Zallen, 2007). (B) Stbm then recruits Prickled to the proximal side of the cell, which antagonizes Dishevelled (Dsh), hence restricting the location of the Dsh-Fz complex to the opposite, distal side. At the same time, interactions between the extracellular domains of Stbm and Fz at adjacent proximal and distal membranes of neighboring cells stabilize the polarized configurations, allowing a small clone of cells to be polarized. (C) A speculative model of PCP (see Tree et al., 2002b), in which the organized asymmetric distribution of core PCP proteins spreads from a small group of polarized organizer cells. A wave of polarized interactions emanates from an initial site of polarization outwards in all directions through the plane of the tissue, establishing a coordinated array of polarized cells (indicated here by the decreasing intensity of blue shading from the center to outer cells). See Jones and Chen (Jones and Chen, 2007).
PCP is thought to occur in three steps (Tree et al., 2002a). First, a directional cue initiates polarity that orients the field with respect to the rest of the embryo (Fig. 2A). The source of this cue is believed to be an extracellular gradient of a morphogen related to the Wnt family of Fz ligands, although its existence has not been proven in most instances of PCP. Next, this signal is interpreted by intracellular factors to produce the asymmetric subcellular localization of core PCP proteins. These asymmetries then spread across the entire cell field, perhaps by mutual inhibition and/or stabilization at cell-cell boundaries (Fig. 2B,C) (Seifert and Mlodzik, 2007), creating global parallel arrays of asymmetric intracellular protein localization. Finally, tissues interpret this subcellular asymmetry during polarized differentiation and morphogenesis.
We propose that LR patterning is a type of PCP. The field of PCP is a complex one that has several important open questions, including the identity of the initial global directional cue that orients the field with respect to the embryonic axes, the mechanisms by which polarized PCP proteins lead to downstream morphogenetic cascades, and the details of PCP protein interactions in vertebrates. However, the main principles of this important pathway are becoming clear. The PCP system exhibits several crucial properties that might illuminate our understanding of how components important in LR patterning can be coordinated to impose LR directionality.
We propose that PCP and LR patterning can be thought of as being linked at a number of levels. The broadest comparison between the two is motivated by the fact that PCP and LR patterning solve the same morphogenetic problem and share a fundamental logic of patterning control (as described in more detail below). In addition to the parallels in logic that underlie PCP and LR patterning, we also highlight similarities in epistatic interactions of PCP and LR mutants, and intriguing overlaps in the phenotypes of several genetic mutants. The hypothesis that a deeper, more molecularly conserved link exists between these two pathways would posit that the spread of LR information could lie downstream of the same molecular pathways that control classical PCP readouts, and that perhaps even certain core PCP proteins might themselves signal in the LR pathway. Although we discuss several genetic mutations that cause both PCP and LR defects, no published studies have specifically examined PCP mutants for LR patterning defects. We hope that our proposal spurs such experiments. However, our hypothesis has relevance aside from any role that the core PCP genes might have in LR patterning, as PCP may in fact be patterned by more than one process (Lawrence et al., 2007). Key features of PCP patterning in new contexts have led to the definition of PCP being extended to polarized structures that are patterned by molecules other than the core group of PCP proteins, such as the dentical structures that line the Drosophila larval cuticle (Zallen, 2007). We argue here that LR patterning is indeed one such context that employs PCP, although future experiments are required to reveal the degree of conservation of the molecular players involved.
PCP and LR patterning share a similar underlying logic
PCP is most commonly described as an epithelial patterning phenomenon, and LR patterning is broadly consistent with this. During LR patterning, the early-cleavage frog embryo is a polarized epithelium (Muller and Hausen, 1995; Chalmers et al., 2003), as are the chick (Stern, 1982; Wei and Mikawa, 2000; Voiculescu et al., 2007) and zebrafish (Oteiza et al., 2008) embryos. Both PCP and LR patterning are processes that direct the consistent, asymmetric orienting of cells within an epithelium, orthogonal to two other prespecified axes. To achieve this, cross-talk between the axes, possibly via the cytoskeleton, is essential (Djiane et al., 2005; Li et al., 2008; Qiu et al., 2005; Aw et al., 2008). In PCP, key proteins such as Fz and Strabismus (Stbm; Van Gogh - FlyBase), as discussed above and shown in Fig. 2, have first to be recruited to the apical surface in order for planar polarity to manifest. The proteins are then asymmetrically distributed within the plane of the cell sheet (Zallen, 2007). Similarly, in Xenopus, the apical-basal axis is important for the correct establishment of asymmetry, and LR-relevant ion transporters, such as the H/K-ATPase (see Table 1), must first reach the apical blastomere membrane before being asymmetrically plane-polarized in a similar manner to canonical PCP proteins (Adams et al., 2006), a process that is controlled by the chirality of the cytoskeleton (Adams et al., 2006; Aw et al., 2008; Morokuma et al., 2008; Qiu et al., 2005). It was recently shown that the four-cell frog embryo is able to integrate information from its apical-basal and planar polarities to calculate intracellular directionality (Aw et al., 2008).
Table 1.
An overview of left-right patterning genes
|
Mutant names and phenotypes
|
|||||||
|---|---|---|---|---|---|---|---|
| Gene | Species | Product/role | Stage/localization | Cilia phenotypes | Left-right phenotypes | PCP phenotypes | References |
| Nodal | Chick, mouse, frog, fish | TGFβ-family signaling molecule | Post-gastrulation, left-sided | Not known | Heterotaxia | Not known | (Collignon et al., 1996; Levin et al., 1995; Lohr et al., 1997; Rebagliati et al., 1998) |
| V-ATPase | Frog | Proton pump | Four cell, right-sided | Reduction in number and length of cilia | Heterotaxia | Not known | (Adams et al., 2006) |
| H/K-ATPase | Chick, frog, rabbit | Potassium/proton exchanger | Four cell, right-sided | Not known | Heterotaxia | Not known | (Hibino et al., 2006; Levin et al., 2002) |
| Left-right dynein (Lrd; Dnahc11) | Human, mouse, fish | Microtubule-based motor protein. Selectively segregates chromatids in mouse cells | Non-ciliated ES cells and blastocysts (mRNA); ciliated cells in adult mice, including node monocilia; Kupffer's vesicle in fish | Immotile cilia | 50% situs inversus, 50% situs solitus | Not known | (Armakolas and Klar, 2007; Okada et al., 1999; Supp et al., 1997) |
| Inversin (Inv; Invs) | Human, mouse, frog | Inhibits canonical Wnt signaling | RNA in two-cell mouse embryo; localization includes basal bodies, cilia membranes, microtubules | No obvious morphologic al changes in cilia, slightly slower beating | Inv mouse mutants exhibit 100% situs inversus | Inv mutant mice exhibit hair pattern defects; in Xenopus, required for convergent extension | (Morgan et al., 1998; Simons et al., 2005) |
| Polaris (Ift88) | Mouse, fish | Intraflagellar transport | Cilia and basal bodies, centrosome | No central cilium in ventral node cells of mouse | Heterotaxia | In mice, misoriented cochlear stereociliary bundles | (Bisgrove et al., 2005; Murcia et al., 2000; Robert et al., 2007) |
| Nephrocystin 3 (Nphp3) | Human, mouse | Inhibits canonical Wnt signaling | Primary cilia, basal bodies, centrosomes | Longer renal monocilia | Situs inversus | Defects in convergent extension in Xenopus | (Bergmann et al., 2008) |
| BBS genes | Human, mouse | Intracellular trafficking, basal body and cilia formation and function | Centrosomes, centrioles, basal bodies, cilia | Shorter, fewer cilia | Heterotaxia | Misoriented cochlear stereociliary bundles, neural tube defects. Interacts genetically with Vangl2 | (Ansley et al., 2003; Kim et al., 2004; Ross et al., 2005; Tobin and Beales, 2007; Yen et al., 2006) |
| Seahorse (Irrc6) | Fish | Inhibits canonical Wnt signaling | Cytoplasmic puncta in ciliated tissues | None | Heterotaxia | Defects in convergent extension in zebrafish gastrulation | (Kishimoto et al., 2008) |
This overview is limited to the genes and proteins discussed in this article.
BBS, Bardet-Biedl syndrome.
PCP and LR patterning share three broad patterning phases (Fig. 3). First, an initial polarity signal aligns itself with two other prespecified axes (Fig. 3A). Next, the initial polarity signal is transmitted and amplified by asymmetric protein function at either the tissue or organismal level, similar to the left-sided Nodal signaling cascade in LR patterning (see Table 1), or via intracellular protein localization in PCP (Fig. 3B). Finally, the molecular asymmetries are interpreted by each tissue to result in asymmetric morphogenesis (Fig. 3C). How orientation is initially achieved remains a controversial question for both pathways, but there are some interesting analogies between the phenotypes of animals that express loss-of-function mutations in core PCP and LR patterning genes (Fig. 3). Since PCP manifests as different patterning phenomena in different tissues, for clarity we focus on the phenotypes that result from mutations in PCP genes in the ommatidia of the Drosophila eye, where upstream genes that initiate polarity have possibly been identified; these are believed to be the atypical cadherins fat (ft), dachsous (ds) and four-jointed (fj) (Yang et al., 2002). The details of the analogous phenotypes between the phases of LR and PCP establishment might deviate in other planar-polarized epithelia. During LR patterning and during PCP establishment in the fly eye, the coordination of downstream events occurs as a step distinct from the initial orientation of global polarity with respect to the major embryonic axes, as signaling to downstream pathways (normally via Nodal in LR patterning or via fz in PCP) can continue despite the loss of the upstream global orientation cue. For example, loss of left-right dynein (Lrd; Dnahc11) (see Table 1) in LR patterning or loss of ds in PCP both result in random but coordinated asymmetry. Because one would expect loss of the polarity initiator to lead to loss of polarity, and not to randomized polarization, this surprising result hints at a similar underlying biophysical mechanism for PCP and LR patterning.
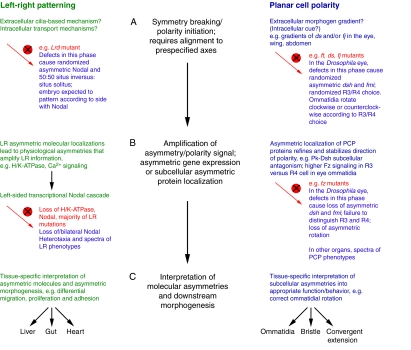
Fig. 3.
LR patterning and PCP steps share similar logic. Three phases of patterning required for LR asymmetry (left, green) and PCP (right, blue). The mutant vertebrate LR and Drosophila PCP eye phenotypes that result from disruption (red circle with a cross) of each phase are described. For simplicity, we focus on phenotypes that result from mutations in PCP genes in the ommatidia of the Drosophila eye. (The details of analogous phenotypes between the phases of LR and PCP establishment might differ in other planar-polarized epithelia.) (A) In the first phase, symmetry breaking and polarity initiation occur. Defects in this phase abolish the directional cue and lead to random selection of polarity direction, resulting in mutants that retain asymmetric, but randomly oriented, expression of key downstream genes such as Nodal and fz. Mutants develop with randomized asymmetry/polarity that follows from the direction of asymmetric gene expression. For example, 50:50 situs inversus:situs solitus in LR left-right dynein (Lrd) mutant or randomized clockwise/counterclockwise rotation of ommatidia in PCP fat (ft), dachsous (ds) and four-jointed (fj) mutants. (B) In the second phase, the asymmetry/polarity cue is amplified and refined over the cell field. In LR patterning, this might occur via asymmetric ion flux or movement of extracellular morphogens by cilia that are ultimately transduced into the left-sided Nodal transcriptional cascade. In PCP, this occurs via the asymmetric subcellular localization of PCP proteins. Mutations in this phase often cause loss of asymmetric gene expression and a spectrum of LR and PCP phenotypes [loss of, or bilateral, Nodal expression and heterotaxia in LR patterning, or loss of asymmetric Frizzled (Fz) and loss of asymmetric rotation in ommatidia, respectively]. (C) In the final phase, the molecular asymmetries are differentially interpreted in the individual tissues to produce the required morphologies. Mechanisms and phenotypes are reviewed elsewhere (Levin, 2006; Seifert and Mlodzik, 2007; Tree et al., 2002a; Wang and Nathans, 2007; Zallen, 2007). pk, prickled; dsh, dishevelled; fmi, flamingo; R, photoreceptor.
Can insights from PCP improve our understanding of LR asymmetry?
Could the mechanisms that underlie PCP help to fill in gaps in our understanding of LR patterning (Aw and Levin, 2008)? Is there a LR coordinator, and, if so, how does asymmetry information spread? Organisms such as the chick, in which asymmetry appears to initiate when the blastoderm contains thousands of small cells, cannot utilize intracellular transport machinery to localize protein cargo to the left or right sides of the embryo [unlike the process that occurs during holoblastic cleavage in Xenopus (Levin, 2006)]. By setting up an alignment of cellular polarization, PCP could allow intracellular events to impose polarity on a complete body axis, even in organisms in which each cell does not span the midline.
It has been suggested that LR information originates locally in a single- or multiple-cell organizer that first derives the orientation of the LR axis from the other axes (Aw et al., 2008; Hyatt and Yost, 1998; Nascone and Mercola, 1997). None of the current models of LR patterning explains how this information might spread. Interestingly, one model of PCP initiation offers a possible mechanism: cell-cell interactions via the protein Pk may establish a polarity between two cells (Fig. 2B), which can then spread outwards as a wave of feedback amplification (Adler, 2002; Strutt and Strutt, 2002; Tree et al., 2002b) (Fig. 2C). Although PCP is thought of as a tissue-level phenomenon, this model allows PCP to be initiated cell-autonomously, and indeed small groups of cells can organize PCP (Adler et al., 2000). Because proteins involved in LR patterning, such as V-ATPase and H/K-ATPase (see Table 1), exhibit asymmetric intracellular localization (Adams et al., 2006; Levin et al., 2002; Morokuma et al., 2008), thereby fulfilling the most fundamental biophysical analogy between LR patterning and PCP, this model of PCP initiation offers the LR patterning field a new way of thinking about how asymmetry can propagate. Another model of PCP holds that each cell in the sheet polarizes simultaneously in response to a global cue, and that cell-cell interactions sharpen the alignment. This model might also be compatible with LR amplification mechanisms because, as argued below and demonstrated clearly by Xu et al. (Xu et al., 2007), individual cells might possess an intracellular polarity that can provide planar (LR) cues and might need a coordination mechanism to synchronize them.
One of the most attractive features of PCP for understanding LR patterning throughout phyla is its scale invariance (that is, its applicability to a field of cells of any size or number), because planar polarity is thought of as being a global property of tissue structure, which requires asymmetries to be established in cells located far from each other (Zallen, 2007). It is therefore possible that a PCP-like orienting mechanism could provide LR directional coordination throughout the embryo, regardless of its size or shape, or even the timing of LR initiation. Because planar orientation in this class of models is maintained through cell-cell interactions between neighbors, new cells entering the plane during blastoderm expansion could derive LR orientation information from their neighbors, and maintain the orientation of relevant multicellular structures, such as unidirectional gap junctions (Levin and Mercola, 1999; Zhang et al., 2003). Although the relevance of PCP in different species remains to be tested, these models are applicable to diverse body plans, addressing another key problem in LR patterning today - the degree to which basic asymmetry mechanisms are conserved across phyla.
Does LR asymmetry originate intracellularly?
Before LR information can be propagated, the LR axis must first be oriented correctly. Although our hypothesis that LR information spreads by PCP mechanisms is compatible with many models of initiation, we propose that the LR axis is first oriented intracellularly, and that the subcellular component that coordinates the three axes (Brown and Wolpert, 1990) is a cytoskeletal-organizing center, such as the centriole or basal body (an organelle derived from the centriole that is found at the base of cilia).
The early events that set up the Drosophila embryo provide a well-understood precedent for using ancient, conserved cytoskeletal nucleating centers, such as centrioles, to set up major body axes (Steinhauer and Kalderon, 2006). The microtubule-organizing center (MTOC) nucleates microtubule assembly (Kellogg et al., 1994) and can position itself in the exact center of even irregularly shaped cells (McNiven and Porter, 1988), suggesting that it can integrate the three-dimensional morphology of the cell. In ciliates, basal bodies have a LR asymmetry (they are chiral stereoisomers that occur only in one enantiomer), which is linked to the overall chirality of the cell (Beisson and Jerka-Dziadosz, 1999; Bell et al., 2008). Consistent with our hypothesis (as we discuss in more detail below), LR patterning defects are often seen in patients with Bardet-Biedl syndrome, a human genetic ciliopathic disorder that leads to basal body dysfunction (Ansley et al., 2003; Kim et al., 2004; Tobin and Beales, 2007).
A cytoskeletal origin of asymmetry is supported by a very interesting recent study that has shown that the Xenopus egg contains a pre-existing `East-West' chirality of the actin cytoskeleton wrapped around the cortex of the egg (Danilchik et al., 2006). The origin of this chirality is unresolved. As four-cell Xenopus embryos already exhibit LR asymmetry in protein localization (Adams et al., 2006), the counterclockwise cytoskeletal chirality of the egg could be converted into a LR directionality in the embryo. The sperm entry point, which defines the DV axis in Xenopus, could select in each embryo a point on the egg's circumference at which the prior East-West chirality's tangent determines an orthogonal LR directionality (Fig. 4A-C).
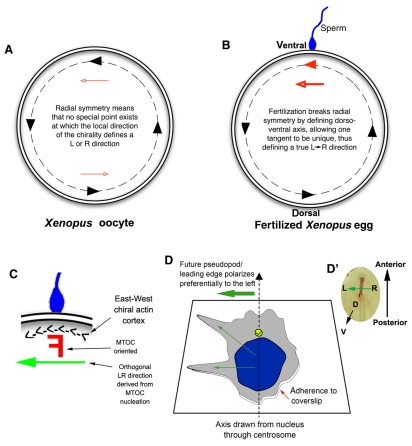
Fig. 4.
Innate chirality allows polarity determination in single cells. (A) A Xenopus egg viewed from the animal pole; the animal-vegetal axis lies perpendicular to the plane of the page. A consistent `East-West' or counterclockwise chirality has been identified in the actin cytoskeleton around the egg's periphery (black dashed line) (Danilchik et al., 2006), which provides different LR directional cues at distinct tangent points (black arrowheads along the dashed line). These cues offer no unique LR orientation because each point is equivalent to the others (red arrowheads show that cues point rightwards on one side and leftwards on the opposite side). (B) At fertilization, sperm entry breaks the radial symmetry and determines a specific point on the circumference through which the midline axis of bilateral symmetry passes. The chiral orientation of the actin cytoskeleton at this point converts the bilateral symmetry into LR asymmetry through a linear cue (red arrowhead defined by sperm entry) along the LR axis. Thus, circumferential chirality can be converted into an organism-wide linear directionality (LR) once the DV axis is determined. (C) Sperm entry point magnified, where a putative chiral `F-molecule' (Brown and Wolpert, 1990) in the microtubule-organizing center (MTOC) can be oriented with respect to the actin cortex and nucleate microtubule transport paths that have a true LR directionality. (D) Intrinsic polarity of a differentiated HL60 cell in culture (Xu et al., 2007). Cells extend pseudopodia preferentially to the left of an arrow pointing from the nucleus to the centrosome (yellow circle), revealing how HL60 cells are intrinsically chiral, utilizing cytoplasmic structures and a polarized axis (adhesion to coverslip versus free medium) to consistently orient the LR axis. (D′) A chick embryo blastoderm is analogous to the cell in D, in that its DV axis is fixed and a leftward signal must be determined to establish sonic hedgehog (Shh) expression on the left side of the node (brown).
Another significant study for the field of LR asymmetry, in which polarity in human neutrophil-like differentiated HL60 cells was investigated, recently revealed that even these non-embryonic cells in culture orient an apparent LR axis (Xu et al., 2007). Neutrophils respond to inflammatory stimuli by crawling towards sites of infection via pseudopods. Strikingly, most cells extended pseudopods to the left of an imaginary line drawn from the middle of the nucleus towards the centrosome of each cell (Fig. 4D). This indicates that differentiated HL60 cells appear to have a functional chirality, with three apparent axes of asymmetry, just as in LR patterning: that of the imaginary line pointing from the nucleus to the centrosome (axis 1, geometrically analogous to the embryonic AP axis in LR patterning); a vertical axis between the coverslip, cell and bathing medium that is perpendicular to the first axis (axis 2, analogous to the embryonic DV axis); and a third lateral axis along the surface of the cell along which biased polarity occurs (axis 3, analogous to the LR axis), in the direction to the left of the first axis (see Fig. 4D).
Crucially, the HL60 data recapitulate all of the important phenotypes obtained during manipulation of embryonic LR patterning. Speculating that the centrosome-related chirality of the cell might be controlled by the cell division cycle 42 (CDC42)-partitioning defective 6 (PAR6) epithelial polarity pathway (Cau and Hall, 2005), Xu et al. (Xu et al., 2007) examined how disrupting members of the pathway affected cell polarity. Disruption of the key upstream elements PAR6, CDC42 or phosphotidylinositol (3,4,5)-triphosphate (PIP3) abolished the neutrophil polarity altogether (analogous to isomerism in LR patterning, in which organs lose LR asymmetric morphology or position). The targeting of downstream elements of the pathway, such as the atypical protein kinase C PKCζ (PRKCγ) and cytoplasmic dynein, randomized polarity and abolished the leftward bias of pseudopod extension (analogous to heterotaxia, in which the position of only some visceral organs is randomly reversed). Expressing a constitutively active mutant of GSK3β reversed polarity (analogous to situs inversus, in which the position of all visceral organs is completely reversed). In both LR patterning and neutrophil polarity, a Wnt pathway regulator fully reverses the asymmetry and causes cells to polarize to the right; in the case of HL60 cells, this is the constitutively active GSK3β, whereas in the case of vertebrate asymmetry it is a mutant of the inversin (Inv; Invs) protein (Morgan et al., 1998) (see Table 1). As in LR asymmetry, the leftward bias of the HL60 cells was shown to require microtubule organization and the dynein motor (Aw et al., 2008; Supp et al., 1999).
The polarization of the HL60 cells is a remarkable illustration of a cell performing the precise calculation that every vertebrate embryo needs to make: to derive a LR direction from two pre-existing axes. It is also a demonstration of how asymmetry can be initiated de novo without recourse to cilia (which neutrophils do not possess). Together, these findings illustrate how individual mammalian cells could utilize existing subcellular structures to consistently orient their LR axis.
These data are consistent with our proposal that cytoskeleton-dependent intracellular trafficking has a role in aligning a major axis of polarity (in both PCP and the LR axis). For example, in the fly wing epithelium, Fz protein is transported through the cells by an oriented cytoskeletal array (Shimada et al., 2006). This is precisely what occurs in LR patterning in at least some other species (Aw et al., 2008). Both PCP and LR asymmetry may use the cytoskeleton as a vector for orienting key polarized molecules within cells.
Overlapping PCP and LR phenotypes in ciliary mutants: a shared underlying cause?
Whether mutations in core PCP genes cause LR randomization has yet to be directly tested. Interestingly, as we discuss below, mutations in several genes involved in basal body/ciliary function (see Table 1) cause specific LR defects and the mispatterning of planar-patterned epithelia, suggesting that a common mechanism underlies epithelial PCP and LR patterning.
The inversin gene is partially deleted in Inv/Inv mice, inducing ∼100% organ inversion (Mochizuki et al., 1998; Morgan et al., 1998). Inv RNA is transcribed from the two-cell stage (Eley et al., 2004; Nurnberger et al., 2006), and its protein exhibits a dynamic distribution in both ciliated and non-ciliated cells: at different parts of the cell cycle, it localizes to basal bodies, primary cilia, cell-cell junctions, plasma membrane, polarized microtubule pools [where it interacts directly with tubulin and N-cadherin in the cytoplasm (Nurnberger et al., 2002)] and the spindle poles (Eley et al., 2004; Nurnberger et al., 2002; Nurnberger et al., 2004). Of note, inversin also localizes to the mother centriole (and not the daughter centriole) of cells even before the primary cilium extends (Watanabe et al., 2003). This finding is particularly interesting because the daughter centriole takes its cues from, and is always formed at right angles to, the mother centriole, which provides spatial patterning and orientation information to organelles such as the nucleus (Feldman et al., 2007). Interestingly, Inv mutant mice exhibit hair pattern changes that resemble those seen in frizzled 6 (Fzd6)-deficient mice, and Inv was recently shown to act as a molecular switch between canonical and non-canonical (PCP) Wnt signaling pathways. In transfected cells, Inv localizes to, and directly binds, PCP proteins such as Stbm and Pk, and interacts and colocalizes with Dsh. Inv protein inhibits canonical Wnt signaling by targeting cytoplasmic Dsh for degradation; it is also required in PCP-dependent gastrulation movements in Xenopus (Simons et al., 2005). Some of the inv loss-of-function phenotypes in zebrafish can be rescued by diversin (ankyrin repeat domain 6 - ZFIN), which exhibits homology to the Drosophila PCP gene diego (Moeller et al., 2006; Schwarz-Romond et al., 2002).
Polaris (Ift88) loss-of-function in mouse and zebrafish also results in LR patterning defects (Bisgrove et al., 2005; Moyer et al., 1994; Murcia et al., 2000; Schrick et al., 1995; Taulman et al., 2001). Polaris encodes a protein that localizes to cilia and basal bodies. It functions in intraflagellar transport and remains associated with the centrosome in non-ciliated cells (Robert et al., 2007). Polaris interacts genetically with the PCP gene Vangl2 (Jones et al., 2008), and Polaris inactivation in mice leads to misoriented stereociliary bundles in the organ of Corti, similar to the phenotype seen in mouse embryos with mutations in Vangl2 and in another PCP gene, Fzd3. In Polaris mutants, the position of misoriented stereociliary bundles strongly correlates with that of the mislocalized basal bodies, consistent with Polaris and the basal body being intracellular sources of polarization information in both LR asymmetry and PCP.
Bardet-Biedl syndrome (BBS) is a pleiotropic human genetic disorder in which LR randomization features. Twelve BBS genes have been identified, and most play roles in intracellular trafficking and in basal body and cilia function (Ansley et al., 2003; Kim et al., 2004; Tobin and Beales, 2007). Among the BBS genes, Bbs4 mouse mutants exhibit classical PCP phenotypes, such as neural tube defects and disrupted cochlea stereociliary bundles (Ross et al., 2005). Bbs1, Bbs4 and Bbs6 interact genetically with the PCP gene Vangl2 in both mouse and zebrafish, further supporting a mechanistic link between basal body/cytoskeleton, intracellular trafficking, PCP and LR asymmetry. BBS mutant mice do not exhibit situs inversus, but occasionally do exhibit multiple accessory spleens (P. Beales, personal communication).
Nephronophthisis (NPH) is an autosomal recessive kidney disease. Loss-of-function of Inv or nephrocystin 3 [nephronophthisis 3 (Nphp3)] leads to situs inversus in mice (Bergmann et al., 2008). Nphp3 directly interacts with Inv, and both can inhibit canonical (β-catenin-dependent) Wnt signaling (Bergmann et al., 2008; Simons et al., 2005). Loss of NPHP3 function in Xenopus leads to defects in PCP-dependent processes such as convergent extension and neural tube closure (Bergmann et al., 2008).
The phenotypes of these genetic mutants reveal consistent links between basal body/ciliary function, PCP and LR patterning. One possibility is that ciliary defects underlie both the LR and PCP phenotypes. However, the ability of many non-ciliated tissues to achieve PCP, and a lack of obvious primary cilia defects in LR mutants that carry mutations in genes expressed in ciliary cells that have ciliary functions, such as in seahorse (leucine rich repeat containing 6 - ZFIN) (see Table 1) and Inv (Kishimoto et al., 2008; Watanabe et al., 2003), lead us to propose that ciliary dysfunction and LR defects might both be parallel, downstream consequences of impaired planar polarity (see Box 1).
Box 1. Models for how PCP and LR patterning might interact in ciliary mutants
Ciliary beating is a sensitive readout of the polarity of a cell (Boisvieux-Ulrich and Sandoz, 1991). However, mutant ciliary proteins also affect PCP. The phenotypes of ciliary/basal body mutants, in particular the prediction of stereocilium position by the basal body in Polaris (see Table 1) mouse mutants (Jones et al., 2008), suggest that basal body proteins might be key to both LR patterning and PCP, by linking centriole orientation to PCP signal directionality. Since a true inversin (Inv) null mouse has not yet been examined, the partial deletion of Inv in these mutant mice might result in a reversed connection between cytoskeletal chirality and downstream amplification mechanisms, hence the near complete penetrance of situs inversus in Inv mutant offspring (Morgan et al., 1998). The occurrence of LR and ciliary phenotypes as parallel consequences of defective cellular polarity is supported by the analysis of the zebrafish gene seahorse (Kishimoto et al., 2008), which distinguishes between PCP and ciliary function in LR patterning. Seahorse protein associates with the core PCP protein Dishevelled, and seahorse loss-of-function mutants exhibit cilia of normal structure and motility, but randomized LR asymmetry.
There are other possible explanations for the LR and PCP phenotypes in ciliary mutants. Cilia may be important in patterning PCP. However, because non-ciliated epithelia also exhibit PCP, for this to hold, PCP mechanisms would have to have evolved separately in ciliated and non-ciliated epithelia. It is also possible that the roles of ciliary proteins in PCP and LR patterning are different and arose independently. However, because the logical structures of the two phenomena are similar, and, in animals such as the chick, LR begins to be patterned in epithelia that already have PCP proteins at work, it seems plausible that the cell's polarity machinery was co-opted to solve the two problems via the same mechanism, instead of functionally buffering them from each other.
PCP as an amplifier of intracellular LR information: model predictions
A fundamental prediction of our model is that LR information propagates by the polar localization of protein complexes within cells in epithelia. Such subcellular asymmetries (of ion transporter proteins, for example) do indeed exist in embryonic epithelia undergoing LR patterning (Adams et al., 2006; Levin et al., 2002; Morokuma et al., 2008). This model also predicts the reversal of LR patterning that results from the surgical rotation of the blastocoel roof in Xenopus embryos (Mangold, 1921; Yost, 1992). It remains to be tested whether the localization of LR components is driven by the same mechanism that establishes polar localization of core PCP proteins.
As discussed above, LR and PCP defects both result from the disruption of key intracellular proteins, as predicted by our model. That these defects are specific, occurring in otherwise largely normal embryos, suggests that the concordance of LR and PCP phenotypes is not due to the interruption of basic housekeeping pathways that are important for other patterning events. Our hypothesis of a link between the canonical planar polarity of various embryonic structures and LR patterning is also consistent with the recently revealed role of PCP in determining hair whorl patterns (Guo et al., 2004; Wang et al., 2006). Our model predicts the formerly mysterious relationship between the direction of hair whorls on the scalp and the mirroring of subtle structures in monozygotic twins (Jansen et al., 2007; Levin, 1999; Weber et al., 2006).
The suggestion in our hypothesis that the orienting factor might be an intracellular structure, and not an extracellular gradient, is independent of the proposed relationships between PCP and LR patterning. This makes a fundamental prediction: that cells can compute the third planar axis from the other two. This prediction has recently been confirmed for LR patterning in Xenopus (Aw et al., 2008), for PCP in the Drosophila wing (Adler et al., 2000), and even in single cells in culture (Xu et al., 2007). Many details of this process are still unclear, including the mechanistic models of cytoskeleton-organizing centers and the basal body proteins. It also remains to be seen whether an intracellular orienting cue will be uncovered in canonical examples of PCP.
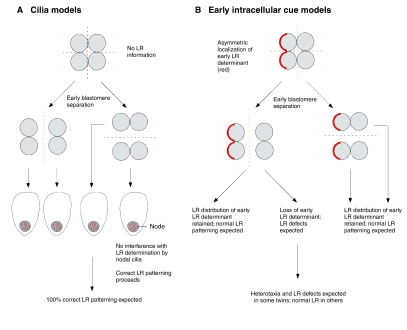
This model also makes another key prediction that distinguishes it from ciliary models of LR initiation: asymmetry phenotypes in non-conjoined monozygotic twins (Fig. 5). If LR-relevant polar localization events occur very early, twins produced by blastomere separation will exhibit some incidence of reversal of asymmetry. By contrast, if LR is initiated by nodal cilia, asymmetry should be 100% normal in early-split twins as no LR information would have been derived before the split, and the cilia will beat in the normal direction in each twin during gastrulation. Indeed, the literature contains numerous unexplained examples of book-ending (mirror imaging of unilateral defects and asymmetric traits) in monozygotic twins (reviewed by Levin, 1999; Levin, 2001), including opposite sidedness of facial defects. Book-ending in monozygotic twins lends support for an intracellular, chiral origin of asymmetry. The conservation of chirality within a pair of splitting cells has been beautifully illustrated by Albrecht-Buehler, who showed that after division, daughters of mammalian cells in culture have mirror-image cytoskeletal organization and subsequent migration trajectories (Albrecht-Buehler, 1977). Interestingly, the mirroring of daughter cells was not completely penetrant, providing a testable quantitative prediction for the incidence of book-ending in monozygotic twins. These data suggest that the initial chiral orientation is disrupted by early cleavage, but that subsequent amplification proceeds normally if the initial polarizing cue is reversed. Newt embryos separated at the two-cell stage also exhibit an 89% incidence of organ laterality reversal in one of the twins (Takano et al., 2007), despite being normal with respect to other morphogenetic events. Thus, the LR axis must have already been orientated by the time of splitting. The twinning data do not therefore support the late-origin ciliary models, but are straightforward predictions of early, intracellular chirality mechanisms.
Fig. 5.
Prediction by intracellular early models of LR patterning of asymmetry phenotypes in embryo-splitting experiments. (A) If asymmetry is initiated by the action of cilia during gastrulation, very early blastomere separation should result in no loss of LR information and in normal nodal cilia. The resulting embryos are expected to exhibit 100% correct LR patterning. (B) In intracellular models, LR information provided by early, asymmetrically localized determinants is lost or altered in some daughter blastomeres upon early splitting, leading to the prediction of LR patterning defects, as is observed in human monozygotic twins and in experiments in amphibians.
Limitations and experimental tests of the model
Do our models fit all of the data? Although we favor an early intracellular origin for the initial alignment of the LR axis, such as a MTOC, known to be crucial for planar polarity (Jones et al., 2008; Nubler-Jung et al., 1987), the amplification aspect of a PCP model is equally compatible with signals being generated by a later organizer, such as the ciliated node. Thus, the origin phase and the amplification phase are independent features of our model.
Although it is clear that, at least in some species, LR components are plane-polarized in epithelia, there is currently a dearth of functional data that directly tests the relationship between core PCP proteins and LR patterning. The PCP gene flamingo is LR asymmetrically expressed in chick embryos (Formstone and Mason, 2005); this is not obviously predicted by our model, although flamingo protein localization has not been examined at the cell level in chick. Canonical PCP genes are expressed in the mouse embryo during streak elongation (Crompton et al., 2007), but dissection of PCP from ciliary mechanisms by conditional gene inactivation remains to be performed in mammalian LR mutants. Likewise, the roles, if any, of the physiological LR signals, such as the movement of ions and serotonin (Levin, 2006; Raya et al., 2004), in PCP have not yet been examined.
Several studies have described the occurrence of PCP defects in the absence of LR randomization, and vice versa (Montcouquiol et al., 2003; Pennekamp et al., 2002). However, the independent occurrence of these phenotypes does not strictly contradict our models because the pathways could utilize similar molecules at one point in the pathway but different ones at others (the pathways could use the same logic but at some point diverge). However, it should be noted that PCP and LR defects can be far from obvious if not specifically looked for in embryos. It is possible that organ of Corti polarization defects might have been missed in LR mutant mice, and the incorrect coiling of the reproductive system, a subtle LR defect (Coutelis et al., 2008), might have easily been missed in the analyses of canonical Drosophila PCP mutants. Furthermore, fly PCP mutants are often genetic mosaics created specifically to examine PCP in the eye or wing; more spatially extensive gene inactivation might be required to elucidate the roles, if any, of PCP proteins in LR patterning
Our model does not constrain additional important questions in the LR field, such as how LR direction is converted into position with respect to the midline, or whether the final positional readout available to organ primordia contains a range of values along the LR axis or only binary right-versus-left information. Many questions as to the details of cytoskeletal orientation in both PCP and LR patterning remain. Our model predicts that, as in LR patterning, certain kinds of mutations of intracellular proteins should alter the orientation of a properly patterned epithelium with respect to the rest of the embryo. For example, there might exist mutants that cause specific reversals of PCP with respect to body axes (rather than loss of coordination), but to our knowledge this has been observed very rarely and to a limited extent only in the Drosophila wing, where regions of reversed polarity have been seen in dachsous mutants (Adler et al., 1998), and in the eye (Simon, 2004). Such phenotypes have not yet been reported in vertebrates. Expression of the Inv mutant gene, which may not be a true null, in Drosophila might reveal a novel PCP phenotype, and such phenotypes should be carefully looked for in both flies and mammals to test our model and to identify upstream orientation signals.
The epithelium of the chick embryo blastoderm is an ideal model in which to look for the interaction of PCP components with early markers of LR asymmetry, such as the left-sided depolarization of cells during streak initiation (Levin et al., 2002). Both vertebrate and invertebrate models of PCP could be used to probe for biochemical and genetic interactions between canonical planar polarity proteins and H+ pumps and K+ channels. We are currently directly testing the PCP model by examining PCP-relevant proteins for LR asymmetrical localization in cells of chick and frog embryos, and testing specific disruptors of PCP for their ability to randomize embryonic LR patterning. Planar-polarized gradients of pH, Ca2+ fluxes and membrane voltage at the level of individual cells should be looked for in chick, frog, mouse and zebrafish epithelia at early stages of LR patterning.
Conclusion
Subtle intracellular asymmetries are revealed by the consistently polarized pseudopodia in human HL60 cells, the mirror image paths of daughter mouse 3T3 cells (Albrecht-Buehler, 1977), and by the mirror asymmetry in monozygotic twins of mammals and amphibia. Our hypothesis suggests that LR asymmetry derives from a cellular chirality that exists in single cells; it is directly apparent in the handed chirality of protozoa, but must be amplified in multicellular species. PCP, which is a conserved and powerful patterning system that solves fundamentally the same morphogenetic problems as those faced by LR asymmetry, is an ideal mechanism for imposing LR signals over large fields of cells.
Our basic hypothesis holds that two predefined axes allow the chiral cytoskeleton to initiate the asymmetric (polarized) intracellular localization of LR machinery. This is supported by existing data and it is plausible that if single cells already have the components necessary to drive and amplify cytoskeletal asymmetry (Aw et al., 2008; Levin, 2006), this toolkit might be reused for asymmetry in diverging multicellular lineages. A number of mutations in basal body/ciliary proteins result in both laterality and PCP phenotypes, and PCP provides a conceptually satisfying system for LR patterning across different scales. However, our proposal that a deeper molecular link exists between LR patterning and PCP remains to be tested, as core PCP proteins have not been linked to the LR pathway and no data yet identify an intracellular molecule in the large-scale orientation of PCP fields. Synthetic in silico modeling at multiple levels of organization coupled with a molecular investigation of the parallels between PCP and LR patterning should test our hypotheses and might reveal fascinating new aspects of developmental and cell biology.
We thank Jennifer Zallen, Janine Beisson, Cliff Tabin, Ann Ramsdell, Chris McManus, Stephane Noselli, Ying Zhang and members of the Levin laboratory for many useful discussions. The authors are supported by the March of Dimes, American Heart Association and NIH (M.L.), and by the Agency for Science, Technology, and Research, Singapore (S.A.). Deposited in PMC for release after 12 months.
References
- Adams, D. S., Robinson, K. R., Fukumoto, T., Yuan, S., Albertson, R. C., Yelick, P., Kuo, L., McSweeney, M. and Levin, M. (2006). Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133, 1657-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, P. N. (2002). Planar signaling and morphogenesis in Drosophila. Dev. Cell 2, 525-535. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., Charlton, J. and Liu, J. (1998). Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development 125, 959-968. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., Taylor, J. and Charlton, J. (2000). The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech. Dev. 96, 197-207. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler, G. (1977). Daughter 3T3 cells. Are they mirror images of each other? J. Cell Biol. 72, 595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansley, S. J., Badano, J. L., Blacque, O. E., Hill, J., Hoskins, B. E., Leitch, C. C., Kim, J. C., Ross, A. J., Eichers, E. R., Teslovich, T. M. et al. (2003). Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 425, 628-633. [DOI] [PubMed] [Google Scholar]
- Armakolas, A. and Klar, A. J. (2007). Left-right dynein motor implicated in selective chromatid segregation in mouse cells. Science 315, 100-101. [DOI] [PubMed] [Google Scholar]
- Aw, S. and Levin, M. (2008). What's left in asymmetry? Dev. Dyn. 237, 3453-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw, S., Adams, D. S., Qiu, D. and Levin, M. (2008). H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech. Dev. 125, 353-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod, J. D. and McNeill, H. (2002). Coupling planar cell polarity signaling to morphogenesis. Scientific World Journal 2, 434-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson, J. and Jerka-Dziadosz, M. (1999). Polarities of the centriolar structure: morphogenetic consequences. Biol. Cell 91, 367-378. [PubMed] [Google Scholar]
- Bell, A. J., Satir, P. and Grimes, G. W. (2008). Mirror-imaged doublets of Tetmemena pustulata: implications for the development of left-right asymmetry. Dev. Biol. 314, 150-160. [DOI] [PubMed] [Google Scholar]
- Bergmann, C., Fliegauf, M., Bruchle, N. O., Frank, V., Olbrich, H., Kirschner, J., Schermer, B., Schmedding, I., Kispert, A., Kranzlin, B. et al. (2008). Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am. J. Hum. Genet. 82, 959-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove, B. W., Snarr, B. S., Emrazian, A. and Yost, H. J. (2005). Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Dev. Biol. 287, 274-288. [DOI] [PubMed] [Google Scholar]
- Boisvieux-Ulrich, E. and Sandoz, D. (1991). Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol. Cell 72, 3-14. [DOI] [PubMed] [Google Scholar]
- Brown, N. A. and Wolpert, L. (1990). The development of handedness in left/right asymmetry. Development 109, 1-9. [DOI] [PubMed] [Google Scholar]
- Brueckner, M. (2001). Cilia propel the embryo in the right direction. Am. J. Med. Genet. 101, 339-344. [DOI] [PubMed] [Google Scholar]
- Cau, J. and Hall, A. (2005). Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 118, 2579-2587. [DOI] [PubMed] [Google Scholar]
- Chalmers, A. D., Strauss, B. and Papalopulu, N. (2003). Oriented cell divisions asymmetrically segregate aPKC and generate cell fate diversity in the early Xenopus embryo. Development 130, 2657-2668. [DOI] [PubMed] [Google Scholar]
- Collignon, J., Varlet, I. and Robertson, E. J. (1996). Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 381, 155-158. [DOI] [PubMed] [Google Scholar]
- Coutelis, J. B., Petzoldt, A. G., Spéder, P., Suzanne, M. and Noselli, S. (2008). Left-right asymmetry in Drosophila. Semin. Cell Dev. Biol. 19, 252-262. [DOI] [PubMed] [Google Scholar]
- Crompton, L. A., Du Roure, C. and Rodriguez, T. A. (2007). Early embryonic expression patterns of the mouse Flamingo and Prickle orthologues. Dev. Dyn. 236, 3137-3143. [DOI] [PubMed] [Google Scholar]
- Danilchik, M. V., Brown, E. E. and Riegert, K. (2006). Intrinsic chiral properties of the Xenopus egg cortex: an early indicator of left-right asymmetry? Development 133, 4517-4526. [DOI] [PubMed] [Google Scholar]
- Djiane, A., Yogev, S. and Mlodzik, M. (2005). The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121, 621-631. [DOI] [PubMed] [Google Scholar]
- Eley, L., Turnpenny, L., Yates, L. M., Craighead, A. S., Morgan, D., Whistler, C., Goodship, J. A. and Strachan, T. (2004). A perspective on inversin. Cell Biol. Int. 28, 119-124. [DOI] [PubMed] [Google Scholar]
- Esser, A. T., Smith, K. C., Weaver, J. C. and Levin, M. (2006). Mathematical model of morphogen electrophoresis through gap junctions. Dev. Dyn. 235, 2144-2159. [DOI] [PubMed] [Google Scholar]
- Feldman, J. L., Geimer, S. and Marshall, W. F. (2007). The mother centriole plays an instructive role in defining cell geometry. PLoS Biol. 5, e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formstone, C. J. and Mason, I. (2005). Expression of the Celsr/flamingo homologue, c-fmi1, in the early avian embryo indicates a conserved role in neural tube closure and additional roles in asymmetry and somitogenesis. Dev. Dyn. 232, 408-413. [DOI] [PubMed] [Google Scholar]
- Fukumoto, T., Kema, I. P. and Levin, M. (2005). Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 15, 794-803. [DOI] [PubMed] [Google Scholar]
- Guo, N., Hawkins, C. and Nathans, J. (2004). Frizzled6 controls hair patterning in mice. Proc. Natl. Acad. Sci. USA 101, 9277-9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino, T., Ishii, Y., Levin, M. and Nishino, A. (2006). Ion flow regulates left-right asymmetry in sea urchin development. Dev. Genes Evol. 216, 265-276. [DOI] [PubMed] [Google Scholar]
- Hyatt, B. A. and Yost, H. J. (1998). The left-right coordinator: the role of Vg1 in organizing left-right axis formation. Cell 93, 37-46. [DOI] [PubMed] [Google Scholar]
- Jansen, A., Lohmann, H., Scharfe, S., Sehlmeyer, C., Deppe, M. and Knecht, S. (2007). The association between scalp hair-whorl direction, handedness and hemispheric language dominance: is there a common genetic basis of lateralization? Neuroimage 35, 853-861. [DOI] [PubMed] [Google Scholar]
- Jones, C. and Chen, P. (2007). Planar cell polarity signaling in vertebrates. BioEssays 29, 120-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C., Roper, V. C., Foucher, I., Qian, D., Banizs, B., Petit, C., Yoder, B. K. and Chen, P. (2008). Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 40, 69-77. [DOI] [PubMed] [Google Scholar]
- Kellogg, D. R., Moritz, M. and Alberts, B. M. (1994). The centrosome and cellular organization. Annu. Rev. Biochem. 63, 639-674. [DOI] [PubMed] [Google Scholar]
- Kim, J. C., Badano, J. L., Sibold, S., Esmail, M. A., Hill, J., Hoskins, B. E., Leitch, C. C., Venner, K., Ansley, S. J., Ross, A. J. et al. (2004). The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat. Genet. 36, 462-470. [DOI] [PubMed] [Google Scholar]
- Kishimoto, N., Cao, Y., Park, A. and Sun, Z. (2008). Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev. Cell 14, 954-961. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker, A. G., Olale, F., Haycraft, C. J., Yoder, B. K., Schier, A. F. and Drummond, I. A. (2005). Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development 132, 1907-1921. [DOI] [PubMed] [Google Scholar]
- Lawrence, P. A., Struhl, G. and Casal, J. (2007). Planar cell polarity: one or two pathways? Nat. Rev. Genet. 8, 555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, M. (1999). Twinning and embryonic left-right asymmetry. Laterality 4, 197-208. [DOI] [PubMed] [Google Scholar]
- Levin, M. (2001). Asymmetry of body and brain: embryological and twin studies. In International Encyclopedia of Social and Behavioral Sciences (ed. N. J. Smelser and P. B. Baltes), pp. 853-859. Oxford, UK: Elsevier.
- Levin, M. (2006). Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res. C Embryo Today 78, 191-223. [DOI] [PubMed] [Google Scholar]
- Levin, M. and Mercola, M. (1999). Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development 126, 4703-4714. [DOI] [PubMed] [Google Scholar]
- Levin, M. and Palmer, A. R. (2007). Left-right patterning from the inside out: widespread evidence for intracellular control. BioEssays 29, 271-287. [DOI] [PubMed] [Google Scholar]
- Levin, M., Johnson, R. L., Stern, C. D., Kuehn, M. and Tabin, C. (1995). A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 82, 803-814. [DOI] [PubMed] [Google Scholar]
- Levin, M., Thorlin, T., Robinson, K. R., Nogi, T. and Mercola, M. (2002). Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 111, 77-89. [DOI] [PubMed] [Google Scholar]
- Li, Z., Wang, L., Hays, T. S. and Cai, Y. (2008). Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity. J. Cell Biol. 180, 31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr, J. L., Danos, M. C. and Yost, H. J. (1997). Left-right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development 124, 1465-1472. [DOI] [PubMed] [Google Scholar]
- Mangold, O. (1921). Situs inversus bei Triton. Arch. Entwickl.-Mech. Org. 48, 505-516. [Google Scholar]
- Marszalek, J. R., Ruiz-Lozano, P., Roberts, E., Chien, K. R. and Goldstein, L. S. (1999). Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc. Natl. Acad. Sci. USA 96, 5043-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J., Somlo, S., Makova, S., Tian, X. and Brueckner, M. (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114, 61-73. [DOI] [PubMed] [Google Scholar]
- McNiven, M. A. and Porter, K. R. (1988). Organization of microtubules in centrosome-free cytoplasm. J. Cell Biol. 106, 1593-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, T., Saijoh, Y., Tsuchiya, K., Shirayoshi, Y., Takai, S., Taya, C., Yonekawa, H., Yamada, K., Nihei, H., Nakatsuji, N. et al. (1998). Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature 395, 177-181. [DOI] [PubMed] [Google Scholar]
- Moeller, H., Jenny, A., Schaeffer, H. J., Schwarz-Romond, T., Mlodzik, M., Hammerschmidt, M. and Birchmeier, W. (2006). Diversin regulates heart formation and gastrulation movements in development. Proc. Natl. Acad. Sci. USA 103, 15900-15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol, M., Rachel, R. A., Lanford, P. J., Copeland, N. G., Jenkins, N. A. and Kelley, M. W. (2003). Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423, 173-177. [DOI] [PubMed] [Google Scholar]
- Morgan, D., Turnpenny, L., Goodship, J., Dai, W., Majumder, K., Matthews, L., Gardner, A., Schuster, G., Vien, L., Harrison, W. et al. (1998). Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat. Genet. 20, 149-156. [DOI] [PubMed] [Google Scholar]
- Morokuma, J., Blackiston, D. and Levin, M. (2008). KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell. Physiol. Biochem. 21, 345-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer, J. H., Lee-Tischler, M. J., Kwon, H. Y., Schrick, J. J., Avner, E. D., Sweeney, W. E., Godfrey, V. L., Cacheiro, N. L., Wilkinson, J. E. and Woychik, R. P. (1994). Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 264, 1329-1333. [DOI] [PubMed] [Google Scholar]
- Muller, H. A. and Hausen, P. (1995). Epithelial cell polarity in early Xenopus development. Dev. Dyn. 202, 405-420. [DOI] [PubMed] [Google Scholar]
- Murcia, N. S., Richards, W. G., Yoder, B. K., Mucenski, M. L., Dunlap, J. R. and Woychik, R. P. (2000). The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127, 2347-2355. [DOI] [PubMed] [Google Scholar]
- Nascone, N. and Mercola, M. (1997). Organizer induction determines left-right asymmetry in Xenopus. Dev. Biol. 189, 68-78. [DOI] [PubMed] [Google Scholar]
- Nonaka, S., Tanaka, Y., Okada, Y., Takeda, S., Harada, A., Kanai, Y., Kido, M. and Hirokawa, N. (1998). Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829-837. [DOI] [PubMed] [Google Scholar]
- Nonaka, S., Shiratori, H., Saijoh, H. and Hamada, H. (2002). Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96-99. [DOI] [PubMed] [Google Scholar]
- Nubler-Jung, K., Bonitz, R. and Sonnenschein, M. (1987). Cell polarity during wound healing in an insect epidermis. Development 100, 163-170. [DOI] [PubMed] [Google Scholar]
- Nurnberger, J., Bacallao, R. L. and Phillips, C. L. (2002). Inversin forms a complex with catenins and N-cadherin in polarized epithelial cells. Mol. Biol. Cell 13, 3096-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger, J., Kribben, A., Opazo Saez, A., Heusch, G., Philipp, T. and Phillips, C. L. (2004). The Invs gene encodes a microtubule-associated protein. J. Am. Soc. Nephrol. 15, 1700-1710. [DOI] [PubMed] [Google Scholar]
- Nurnberger, J., Kavapurackal, R., Zhang, S. J., Opazo Saez, A., Heusch, G., Philipp, T., Pietruck, F. and Kribben, A. (2006). Differential tissue distribution of the Invs gene product inversin. Cell Tissue Res. 323, 147-155. [DOI] [PubMed] [Google Scholar]
- Okada, Y., Nonaka, S., Tanaka, Y., Saijoh, Y., Hamada, H. and Hirokawa, N. (1999). Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol. Cell 4, 459-468. [DOI] [PubMed] [Google Scholar]
- Oteiza, P., Koppen, M., Concha, M. L. and Heisenberg, C. P. (2008). Origin and shaping of the laterality organ in zebrafish. Development 135, 2807-2813. [DOI] [PubMed] [Google Scholar]
- Pennekamp, P., Karcher, C., Fischer, A., Schweickert, A., Skryabin, B. et al. (2002). The ion channel polycystin-2 is required for left-right axis determination in mice. Curr. Biol. 12, 938-943. [DOI] [PubMed] [Google Scholar]
- Qiu, D., Cheng, S. M., Wozniak, L., McSweeney, M., Perrone, E. and Levin, M. (2005). Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev. Dyn. 234, 176-189. [DOI] [PubMed] [Google Scholar]
- Ramsdell, A. F. (2005). Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 288, 1-20. [DOI] [PubMed] [Google Scholar]
- Raya, A., Kawakami, Y., Rodriguez-Esteban, C., Ibanes, M., Rasskin-Gutman, D., Rodriguez-Leon, J., Buscher, D., Feijo, J. A. and Izpisua Belmonte, J. C. (2004). Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature 427, 121-128. [DOI] [PubMed] [Google Scholar]
- Rebagliati, M. R., Toyama, R., Fricke, C., Haffter, P. and Dawid, I. B. (1998). Zebrafish nodal-related genes are implicated in axial patterning and establishing left-right asymmetry. Dev. Biol. 199, 261-272. [DOI] [PubMed] [Google Scholar]
- Robert, A., Margall-Ducos, G., Guidotti, J. E., Bregerie, O., Celati, C., Brechot, C. and Desdouets, C. (2007). The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci. 120, 628-637. [DOI] [PubMed] [Google Scholar]
- Ross, A. J., May-Simera, H., Eichers, E. R., Kai, M., Hill, J., Jagger, D. J., Leitch, C. C., Chapple, J. P., Munro, P. M., Fisher, S. et al. (2005). Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 37, 1135-1140. [DOI] [PubMed] [Google Scholar]
- Schrick, J. J., Onuchic, L. F., Reeders, S. T., Korenberg, J., Chen, X. N., Moyer, J. H., Wilkinson, J. E. and Woychik, R. P. (1995). Characterization of the human homologue of the mouse Tg737 candidate polycystic kidney disease gene. Hum. Mol. Genet. 4, 559-567. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond, T., Asbrand, C., Bakkers, J., Kuhl, M., Schaeffer, H. J., Huelsken, J., Behrens, J., Hammerschmidt, M. and Birchmeier, W. (2002). The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 16, 2073-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweickert, A., Weber, T., Beyer, T., Vick, P., Bogusch, S., Feistel, K. and Blum, M. (2007). Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 17, 60-66. [DOI] [PubMed] [Google Scholar]
- Seifert, J. R. and Mlodzik, M. (2007). Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126-138. [DOI] [PubMed] [Google Scholar]
- Shimada, Y., Yonemura, S., Ohkura, H., Strutt, D. and Uemura, T. (2006). Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev. Cell 10, 209-222. [DOI] [PubMed] [Google Scholar]
- Simon, M. A. (2004). Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development 131, 6175-6184. [DOI] [PubMed] [Google Scholar]
- Simons, M., Gloy, J., Ganner, A., Bullerkotte, A., Bashkurov, M., Kronig, C., Schermer, B., Benzing, T., Cabello, O. A., Jenny, A. et al. (2005). Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 37, 537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spéder, P., Petzoldt, A., Suzanne, M. and Noselli, S. (2007). Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr. Opin. Genet. Dev. 17, 351-358. [DOI] [PubMed] [Google Scholar]
- Steinhauer, J. and Kalderon, D. (2006). Microtubule polarity and axis formation in the Drosophila oocyte. Dev. Dyn. 235, 1455-1468. [DOI] [PubMed] [Google Scholar]
- Stern, C. (1982). Experimental reversal of polarity in chick embryo epiblast sheets in vitro. Exp. Cell Res. 140, 468-471. [DOI] [PubMed] [Google Scholar]
- Strutt, H. and Strutt, D. (2002). Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev. Cell 3, 851-863. [DOI] [PubMed] [Google Scholar]
- Supp, D. M., Witte, D. P., Potter, S. S. and Brueckner, M. (1997). Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature 389, 963-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp, D. M., Brueckner, M., Kuehn, M. R., Witte, D. P., Lowe, L. A., McGrath, J., Corrales, J. and Potter, S. S. (1999). Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development Suppl. 126, 5495-5504. [DOI] [PMC free article] [PubMed]
- Tabin, C. (2005). Do we know anything about how left-right asymmetry is first established in the vertebrate embryo? J. Mol. Histol. 36, 317-323. [DOI] [PubMed] [Google Scholar]
- Takano, K., Ito, Y., Obata, S., Oinuma, T., Komazaki, S., Nakamura, H. and Asashima, M. (2007). Heart formation and left-right asymmetry in separated right and left embryos of a newt. Int. J. Dev. Biol. 51, 265-272. [DOI] [PubMed] [Google Scholar]
- Taulman, P. D., Haycraft, C. J., Balkovetz, D. F. and Yoder, B. K. (2001). Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol. Biol. Cell 12, 589-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, J. L. and Beales, P. L. (2007). Bardet-Biedl syndrome: beyond the cilium. Pediatr. Nephrol. 22, 926-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree, D. R., Ma, D. and Axelrod, J. D. (2002a). A three-tiered mechanism for regulation of planar cell polarity. Semin. Cell Dev. Biol. 13, 217-224. [DOI] [PubMed] [Google Scholar]
- Tree, D. R., Shulman, J. M., Rousset, R., Scott, M. P., Gubb, D. and Axelrod, J. D. (2002b). Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109, 371-381. [DOI] [PubMed] [Google Scholar]
- Voiculescu, O., Bertocchini, F., Wolpert, L., Keller, R. E. and Stern, C. D. (2007). The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449, 1049-1052. [DOI] [PubMed] [Google Scholar]
- Wang, Y. and Nathans, J. (2007). Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134, 647-658. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Badea, T. and Nathans, J. (2006). Order from disorder: self-organization in mammalian hair patterning. Proc. Natl. Acad. Sci. USA 103, 19800-19805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, D., Saijoh, Y., Nonaka, S., Sasaki, G., Ikawa, Y., Yokoyama, T. and Hamada, H. (2003). The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development 130, 1725-1734. [DOI] [PubMed] [Google Scholar]
- Weber, B., Hoppe, C., Faber, J., Axmacher, N., Fliessbach, K., Mormann, F., Weis, S., Ruhlmann, J., Elger, C. E. and Fernandez, G. (2006). Association between scalp hair-whorl direction and hemispheric language dominance. Neuroimage 30, 539-543. [DOI] [PubMed] [Google Scholar]
- Wei, Y. and Mikawa, T. (2000). Formation of the avian primitive streak from spatially restricted blastoderm: evidence for polarized cell division in the elongating streak. Development 127, 87-96. [DOI] [PubMed] [Google Scholar]
- Whitman, M. and Mercola, M. (2001). TGF-beta superfamily signaling and left-right asymmetry. Sci. STKE 2001, RE1. [DOI] [PubMed] [Google Scholar]
- Xu, J., Van Keymeulen, A., Wakida, N. M., Carlton, P., Berns, M. W. and Bourne, H. R. (2007). Polarity reveals intrinsic cell chirality. Proc. Natl. Acad. Sci. USA 104, 9296-9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. H., Axelrod, J. D. and Simon, M. A. (2002). Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108, 675-688. [DOI] [PubMed] [Google Scholar]
- Yen, H. J., Tayeh, M. K., Mullins, R. F., Stone, E. M., Sheffield, V. C. and Slusarski, D. C. (2006). Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum. Mol. Genet. 15, 667-677. [DOI] [PubMed] [Google Scholar]
- Yost, H. J. (1992). Regulation of vertebrate left-right asymmetries by extracellular matrix. Nature 357, 158-161. [DOI] [PubMed] [Google Scholar]
- Zallen, J. A. (2007). Planar polarity and tissue morphogenesis. Cell 129, 1051-1063. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. Q., Hu, Y., Wang, B. J., Lin, Z. X., Naus, C. C. and Nicholson, B. J. (2003). Effective asymmetry in gap junctional intercellular communication between populations of human normal lung fibroblasts and lung carcinoma cells. Carcinogenesis 25, 473-482. [DOI] [PubMed] [Google Scholar]