Abstract
Trypanosoma brucei is the causative agent of African Sleeping Sickness in humans and one of the causes of Nagana in cattle. This protozoan parasite evades the host immune system by antigenic variation, a periodic switching of its variant surface glycoprotein (VSG) coat. VSG switching is spontaneous and occurs at a rate of about 10-2 –10-3 per population doubling in recent isolates from nature, but at a dramatically reduced rate (10-5-10-6) in laboratory-adapted strains1-3. VSG switching is thought to occur predominantly through gene conversion, a form of homologous recombination (HR) initiated by a DNA lesion that is used by other pathogens (e.g. Candida albicans, Borrelia sp. and Neisseria gonorrhoeae) to generate surface protein diversity, and by B lymphocytes of the vertebrate immune system to generate antibody diversity. Very little is known about the molecular mechanism of VSG switching in T. brucei. Here we demonstrate that the introduction of a DNA double-stranded break (DSB) adjacent to the ∼70-bp repeats upstream of the transcribed VSG increases switching in vitro ∼250-fold, producing switched clones with a frequency and features similar to those generated early in an infection. We were also able to detect spontaneous DSBs within the 70-bp repeats upstream of the actively transcribed VSG, suggesting that a DSB is a natural intermediate of VSG gene conversion and that VSG switching is the result of the resolution of this DSB by break-induced replication (BIR).
The T. brucei genome contains >1,000 VSG genes and pseudogenes, yet the single transcribed VSG is invariably found in one of ∼15 large (40-60 kb) telomeric expression sites (ESs)4-6. VSG switching can be achieved by shifting transcription from one ES to another (in situ switch) or by reciprocal translocations between two ESs (telomere exchange), but most switching occurs by copying a new VSG into the actively transcribed ES by duplicative gene conversion2, 7-12. Antigenic switching by gene conversion has been proposed to be initiated by a DSB within or upstream of the actively transcribed VSG9, 10, but physical evidence for a DSB has been lacking. To determine whether a DSB within the transcribed ES is sufficient to precipitate an antigenic switch, we introduced the heterologous recognition sequence (RS) for the yeast mitochondrial endonuclease I-SceI adjacent to the 70-bp repeat region upstream of the VSG 221 locus (70.II cell line; Fig. 1a). I-SceI has previously been used to introduce targeted DSBs in several organisms, including T. brucei13-16. Regulation of the I-SceI enzyme was achieved through stable transfection under the control of an inducible promoter.
Figure 1. Antigenic switching is induced by a single I-SceI-generated DSB.
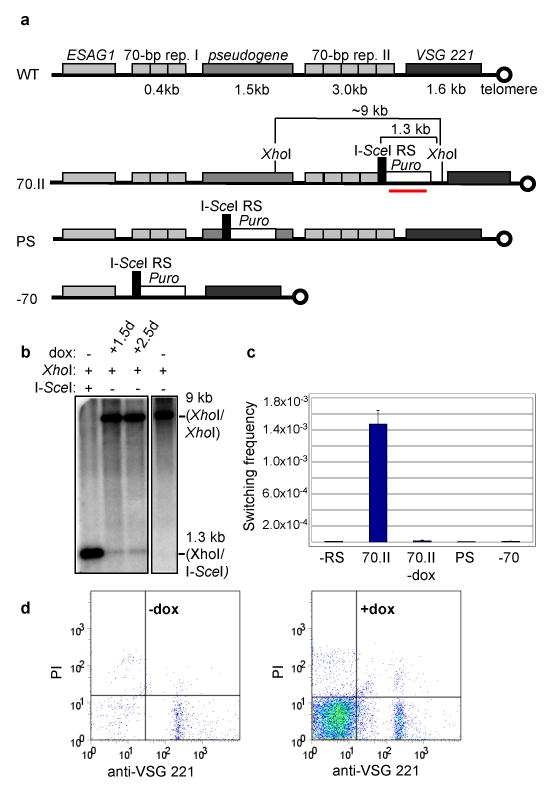
a, Schematic of the telomeric region of the VSG 221 ES (WT). An I-SceI RS was introduced adjacent to the 70-bp repeat region (70.II), within the pseudogene (PS), and in place of the 70-bp repeats (-70). b, I-SceI cuts in vivo. DNA was cut with recombinant I-SceI and XhoI in lane 1 and XhoI in lanes 2, 3, and 4. The Southern blot was probed with a Puro probe (underlined in red in a). c, Switching frequency in 70.II is increased ∼250× above levels in the absence of an RS (-RS) or without I-SceI induction (70.II -dox). This increase was not observed for PS or −70. Error bars represent s.e.m. for ≥3 experiments. d, Representative flow cytometry plots for uninduced (-dox) and induced (+dox) 70.II cells. Events in the lower left (221-) and right (221+) quadrants represent switchers and cells not bound by the column, respectively.
The activity of I-SceI was monitored by induction of the enzyme for 1.5 and 2.5 days and subsequent quantitative Southern blotting (Fig. 1b). As expected, an ∼9kb XhoI/XhoI fragment (Fig. 1a) was reduced to ∼1.3 kb upon I-SceI induction (Fig. 1b, lanes 2 and 3), which corresponds to the size shift seen when genomic DNA was digested with XhoI and recombinant I-SceI (Fig. 1b, lane 1). This smaller fragment was not seen when DNA from uninduced cells was digested with XhoI alone (Fig. 1b, lane 4). By measuring the intensity of the bands, we estimated that the action of I-SceI leads to a DSB in ∼1% of the cells.
To accurately measure changes in switching frequency upon I-SceI induction, we developed a magnetic activated cell sorting (MACS) assay in conjunction with conventional flow cytometry. MACS was optimized to enrich for trypanosomes that had switched their VSG (see Methods). The induction of a DSB increased the switching frequency ∼250-fold compared to cells without an I-SceI RS or in the absence of I-SceI induction (1.5 × 10-3, 5.9 × 10-6, and 1.5 × 10-5 per population, respectively) (Figs. 1c and d). This far exceeds any switching frequency reported for laboratory-adapted strains, and is more representative of switching frequencies seen in the early stages of a natural infection. The results indicated that roughly half the cells in which a DSB was generated switched their VSG. The increased switching frequency was not observed when the DSB was induced in the VSG pseudogene upstream of the 70-bp repeat region (PS cell line; Figs. 1a and c), suggesting that the location of the DSB adjacent to the 70-bp repeats is critical to the high frequency of switching seen here.
Repetitive sequences can provide homology for HR17, 18. In T. brucei, all ES-associated VSGs (and probably most silent VSGs) are found downstream of imperfect 70-bp repeats, which have been mapped to the upstream border of VSG switching events10, 19. Removal of the 70-bp repeats, however, did not decrease an already low rate of VSG switching20. To determine whether the 70-bp repeats are necessary for the high frequency of DSB-induced switching, we replaced them with an I-SceI RS (-70 cell line; Fig. 1a). A DSB in the absence of the 70-bp repeats did not increase VSG switching (Fig. 1c), suggesting that the 70-bp repeats do facilitate VSG switching.
The order in which VSGs are expressed during the course of an infection has been described as “semi-predictable” and is thought to be key to protracted illness2, 21. Telomere-proximal VSGs, such as those in silent ESs or mini-chromosomes, are activated first, followed by those in sub-telomeric arrays2, 21, 22. To determine the chromosomal location of the donor VSG and to elucidate whether switching occurred by duplication, reciprocal telomere exchange or in situ switching, we cloned the progeny and identified the expressed VSG in 42 switched clones from several independent experiments, further characterizing 18 clones by rotating agarose gel electrophoresis (RAGE) and Southern blotting. As shown in Fig. 2 and Supplementary Table S1, all of the switchers showed loss of VSG 221 and duplication of a new VSG into the transcribed locus that was previously occupied by VSG 221. In 15 out of 18 switchers (including five involving VSG 224) the donor VSGs resided in another ES (Fig. 2a and Supplementary Table S1), whereas in the other three, the donor VSGs resided on mini-chromosomes (Fig. 2b and Supplementary Table S1). These results are similar to VSG switching during early stages of infections2, 21.
Figure 2. I-SceI-induced antigenic switching occurs by duplicative gene conversion.
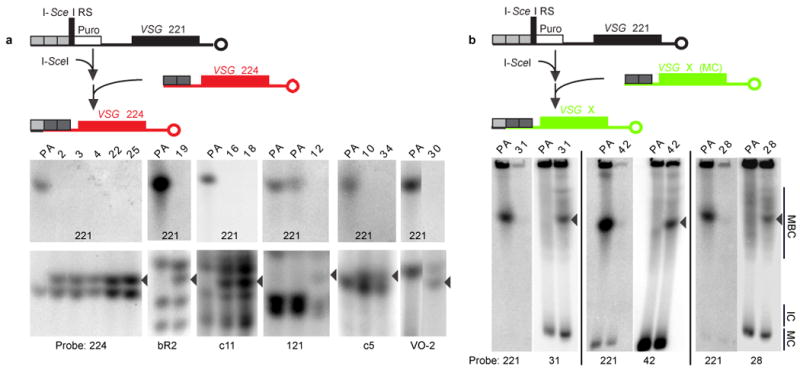
Chromosomes were separated by RAGE and analyzed by Southern blotting. Representative clones are shown. VSG 221 is present in the parental (PA) strain and lost upon I-SceI induction (221 panels). In all switchers (clone numbers are marked on top of each lane), the lost VSG 221 gene is replaced by a VSG gene duplicated from a, a silent ES (224, bR-2, c11, 121, c5, VO-2) or b, a mini-chromosome (MC) (31, 42, 28) that is copied into the ES previously occupied by VSG 221 (arrowheads). Multiple bands represent >1 copy of the VSG gene in the genome.
Since homology is crucial for strand invasion during recombination17, we investigated how the I-SceI-generated DSB was processed after cleavage. We sequenced the repaired region from the five clones that switched to VSG 224. The data revealed that four 70-bp repeats (∼500 bp) in the recipient VSG 221 ES were eliminated, while the processed DSB invaded the first homologous region proximal to the donor VSG 224 ES (Fig. 3a and Supplementary Fig. S1). No remnants of the I-SceI RS were detected.
Figure 3. PCR and sequencing analyses of recipient (VSG 221 ES) and donor (VSG 224 ES).
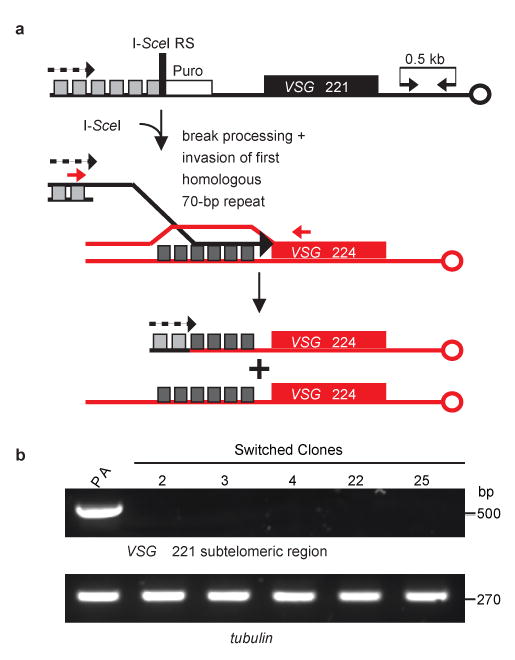
a, PCR and sequencing analyses indicate loss of the I-SceI RS, exonucleolytic degradation and DSB processing, and invasion of the first homologous region in the VSG 224 ES proximal to the VSG. Primers used for PCR are indicated by red arrows. The transcribed ES is indicated by a dotted arrow. For sequence data see Supplementary Fig. S1. b, PCR showing loss of the VSG 221 subtelomeric region (black arrows in panel a) in the switched clones. PA, parental; tubulin is shown as a control.
To distinguish whether antigenic switching was achieved by two crossover events (in the 70-bp repeats and within the C-terminus or 3′ UTR of VSG 221) or by BIR (resolution of a single DSB followed by replication through the telomere), we PCR-amplified the unique region between VSG 221 and its telomere. In the 70.II cell line (parental, PA), an ∼500 bp fragment was amplified (Fig. 3b and Supplementary Fig. S2). In all switched clones, this VSG 221-specific sub-telomeric region was lost (Fig. 3b and Supplementary Fig. S2) and presumably replaced by the sub-telomeric region from the incoming VSG. Although we cannot rule out a second crossover within the telomere tract, these results implicate BIR as the predominant mechanism for early VSG switching.
Thus far, our experiments demonstrate that an exogenous DNA break adjacent to the 70-bp repeats of the active ES is a potent stimulator of VSG switching. To directly determine whether such breaks occur naturally in vivo, we performed ligation-mediated (LM)-PCR on DNA derived from unmanipulated, wild-type trypanosomes. LM-PCR consists of the ligation of a double-stranded DNA linker to high-quality genomic DNA followed by amplification of the region adjacent to the break using linker-specific and locus-specific primers, and detection of specific bands by Southern blotting and hybridization with locus-specific probes. Using this method we could readily detect DNA breaks distributed over the 70-bp repeat region (70-bp II) in the active VSG 221 ES (Fig. 4a). We also detected less frequent DNA breaks upstream of the pseudogene that were co-incident with a much smaller tract of 70-bp repeats (70-bp I), both by size (Fig. 4b) and sequence (i.e. the bands present in Fig. 4b were identical to those revealed when the Southern blot was probed with a 70-bp repeat probe; data not shown). We were unable to detect DNA breaks within the 70-bp repeats of an inactive ES (Fig. 4c) or at a chromosome-internal locus (histone-3 variant, Fig. 4d). The majority of breaks were staggered, and needed to be blunted by T4 polymerase for the LM-PCR primers to be ligated (Fig. 4a, left vs. right). These results demonstrate that DSBs occur frequently and specifically within the 70-bp tracts of the active ES of unmanipulated, wild-type trypanosomes. Alternatively, DSBs could occur throughout the ES, but only persist long enough within the 70-bp repeats to allow detection. It is possible that DSBs occur more frequently in trypanosomes that have not been laboratory adapted, which would be consistent with our previously proposed model in which active ES telomere length and breakage modulate VSG switching23, 24.
Figure 4. Wild-type trypanosomes incur staggered DSBs specifically at the 70-bp repeat regions of the active ES.
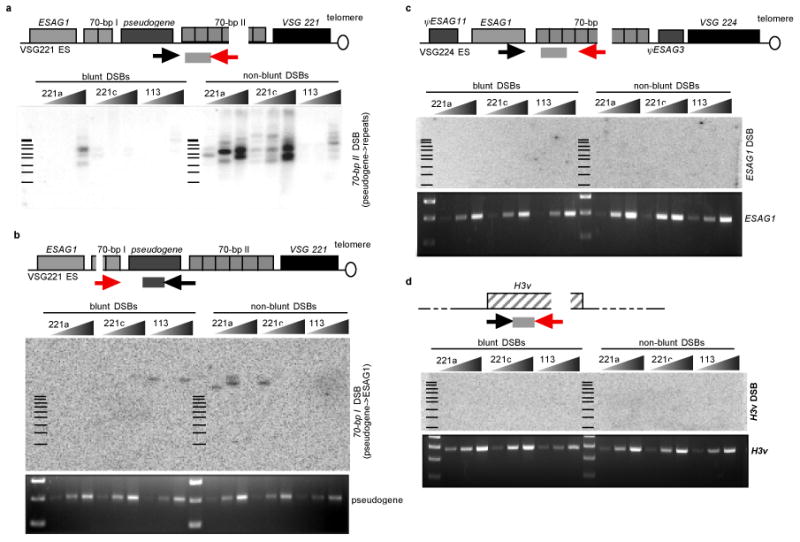
a, LM-PCR over the active ES reveals DSBs within the 70-bp repeat region. A schematic appears over the autoradiogram and the location of LM-PCR primers and DNA probe are indicated as follows: red arrow, DSB-specific (linker-specific) primer; black arrow, locus-specific primer; gray bar, probe. Triangles denote 5-fold dilutions of input DNA from 2 VSG 221-expressing (221a, 221c) and 1 VSG 1.13-expressing (113) cell lines. Bars indicate location of 100-bp ladder. b, Top: probing the active ES for DSBs upstream of the pseudogene reveals infrequent breaks. The sizes of the amplicons indicate that the breakpoints are within the upstream 70-bp repeat region (70-bp I). Bottom: amplification of the pseudogene locus with the forward and reverse primers used for LM-PCR in (a) and (b) serves as loading control. c and d, Top: LM-PCR for the presence of DSBs at a silent ES (VSG 224) and chromosome internal locus (histone-3 variant). Bottom: loading control.
Although the hypothesis that antigenic switching by gene conversion is initiated by a DSB is not new, it had not been experimentally investigated. It has been proposed that a DSB could be generated by an unidentified endogenous endonuclease or that transcription over highly repetitive sequences, such as the 70-bp repeats, could destabilize the active ES locus and cause a DSB10. We favor the latter hypothesis, especially because the TAA:TTA motif that is present within the 70-bp repeats has an intrinsic propensity to destabilize the DNA helix25.
Our results suggest that a DSB is a natural trigger for VSG switching, that repair of the DSB is likely achieved through BIR, and that the mechanistic function of the 70-bp repeats is to facilitate BIR through homology recognition. These results provide insights into the molecular mechanisms of VSG switching and may be relevant to other pathogens that express genes essential for host immune evasion from telomeric loci, as well as to the telomeric immunoglobulin heavy chain locus that diversifies in B lymphocytes.
Methods Summary
For the MACS assay, I-SceI was induced with 0.1 ug/ml doxycycline (Sigma) for 3 days. ∼7.5 × 107 cells were harvested by centrifugation and incubated with 175 ul rabbit α-VSG 221 serum (1:100 in HMI-926) at 4°C for 10 minutes while gently vortexing. Cells were washed with HMI-9 and incubated with 110 ul goat α-rabbit Microbeads (Miltenyi Biotech) as above. Cells were washed with HMI-9 and applied to a MidiMACS Separator Column (Miltenyi Biotech) that had been primed with HMI-9. The column was washed with HMI-9. The effluent (i.e. VSG 221- cells) was centrifuged, resuspended in 150 ul Alexa488-conjugated α-VSG 221 Ab (1:400), and incubated for 15 minutes as above. Cells were washed and resuspended in 300 ul HMI-9. Propidium iodide (BD Pharmingen) and CountBright Beads (Invitrogen) were added prior to analysis by flow cytometry. Cells bound to the column (i.e. VSG 221+) were removed with a plunger and counted. Switching frequency was calculated by dividing VSG 221- and PI- cells by the bead count, multiplying by the number of beads added to the sample (co-efficient provided by Invitrogen), and then dividing by the total number of cells plunged from the MACS column.
Supplementary Material
Acknowledgments
This work was supported by awards from the C.H. Revson Foundation (C.E.B.), German National Academic Foundation (K.I.L.), Otto Ritter Foundation (K.I.L.), W.M. Keck Foundation (F.N.P.), Irma T. Hirschl Foundation (F.N.P.) and by Grant No. R01AI021729 from the National Institute of Allergy and Infectious Diseases of the U.S. National Institutes of Health (G.A.M.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH.
Footnotes
Author Contributions All authors conceived of and designed the experiments. C.E.B and T.L. are primarily responsible for the experiments shown in Fig. 1. C.E.B and O.D. are primarily responsible for the experiments shown in Figs. 2 and 3. F.N.P. is primarily responsible for the experiments in Fig. 4. C.E.B., O.D., G.A.M.C., and F.N.P. wrote the manuscript.
Supplementary Information accompanies the paper on www.nature.com/nature.
References
- 1.Horn D, Cross GAM. Analysis of Trypanosoma brucei vsg expression site switching in vitro. Mol Biochem Parasitol. 1997;84:189–201. doi: 10.1016/s0166-6851(96)02794-6. [DOI] [PubMed] [Google Scholar]
- 2.Robinson NP, Burman N, Melville SE, Barry JD. Predominance of duplicative VSG gene conversion in antigenic variation in African trypanosomes. Mol Cell Biol. 1999;19:5839–46. doi: 10.1128/mcb.19.9.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner CM. The rate of antigenic variation in fly-transmitted and syringe-passaged infections of Trypanosoma brucei. FEMS Microbiol Lett. 1997;153:227–31. doi: 10.1111/j.1574-6968.1997.tb10486.x. [DOI] [PubMed] [Google Scholar]
- 4.Berriman M, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–22. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 5.de Lange T, Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982;299:451–3. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- 6.Hertz-Fowler C, et al. Telomeric expression sites are highly conserved in Trypanosoma brucei. PLoS ONE. 2008;3:e3527. doi: 10.1371/journal.pone.0003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernards A, et al. Activation of trypanosome surface glycoprotein genes involves a duplication-transposition leading to an altered 3′ end. Cell. 1981;27:497–505. doi: 10.1016/0092-8674(81)90391-3. [DOI] [PubMed] [Google Scholar]
- 8.Hoeijmakers JH, Frasch AC, Bernards A, Borst P, Cross GAM. Novel expression-linked copies of the genes for variant surface antigens in trypanosomes. Nature. 1980;284:78–80. doi: 10.1038/284078a0. [DOI] [PubMed] [Google Scholar]
- 9.Horn D. The molecular control of antigenic variation in Trypanosoma brucei. Curr Mol Med. 2004;4:563–76. doi: 10.2174/1566524043360078. [DOI] [PubMed] [Google Scholar]
- 10.Barry JD, McCulloch R. Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite. Adv Parasitol. 2001;49:1–70. doi: 10.1016/s0065-308x(01)49037-3. [DOI] [PubMed] [Google Scholar]
- 11.Borst P, Ulbert S. Control of VSG gene expression sites. Mol Biochem Parasitol. 2001;114:17–27. doi: 10.1016/s0166-6851(01)00243-2. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JE, Rudenko G. Switching trypanosome coats: what's in the wardrobe? Trends Genet. 2006;22:614–20. doi: 10.1016/j.tig.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Glover L, Alsford S, Beattie C, Horn D. Deletion of a trypanosome telomere leads to loss of silencing and progressive loss of terminal DNA in the absence of cell cycle arrest. Nucleic Acids Res. 2007;35:872–80. doi: 10.1093/nar/gkl1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glover L, McCulloch R, Horn D. Sequence homology and microhomology dominate chromosomal double-strand break repair in African trypanosomes. Nucleic Acids Res. 2008;36:2608–18. doi: 10.1093/nar/gkn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haber JE. Transpositions and translocations induced by site-specific double-strand breaks in budding yeast. DNA Repair (Amst) 2006;5:998–1009. doi: 10.1016/j.dnarep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Zarrin AA, et al. Antibody class switching mediated by yeast endonuclease-generated DNA breaks. Science. 2007;315:377–81. doi: 10.1126/science.1136386. [DOI] [PubMed] [Google Scholar]
- 17.Llorente B, Smith CE, Symington LS. Break-induced replication: what is it and what is it for? Cell Cycle. 2008;7:859–64. doi: 10.4161/cc.7.7.5613. [DOI] [PubMed] [Google Scholar]
- 18.Barzel A, Kupiec M. Finding a match: how do homologous sequences get together for recombination? Nat Rev Genet. 2008;9:27–37. doi: 10.1038/nrg2224. [DOI] [PubMed] [Google Scholar]
- 19.de Lange T, Kooter JM, Luirink J, Borst P. Transcription of a transposed trypanosome surface antigen gene starts upstream of the transposed segment. EMBO J. 1985;4:3299–306. doi: 10.1002/j.1460-2075.1985.tb04080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCulloch R, Rudenko G, Borst P. Gene conversions mediating antigenic variation in Trypanosoma brucei can occur in variant surface glycoprotein expression sites lacking 70-base-pair repeat sequences. Mol Cell Biol. 1997;17:833–43. doi: 10.1128/mcb.17.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison LJ, Majiwa P, Read AF, Barry JD. Probabilistic order in antigenic variation of Trypanosoma brucei. Int J Parasitol. 2005;35:961–72. doi: 10.1016/j.ijpara.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Marcello L, Barry JD. Analysis of the VSG gene silent archive in Trypanosoma brucei reveals that mosaic gene expression is prominent in antigenic variation and is favored by archive substructure. Genome Res. 2007;17:1344–52. doi: 10.1101/gr.6421207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreesen O, Cross GAM. Consequences of telomere shortening at an active VSG expression site in telomerase-deficient Trypanosoma brucei. Eukaryot Cell. 2006;5:2114–9. doi: 10.1128/EC.00059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreesen O, Li B, Cross GAM. Telomere structure and function in trypanosomes: a proposal. Nat Rev Microbiol. 2007;5:70–5. doi: 10.1038/nrmicro1577. [DOI] [PubMed] [Google Scholar]
- 25.Ohshima K, Kang S, Larson JE, Wells RD. TTA.TAA triplet repeats in plasmids form a non-H bonded structure. J Biol Chem. 1996;271:16784–91. doi: 10.1074/jbc.271.28.16784. [DOI] [PubMed] [Google Scholar]
- 26.Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75:985–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


