Abstract
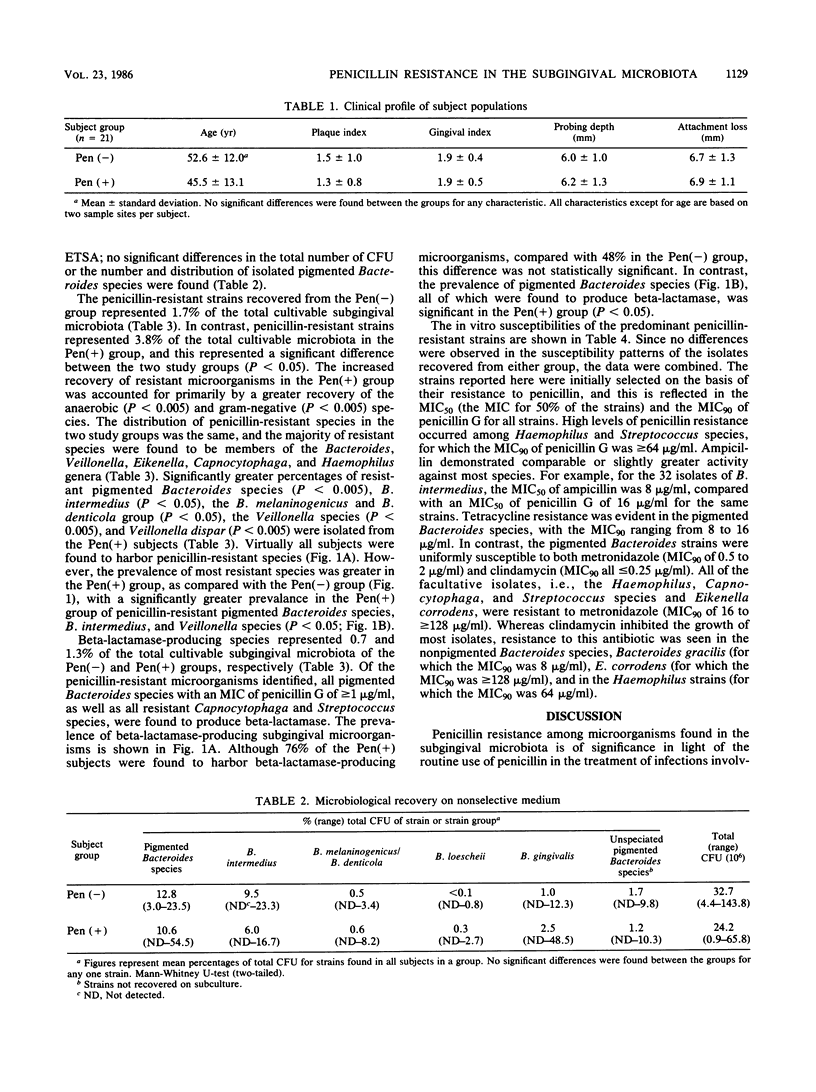
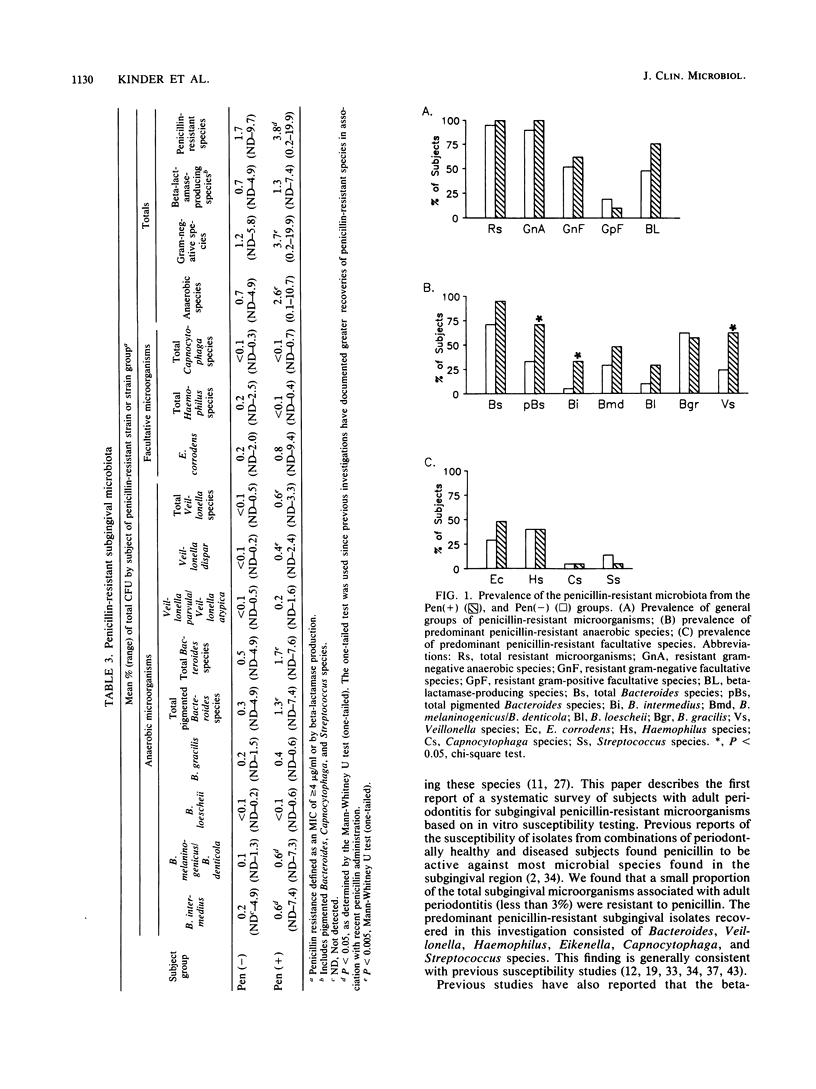
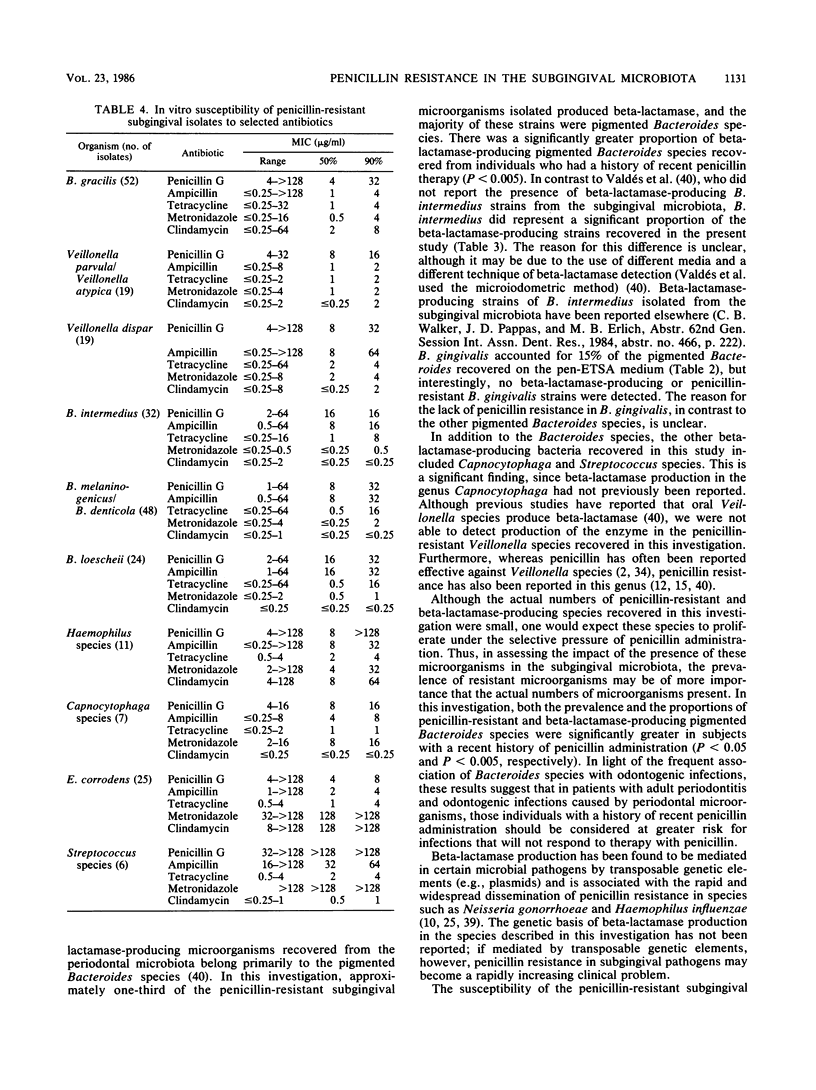
In this investigation, the penicillin-resistant and beta-lactamase-producing subgingival microbiota associated with adult periodontitis was identified, and the impact of a recent exposure to penicillin on the recovery of resistant organisms from this microbiota was assessed. Subjects with adult periodontitis were examined clinically and microbiologically. Twenty-one subjects had a documented history of penicillin therapy within the previous 6 months whereas an additional 21 subjects had no history of antibiotic use within 1 year. Subgingival plaque samples were cultured anaerobically on nonselective and penicillin-containing elective media. MICs and beta-lactamase production were determined for the isolates from the elective medium. The penicillin-resistant microbiota consisted primarily of gram-negative organisms, including Bacteroides, Veillonella, Haemophilus, Eikenella, and Capnocytophaga species. The prevalence (P less than 0.05) and proportions (P less than 0.005) of both penicillin-resistant pigmented Bacteroides and Veillonella species were significantly greater in subjects with recent penicillin exposure. Of the penicillin-resistant genera identified, beta-lactamase production was detected in species of pigmented Bacteroides, Capnocytophaga, and Streptococcus. The prevalence of beta-lactamase-producing Bacteroides species was significantly greater in subjects with recent penicillin exposure (P less than 0.05). Of the antibiotics examined, no single agent was uniformly effective against all of the penicillin-resistant strains, but metronidazole and clindamycin were active against all of the penicillin-resistant pigmented Bacteroides strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahn S. L., Ciola B., Segal A. G. Penicillin-resistant bacteroides melaninogenicus infection of the mandible. J Oral Surg. 1981 Mar;39(3):221–223. [PubMed] [Google Scholar]
- Baker P. J., Slots J., Genco R. J., Evans R. T. Minimal inhibitory concentrations of various antimicrobial agents for human oral anaerobic bacteria. Antimicrob Agents Chemother. 1983 Sep;24(3):420–424. doi: 10.1128/aac.24.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. G., O'Keefe P. The bacteriology of perimandibular space infections. J Oral Surg. 1979 Jun;37(6):407–409. [PubMed] [Google Scholar]
- Brook I., Gober A. E. Emergence of beta-lactamase-producing aerobic and anaerobic bacteria in the oropharynx of children following penicillin chemotherapy. Clin Pediatr (Phila) 1984 Jun;23(6):338–341. doi: 10.1177/000992288402300608. [DOI] [PubMed] [Google Scholar]
- Brook I., Grimm S., Kielich R. B. Bacteriology of acute periapical abscess in children. J Endod. 1981 Aug;7(8):378–380. doi: 10.1016/S0099-2399(81)80060-X. [DOI] [PubMed] [Google Scholar]
- Brook I. beta-Lactamase-producing bacteria recovered after clinical failures with various penicillin therapy. Arch Otolaryngol. 1984 Apr;110(4):228–231. doi: 10.1001/archotol.1984.00800300020004. [DOI] [PubMed] [Google Scholar]
- Chow A. W., Roser S. M., Brady F. A. Orofacial odontogenic infections. Ann Intern Med. 1978 Mar;88(3):392–402. doi: 10.7326/0003-4819-88-3-392. [DOI] [PubMed] [Google Scholar]
- Edson R. S., Rosenblatt J. E., Lee D. T., McVey E. A., 3rd Recent experience with antimicrobial susceptibility of anaerobic bacteria: increasing resistance to penicillin. Mayo Clin Proc. 1982 Dec;57(12):737–741. [PubMed] [Google Scholar]
- Genco C. A., Knapp J. S., Clark V. L. Conjugation of plasmids of Neisseria gonorrhoeae to other Neisseria species: potential reservoirs for the beta-lactamase plasmid. J Infect Dis. 1984 Sep;150(3):397–401. doi: 10.1093/infdis/150.3.397. [DOI] [PubMed] [Google Scholar]
- Heimdahl A., von Konow L., Nord C. E. Beta-lactamase-producing Bacteroides species in the oral cavity in relation to penicillin therapy. J Antimicrob Chemother. 1981 Sep;8(3):225–229. doi: 10.1093/jac/8.3.225. [DOI] [PubMed] [Google Scholar]
- Heimdahl A., von Konow L., Nord C. E. Isolation of beta-lactamase-producing Bacteroides strains associated with clinical failures with penicillin treatment of human orofacial infections. Arch Oral Biol. 1980;25(10):689–692. doi: 10.1016/0003-9969(80)90102-8. [DOI] [PubMed] [Google Scholar]
- Heimdahl A., von Konow L., Satoh T., Nord C. E. Clinical appearance of orofacial infections of odontogenic origin in relation to microbiological findings. J Clin Microbiol. 1985 Aug;22(2):299–302. doi: 10.1128/jcm.22.2.299-302.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman K. S., Holt S. C., Robertson P. B. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J Periodontal Res. 1981 Jul;16(4):363–371. doi: 10.1111/j.1600-0765.1981.tb00987.x. [DOI] [PubMed] [Google Scholar]
- LOE H., SILNESS J. PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand. 1963 Dec;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Laatsch L. J., Hohenfeldt P. R., Kos W. L. Antibiotic susceptibility of black-pigmented Bacteroides isolates from the human oral cavity. Antimicrob Agents Chemother. 1982 Oct;22(4):698–700. doi: 10.1128/aac.22.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C. Exological relationships of bacteria involved in a simple, mixed anaerobic infection. Infect Immun. 1980 Jan;27(1):44–50. doi: 10.1128/iai.27.1.44-50.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Rosenblatt J. E. Penicillin resistance and penicillinase production in clinical isolates of Bacteroides melaninogenicus. Antimicrob Agents Chemother. 1977 Apr;11(4):605–608. doi: 10.1128/aac.11.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The emergence of bacterial resistance and its influence on empiric therapy. Rev Infect Dis. 1983 Mar-Apr;5 (Suppl 1):S9–20. doi: 10.1093/clinids/5.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- Pearlman E., Engleberg N. C., Eisenstein B. I. Identification of protein antigens of Legionella pneumophila serogroup 1. Infect Immun. 1985 Jan;47(1):74–79. doi: 10.1128/iai.47.1.74-79.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILNESS J., LOE H. PERIODONTAL DISEASE IN PREGNANCY. II. CORRELATION BETWEEN ORAL HYGIENE AND PERIODONTAL CONDTION. Acta Odontol Scand. 1964 Feb;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Wong J., Wilkins T. D. Beta-Lactamase activity in strains of Bacteroides melaninogenicus and Bacteroides oralis. Antimicrob Agents Chemother. 1977 Jan;11(1):142–146. doi: 10.1128/aac.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist G. K., Eckerbom M. I., Larsson A. P., Sjögren U. T. Capacity of anaerobic bacteria from necrotic dental pulps to induce purulent infections. Infect Immun. 1979 Aug;25(2):685–693. doi: 10.1128/iai.25.2.685-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Susceptibility of anaerobic bacteria to 23 antimicrobial agents. Antimicrob Agents Chemother. 1976 Oct;10(4):736–752. doi: 10.1128/aac.10.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Jones M. J., Ghoneim A. T. Antimicrobial susceptibilities of bacteria associated with periodontal disease. Antimicrob Agents Chemother. 1983 Mar;23(3):483–486. doi: 10.1128/aac.23.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Survival of human dental plaque flora in various transport media. Appl Microbiol. 1972 Oct;24(4):638–644. doi: 10.1128/am.24.4.638-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Svanberg M., Svanberg G. The predominant cultivable dental plaque flora of beagle dogs with gingivitis. J Periodontal Res. 1980 Mar;15(2):123–136. doi: 10.1111/j.1600-0765.1980.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Ampicillin-resistant Haemophilus influenzae. 1. Incidence, mechanism, and detection. Postgrad Med. 1982 Jan;71(1):133-6, 140, 144-5. doi: 10.1080/00325481.1982.11715965. [DOI] [PubMed] [Google Scholar]
- Valdés M. V., Lobbins P. M., Slots J. Beta-lactamase producing bacteria in the human oral cavity. J Oral Pathol. 1982 Feb;11(1):58–63. doi: 10.1111/j.1600-0714.1982.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Whitcher B. L., Beirne O. R., Smith R. A. Beta-lactamase-producing Bacteroides melaninogenicus and osteomyelitis of the mandible. J Oral Med. 1983 Jan-Mar;38(1):17–20. [PubMed] [Google Scholar]
- White D., Mayrand D. Association of oral Bacteroides with gingivitis and adult periodontitis. J Periodontal Res. 1981 May;16(3):259–265. doi: 10.1111/j.1600-0765.1981.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Williams B. L., McCann G. F., Schoenknecht F. D. Bacteriology of dental abscesses of endodontic origin. J Clin Microbiol. 1983 Oct;18(4):770–774. doi: 10.1128/jcm.18.4.770-774.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Konow L., Nord C. E., Nordenram A. Anaerobic bacteria in dentoalveolar infections. Int J Oral Surg. 1981 Oct;10(5):313–322. doi: 10.1016/s0300-9785(81)80027-0. [DOI] [PubMed] [Google Scholar]
- von Konow L., Nord C. E. Ornidazole compared to phenoxymethylpenicillin in the treatment of orofacial infections. J Antimicrob Chemother. 1983 Mar;11(3):207–215. doi: 10.1093/jac/11.3.207. [DOI] [PubMed] [Google Scholar]


