Abstract
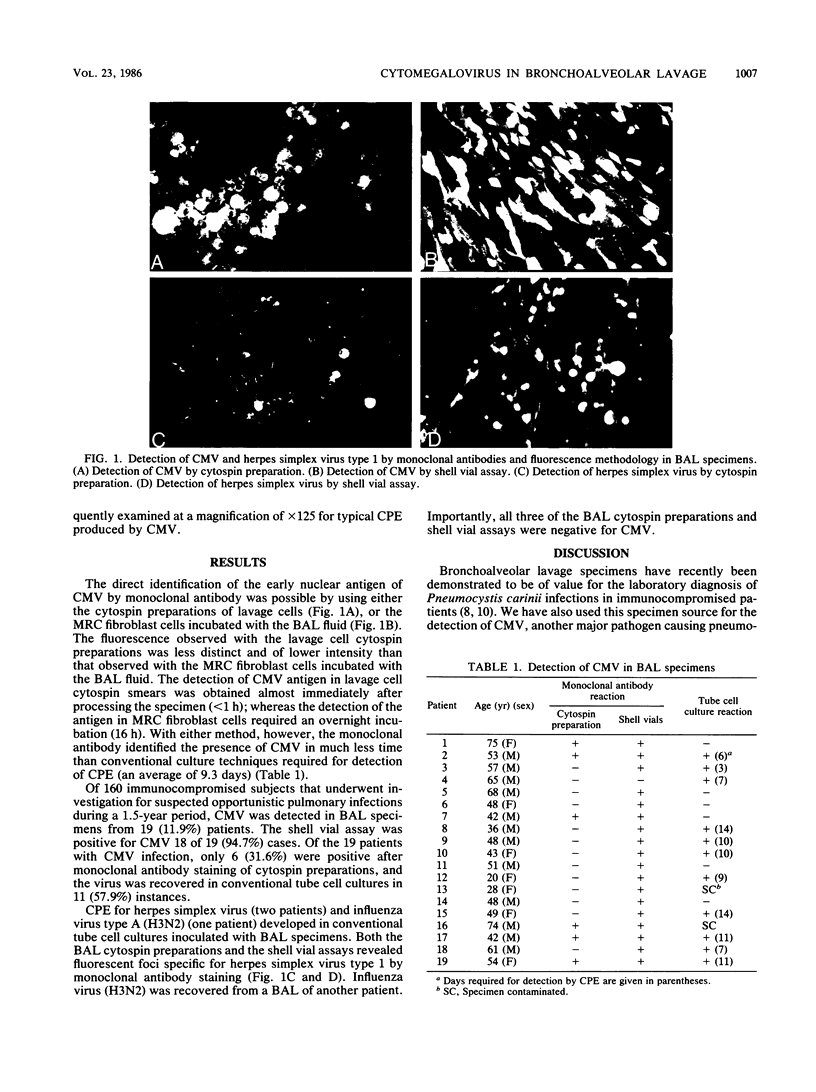
Cytomegalovirus (CMV), a common cause of pneumonia in immunocompromised subjects, is conventionally diagnosed in the laboratory by tube cell culture assays or by detection of characteristic inclusions in histologic sections. Of 160 immunocompromised patients, CMV infection was diagnosed in 19 subjects by bronchoalveolar lavage (BAL), using a monoclonal antibody directed against an early nuclear antigen of the virus. Cytospin preparations from BAL and MRC-5 cell cultures inoculated with the BAL specimens yielded positive results for 6 (31.6%) and 18 (94%) of the 19 subjects, respectively, within hours of the bronchoscopic procedure, whereas conventional tube cell cultures were positive for 11 of the 19 subjects (57.9%) only after an average of 9.3 days. The monoclonal antibody method permitted easy and rapid detection of CMV in BAL specimens.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Comparison of standard tube and shell vial cell culture techniques for the detection of cytomegalovirus in clinical specimens. J Clin Microbiol. 1985 Feb;21(2):217–221. doi: 10.1128/jcm.21.2.217-221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984 Jun;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Wilson D. J., Wold A. D., Smith T. F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985 Jan;21(1):29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Williams D. E., Dines D. E., Sanderson D. R. Interstitial lung disease. Assessment by bronchoalveolar lavage. Mayo Clin Proc. 1983 Nov;58(11):751–757. [PubMed] [Google Scholar]
- Meyers J. D., Leszczynski J., Zaia J. A., Flournoy N., Newton B., Snydman D. R., Wright G. G., Levin M. J., Thomas E. D. Prevention of cytomegalovirus infection by cytomegalovirus immune globulin after marrow transplantation. Ann Intern Med. 1983 Apr;98(4):442–446. doi: 10.7326/0003-4819-98-4-442. [DOI] [PubMed] [Google Scholar]
- Ognibene F. P., Shelhamer J., Gill V., Macher A. M., Loew D., Parker M. M., Gelmann E., Fauci A. S., Parrillo J. E., Masur H. The diagnosis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome using subsegmental bronchoalveolar lavage. Am Rev Respir Dis. 1984 Jun;129(6):929–932. doi: 10.1164/arrd.1984.129.6.929. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Balfour H. H., Jr, Marker S. C., Fryd D. S., Howard R. J., Simmons R. L. Cytomegalovirus disease in renal allograft recipients: a prospective study of the clinical features, risk factors and impact on renal transplantation. Medicine (Baltimore) 1980 Jul;59(4):283–300. [PubMed] [Google Scholar]
- Stover D. E., Zaman M. B., Hajdu S. I., Lange M., Gold J., Armstrong D. Bronchoalveolar lavage in the diagnosis of diffuse pulmonary infiltrates in the immunosuppressed host. Ann Intern Med. 1984 Jul;101(1):1–7. doi: 10.7326/0003-4819-101-1-1. [DOI] [PubMed] [Google Scholar]



