Abstract
Murine haematopoietic stem cells (HSCs) are contained in the Kit+Sca1+Lin− (KSL) population of bone marrow and are able to repopulate lethally irradiated mice. Myeloproliferative disorders (MPDs) are thought to be clonogenic diseases arising at the level of the HSC. Here, we show that mice expressing low levels of the transcription factor c-Myb, as the result of genetic knockdown, develop a transplantable myeloproliferative phenotype that closely resembles the human disease essential thrombocythaemia (ET). Unlike wild-type cells, the KSL population in c-myb knockdown bone marrow cannot repopulate irradiated mice and does not transfer the disease. Instead, cells positive for Kit and expressing low to medium levels of CD11b acquire self-renewing stem cell properties and are responsible for the perpetuation of the myeloproliferative phenotype.
Keywords: c-Myb, differentiation commitment, haematopoietic stem cell, myeloproliferative disorder, self-renewal
Introduction
At the top of the hierarchy of cells giving rise to the blood cell system sit a small number of haematopoietic stem cells (HSCs) that are able to keep supplying the system without themselves becoming depleted. It is essential that a single, identical cell replaces each HSC committing towards a haematopoietic progenitor fate—if this process of self-renewal is not achieved then the complement of HSCs will eventually be depleted. By contrast, an excess of self-renewal could result in over expansion potentially leading to leukaemia (Dick, 2003). The concept that haematological malignancies have a stem cell component that is responsible for their aberrant self-renewal properties arose originally from studies on acute myeloid leukaemia (AML) in which it was found that a small subpopulation of the leukaemic cells was capable of initiating the leukaemia when transplanted into immunocompromised mice (Bonnet and Dick, 1997). Subsequently, evidence has emerged for a similar stem cell-like component in other haematological malignancies, including chronic myeloid leukaemia (CML) (Jamieson et al, 2004), acute lymphocytic leukaemia T-ALL (Cox et al, 2007) and myeloma (Matsui et al, 2004).
The regulation of gene transcription is recognised as a central component of the HSCs control, and a number of recent studies, involving gene knockout or overexpression in mice, have indicated a function for specific transcription factors (Lessard et al, 2004). In parallel with the emerging concept of the leukaemic stem cell, it is becoming clear that at least some leukaemias arise from transforming mutations in transcription factor genes that lead to aberrant self-renewal capacity occurring in either the HSC itself or in downstream progenitors (Huntly and Gilliland, 2005), whereas changes in the expression of certain crucial regulators can predispose mice to the development of specific leukaemias (Rosenbauer et al, 2005).
The c-Myb transcription factor, originally identified as the oncogenic component of two different avian leukaemia viruses (Lipsick and Wang, 1999), is the founding member of a family of proteins, characterised by a highly conserved DNA-binding domain (Frampton et al, 1989), which are implicated in regulatory decisions affecting cell proliferation, differentiation and apoptosis (Oh and Reddy, 1999).
The c-Myb has been shown to be crucial in the development of the haematopoietic system and in c-myb knockout mice dying of anemia at day 15 of gestation because of a failure to develop adult haematopoiesis in the foetal liver (Mucenski et al, 1991). Nevertheless, commitment to definitive haematopoiesis can occur in the absence of c-Myb (Allen et al, 1999; Sumner et al, 2000). Analysis of mice expressing a knockdown allele of c-myb, undergoing conditional gene deletion, or overexpressing miR-150, a micro-RNA (miRNA) that targets the c-myb transcript, showed that the level of c-Myb expression controls the differentiation along individual blood cell lineages (Allen et al, 1999; Emambokus et al, 2003; Bender et al, 2004; Thomas et al, 2005; Vegiopoulos et al, 2006; Xiao et al, 2007), but these studies did not investigate any direct influence of c-Myb on HSCs. More recently, examination of mice homozygous for a point mutation in the p300/CBP interacting domain of c-Myb (M303V) showed an increase in the relative number of HSCs, possibly through an influence on quiescence (Sandberg et al, 2005).
Reflecting the central importance of c-Myb in haematopoiesis, evidence has recently emerged for a role of c-Myb in leukaemia. Hence, c-myb gene duplications or translocations of the c-myb gene to the TCRβ locus have been observed in a significant proportion of childhood T-ALLs (Clappier et al, 2007; Lahortiga et al, 2007), whereas a less direct involvement of c-Myb, as a downstream target gene of the t11:19 translocation–fusion transcriptional regulator MLL–ENL in myeloid leukaemia, has also recently been shown (Hess et al, 2006).
Here, through studies using a knockdown allele of the c-myb gene and the generation of radiation chimeras, we show for the first time that c-Myb has a crucial role in the control of both the proliferation and differentiation fate of the HSC, and show that compromise of this control leads to the development of a myeloproliferative disease (MPD)-like phenotype. Downregulation of c-Myb leads to the appearance of a phenotypic KSL population that lacks self-renewal capacity and quiescence, presents an altered transcriptional program and loses the capacity of bone marrow repopulation after transplantation. More importantly, we show that the MPD is not maintained at the level of the KSL HSC but rather by a Kit+CD11blow/med population that acquires self-renewal potential and the capacity to transfer the MPD-like phenotype to recipient animals. The perpetuation of atypical repopulating stem cells in mice transplanted with cells defective in c-myb expression suggests that such mice represent a model for the study of the stem cell component of potentially related human MPDs and for factors that might influence progression towards leukaemia.
Results
Reduced c-Myb levels lead to a myeloid disorder reminiscent of essential thrombocythemia
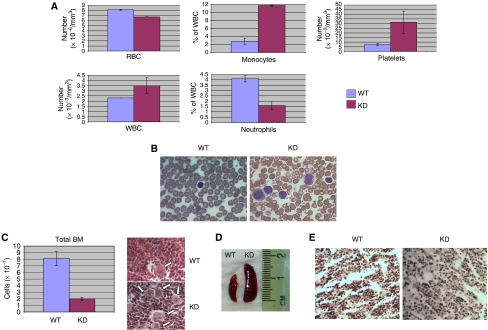
Earlier, we have shown in E14 embryos how homozygosity for a knockdown mutation of c-myb perturbs foetal liver haematopoiesis, most notably by reducing erythroid and granulocytic differentiation and increasing thrombopoiesis (Emambokus et al, 2003). Furthermore, others have shown that point mutations in c-Myb, which partially disable specific functional domains of the protein, or a transgene insertion in an enhancer located 77 kb upstream of c-myb gene cause an increase in platelet numbers (Carpinelli et al, 2004; Sandberg et al, 2005; Mukai et al, 2006). In order to determine how the more profound decrease in all c-Myb functions that are characteristic of our knockdown mutant affects adult haematopoiesis, we analysed peripheral blood and bone marrow of 4- to 6-week-old c-myb knockdown animals. In agreement with our earlier observations on foetal liver haematopoiesis, we were able to show a decrease in red cells and neutrophils, contrasted by an increase in macrophage and platelets, in the blood of c-myb knockdown versus wild type adult mice (Figure 1A). We observed a decreased bone-marrow cellularity (>5-fold) in c-myb knockdown animals, whereas thrombocytosis, as seen in peripheral blood (Figure 1B), was reflected by a dramatic increase in bone marrow megakaryocytes, often arranged in clusters (Figure 1C). Paralleling the anemia, the Ter119/CD71 profiles showed that c-myb knockdown bone marrow showed an increased ratio of immature to mature erythroid cells (Supplementary Figure 1A), whereas the lower percentage of peripheral neutrophils and the reciprocal increase in macrophage was reflected by a relative loss of CD11bhighGr1high cells and an increase in CD11bmedGr1low cells in the bone marrow (Supplementary Figure 1B). The increase in bone marrow megakaryocytes was also apparent from the presence of a high proportion of c-Kit+CD41+ cells (Supplementary Figure 1C), suggestive of an expansion in the number of megakaryocyte-lineage progenitors. Splenomegaly was also a characteristic of the c-myb knockdown animals (Figure 1D). The absence of reticulin staining in the bone marrow in the c-myb knockdowns, characteristic of myelofibrosis, suggests that the phenotype is very similar to that seen in the MPD essential thrombocytosis (ET) (Figure 1E).
Figure 1.
The c-myb knockdown has profound effects on adult haematopoiesis. (A) Histograms comparing the numbers of red blood cells (RBC), platelets, and white blood cells (WBC) and the percentage of monocytes and neutrophils in the WBC fraction in wild-type (WT) and c-myb knockdown (KD) peripheral blood. (B) Diff-Quick staining from WT and KD blood smears. (C) Histogram comparing the total number of cells in WT and KD bone marrow after red cell lysis (mean±s.d., n=7). The micrographs on the right show hematoxylin and eosin-stained paraffin sections of WT and KD bone marrow, the arrows indicating megakaryocytes. (D) Comparative picture of the spleens from WT and KD mice. (E) Reticulin staining from WT and KD bone marrow.
Altered c-Myb activity also profoundly affects haematopoietic stem cells
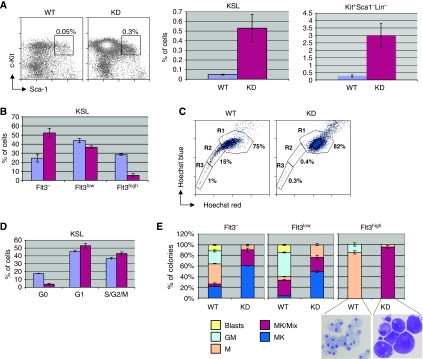
As MPDs are thought to arise clonally at the level of the HSC or multipotential progenitors, we decided to study this compartment in our animal model. We compared the Kit+Sca1+Lin− (KSL) (Pohlmann et al, 2001) and Kit+Sca1−Lin− (committed progenitor) fraction of wild-type and c-myb-knockdown bone marrow. Mutant bone marrow contained approximately fivefold more progenitors Kit+Sca1−Lin− (GMP, MEP, CMP; (Akashi et al, 2000)), as well as KSL cells; however, taking into account the reduced cellularity of the c-myb knockdown bone marrow, this meant that their absolute number was approximately equal in the wild-type and mutant mice (Figure 2A). Unlike Sandberg et al (2005), but paralleling our recent findings on erythroid precursors (Vegiopoulos et al, 2006), we found that c-Kit expression in the KSL population was ∼2–4-fold lower in the c-myb knockdown, whereas other stem-cell surface markers such as CD31, CD34 and CD105 (endoglin) were not altered (data not shown). The KSL population can be further subdivided into functionally distinct cell types based on the expression of CD34 and Flt3 (Adolfsson et al, 2005). Hence, cells capable of long-term reconstitution (LT-HSC; CD34−Flt3−), short-term reconstitution (ST-HSC; CD34+Flt3−), or that are multipotent progenitors incapable of reconstitution (MPP; CD34+Flt3+) can be defined. Comparing the expression of Flt3 on KSL cells, we found that the proportion of Flt3high cells was reduced from 28% in wild-type bone marrow to 6% in c-myb knockdown, with a corresponding increase in Flt3− cells (Figure 2B and Supplementary Figure 2A). This indicates that less MPP are present and therefore the increase seen in the number of KSL should correspond to LT-HSC and ST-HSC.
Figure 2.
The c-myb knockdown retains phenotypically defined HSCs, but these show changes in quiescence and stem cell phenotype and differentiation capacity. (A) Flow cytometric profiles of red-cell-depleted WT and KD bone marrow stem cells (KSL: Kit+Sca1+Lin−). The histogram shows a comparison of the number of KSL cells (right) and Kit+Sca1−Lin− (left) in WT and KD as a percentage of the total number of bone marrow cells (mean±s.d., n=7). (B) Histogram representing a quantification of the relative proportions of KSL cells defined on the basis of their expression of Flt3 (mean±s.d., n=7). (C) Flow cytometric profiles comparing staining with Hoechst 33342 of WT and KD KSL cells. The side population cells are defined as regions R2 and R3. (D) Histograms representing a quantification of gated KSL cells in different cell-cycle phases staining with an antibody specific for the nuclear antigen Ki67 and with propidium iodide to determine the DNA content (mean±s.d., n=3). (E) WT and KD KSL cells subdivided for Flt3 expression were seeded in methylcellulose as described in Materials and methods. Colonies were counted and their morphology assessed after 6 days (mean±s.d., n=3). The May–Grünwald Giemsa-stained cells illustrate the phenotype of the predominant colony types seen in the KSL Flt3high population isolated from WT (macrophage) and KD (megakaryocyte).
In order to further define and verify the effect of reduced c-Myb levels on HSC, we stained KSL cells with the dye Hoechst 33342 and determined by flow cytometry what proportion excluded the dye. Such so-called ‘side population' (SP) cells, which represent predominantly immature, quiescent, niche-associated HSCs (Goodell et al, 1996), were considerably reduced in the c-myb knockdown bone marrow (Figure 2C). The apparent loss of quiescent cells from the KSL population was confirmed by staining of cells simultaneously for expression of the nuclear antigen Ki67 and for DNA content, which showed a relative reduction of cells in G0 in the c-myb knockdown compared with wild type (Figure 2D). It would seem that the change in Flt3 expression described above did not simply reflect a shift towards more immature/quiescent cells as the c-myb knockdown KSL population showed a loss of SP cells, most of which fall in the Flt3−/low fraction in the wild type (Supplementary Figure 2B).
To assess any difference in the differentiation potential of wild-type and c-myb knockdown stem cells, bone-marrow KSL cells sorted into Flt3−, Flt3low and Flt3high fractions were compared for their ability to form colonies in methylcellulose containing growth factors compatible with multilineage haematopoietic differentiation (Figure 2E). These assays revealed that c-myb knockdown cells showed a dramatically higher potential for megakaryocyte differentiation and a corresponding decrease in macrophage/granulocyte colony-forming ability. Wild-type Flt3high KSL cells, corresponding to the MPP, gave rise almost entirely to macrophage/granulocytes, whereas, strikingly, virtually all c-myb knockdown-derived colonies were mixed with a predominantly megakaryocytic content.
The myeloid disorder in c-myb knockdown mice has a self-renewing component
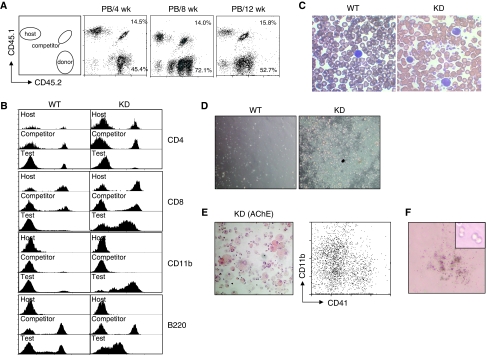
To determine the functional properties of HSCs and multipotent progenitors (i.e. the KSL population) with decreased levels of c-Myb, we carried out competitive repopulation assays (Szilvassy et al, 1990) by transplanting total wild-type or c-myb knockdown bone marrow cells (CD45.2) together with wild-type competitor cells (CD45.1/CD45.2) into lethally irradiated mice (CD45.1). After transplantation the ratios of total donor to competitor cells were determined in peripheral blood every 4 weeks. The input donor test:competitor ratios were maintained throughout the experiment using either wild-type or c-myb knockdown donor cells (Figure 3A), implying that both were capable of short-term and long-term reconstitution.
Figure 3.
Short-term engraftment by c-myb knockdown bone marrow. Irradiated hosts were injected with 2.5 × 106 test WT or KD total bone marrow cells together with 5 × 105 competitor WT from CD45.1/CD45.2 animals (n=3). The percentages of the wild-type competitor and test donor populations are indicated. The ratios of wild-type competitor to test donor for all animals transplanted gave means and s.d. of 3±0.28, 6±1.4 and 3.2±0.15 for the peripheral blood (PB) from 4, 8 and 12 weeks, respectively. (A) Flow cytometric profiles of peripheral blood taken from primary engrafted animals 4, 8 and 12 weeks after transplantation with identifying host, competitor and KD donor cell populations based on CD45.1/CD45.2 staining. The competitor/donor ratio (C:D) is indicated in the top right corner of each histogram. (B) CD4, CD8, CD11b and B220 expression on peripheral blood cells of host, competitor and donor origin from primary engrafted animals 12 weeks after transplantation with WT or KD donor cells. (C) Blood smears from primary engrafted animals 6 months after transplantation with WT or KD donor cells. (D) Peripheral blood cells from primary engrafted animals 6 months after transplantation with WT or KD donor cells were cultured for 6 days. (E) Acetylcholine esterase staining (left panel) and analysis of CD41 and CD11b expression (right panel) in 6 day-cultured peripheral blood from a recipient transplanted with KD donor cells. (F) Methylcellulose culture of peripheral blood from a recipient transplanted with KD donor cells, showing a typical colony present after 2 weeks.
A closer examination showed that the mutant cells adopted a distinct differentiated phenotype. A first indication of the behaviour of the c-myb knockdown cells came from blood samples taken 12 weeks after transplantation. The profiles of cells of wild-type origin were largely similar whether they were test donor or competitor cells; however, c-myb knockdown test donor cells seemed to give rise to differentiated cells that were predominantly CD11b+. Intriguingly, most of these cells also expressed moderate levels of CD8 (but lower than normally seen on CD8+ T-cells) and low levels of B220, and there was an almost complete absence of CD4+ T-cells or B-cells expressing high levels of B220 (Figure 3B).
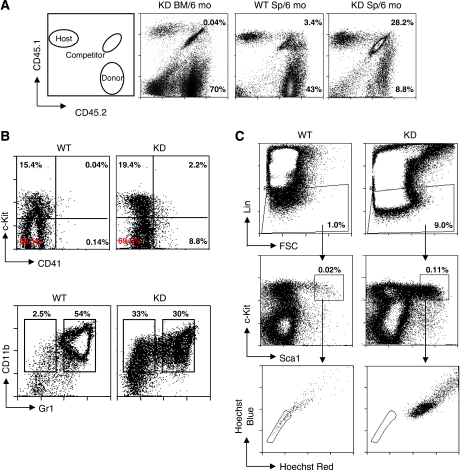
Paralleling the phenotype observed in c-myb knockdown animals, blood smears from primary transplanted animals receiving c-myb knockdown cells showed the presence of large numbers of platelets and an increased prevalence of monocytic cells (Figure 3C), reminiscent of blood in patients with ET (Campbell and Green, 2006). To further investigate the atypical peripheral white-blood cells, we isolated buffy coat cells from animals 6 months post-transplantation and put them in culture in the presence of a cocktail of cytokines to support multilineage myeloid differentiation (Figure 3D). Cultures of blood cells derived from animals transplanted with wild-type test donor cells showed no signs of cellular expansion. By contrast, cells obtained from c-myb knockdown transplants proliferated extensively. Staining of the expanded cells with haematoxylin and for acetylcholine esterase (Figure 3E, left) and analysis of CD11b and CD41 expression (Figure 3E, right) confirmed the presence of macrophage and megakaryocytes. The continuing presence of proliferating blasts was also shown by replating the cultured cells in methylcellulose (Figure 3F). As anticipated from the peripheral blood results, examination of the bone marrow of the primary transplants at 6 months showed good reconstitution by both the test and competitor donor cells, the c-myb knockdown contribution being, if anything, greater than expected based on the input ratio (Figure 4A). Detailed examination of the CD45.2+ wild-type and c-myb knockdown test donor cells showed a profile of immature and differentiated cells reminiscent of that seen in the donor animals (Figure 4B). Hence, the c-myb knockdown donor-derived population contained an increased proportion of Kit+ cells, increased CD41+ cells and, among the myelomonocytic cells, a shift towards a monocytic phenotype (CD11bmedGr1low) at the expense of the neutrophil component (CD11bhighGr1high). Although aberrant in terms of the efficiency of differentiation, there was evidence for the presence of c-myb knockdown donor-derived lymphoid cells including small numbers of CD4+ cells (Supplementary Figure 3). Focusing on immature progenitors and stem cells, it was again apparent that c-myb knockdown cells adopt a profile similar to that seen in the donor animals in that the proportion of Lin− and KSL cells was higher (Figure 4C). Most interestingly, staining with Hoechst 33342 showed that although both wild-type and c-myb knockdown donor cells were able to reconstitute, only the wild-type KSL cells adopted side population characteristics (Figure 4C).
Figure 4.
c-myb knockdown bone marrow is capable of long-term engraftment. (A) Flow cytometric profiles of CD45.1/CD45.2 expression in bone marrow (BM) and spleen (Sp) from primary engrafted animals described in Figures 3 and 6 months after transplantation. The percentages of the wild-type competitor and test donor populations are indicated. The ratios of wild-type competitor to test donor for all animals transplanted gave means and s.d. of 15±1.8, 12±0.45 and 0.45±0.2 for the KD BM, WT Sp and KD Sp, respectively. (B, C) Cells from donor origin (CD45.2) were gated and analysed for (B) Kit/CD41 or CD11b/Gr1, and (C) lineage antigens (Lin) versus cell size (FSC). Lin− cells (indicated by the gated region in the upper panels) were further analysed for expression of c-Kit and Sca1, the KSL population being defined as those cells in the gated region (C, central panels). KSL cells were further analysed by staining with Hoechst 33342 (C, lower panels), side population cells being indicated by the gated region.
Collectively, the results from the analysis of the primary transplants implied that both wild-type and c-myb knockdown bone marrow cells are capable of long-term reconstitution but that, unlike the wild-type cells, those from the mutant animals have a distinct surface antigen phenotype, do not become established in a quiescent state and instead continue to proliferate extensively and give rise to mature cells largely of the monocyte/macrophage and megakaryocyte/platelet lineages. Intriguingly, these differentiated cells seem to derive from a population of proliferating progenitors, some of which are present in the peripheral circulation.
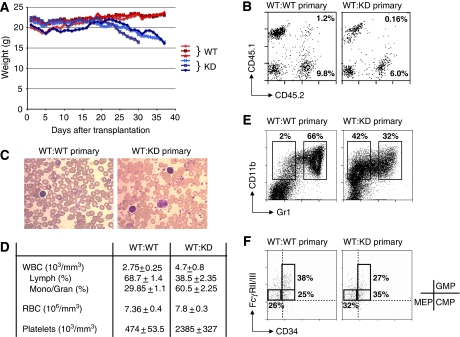
Next, we carried out secondary transplantations to determine whether c-myb knockdown long-term reconstituting stem cells retain the capacity for self-renewal and repopulation. However, at 3 weeks post-transplantation into secondary hosts, before it was possible to make an assessment of long-term repopulation, we noticed that the animals receiving bone marrow from the primary c-myb knockdown transplants started to lose weight (Figure 5A). Although these animals were showing short-term repopulation on the basis of the presence of c-myb knockdown donor-derived cells (Figure 5B), this evidently was not sufficient or was disruptive of normal haematopoiesis and the animals had to be culled between 30–37 days after transplantation. As seen in the primary transplants, analysis of peripheral blood at 28 days showed increased monocytic cells and platelets in animals receiving c-myb knockdown cells (Figure 5C and D). Likewise, CD45.2+ c-myb knockdown donor cells in the bone marrow showed the characteristic features seen in c-myb knockdown animals; that is, increased KSL cells, decreased neutrophils paralleled by increased monocyte/macrophage, increased CD41+ cells (Figure 5E and data not shown) and a relative decrease in progenitors committed to the neutrophil lineage (GMPs—Figure 5F).
Figure 5.
Primary and secondary transplanted animals receiving c-myb knockdown bone marrow cells develop a myeloproliferative disorder. Irradiated hosts were injected with 2 × 106 (n=3) bone marrow cells derived from primary transplants that had received WT or KD total bone marrow cells. (A) Weight curve of secondary engrafted animals. (B) CD45.1/CD45.2 staining of peripheral blood taken from secondary engrafted animals 4 weeks after transplantation with donor cells from either a WT competitor/WT test (WT:WT primary) or WT competitor/KD test (WT:KD primary) primary transplant. The percentages of the wild-type competitor and test donor populations are indicated. The ratios of wild-type competitor to test donor for all animals transplanted gave means and s.d. of 12±6.3 and 29.6±7.2 WT:WT primary and WT:KD primary, respectively). (C, D) Blood smears (C) and blood cell counts (D) for secondary engrafted animals described in (B). (E) Staining for CD11b/Gr1 expression in bone marrow cells of test donor origin (CD45.2+) from secondary engrafted animals as described in (C). (F) Analysis of multipotent progenitors (GMP, CMP and MEP) of test donor (CD45.2+) origin, depicting FcγRII/III/CD34 expression on the gated Kit+Sca1−Lin−IL-7R− population. Staining boundaries for the relevant isotype controls are indicated by the dashed lines.
KSL cells from c-myb knockdown animals do not have repopulation capacity and are not responsible for the myeloproliferative phenotype
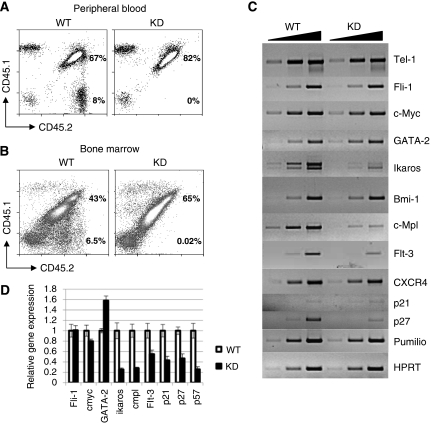
As the first set of transplantations had been carried out using equal numbers of total donor bone marrow cells, which, from our analyses, we knew would have contained different proportions of immature progenitors, we injected either 500 or 2000 sorted KSL cells from wild-type or c-myb knockdown CD45.2 donors into irradiated hosts together with 1 × 106 CD45.1/CD45.2 wild-type total bone marrow cells. Analysis of peripheral blood of the transplanted animals after 4, 8 and 12 weeks indicated that wild-type competitor and test donor cells had yielded successful reconstitution, but, surprisingly, the c-myb knockdown KSL donor cells had not (Figure 6A). Similarly, when the bone marrow was analysed, no cells of c-myb knockdown donor origin were present (Figure 6B).
Figure 6.
Engrafting cells from c-myb knockdown bone marrow are not in the KSL population. Irradiated hosts were injected with 500 (n=6) or 2 × 103 (n=6) WT or KD sorted KSL cells together with 1 × 106 CD45.1/CD45.2 WT competitor cells. (A) Peripheral blood and (B) bone marrow taken from animals engrafted with KSL cells 12 weeks after transplantation, distinguishing host and donor cells based on CD45.1/CD45.2 staining as described in Figure 3. The percentages of the wild-type competitor and test donor populations are indicated. The ratios of wild-type competitor to test donor for all animals transplanted gave means and s.d. of 0.15±0.05 and 0.17+0.02 for WT in the peripheral blood and the bone marrow, respectively. (C) Semi-quantitative RT–PCR of KSL-cell RNA from WT versus KD animals using primers corresponding to the indicated gene (see Supplementary Table 2). Each reaction was sampled at three-cycle intervals in the exponential range of the amplification. (D) Histogram representing the results of quantitative RT–PCR of KSL-cell RNA from WT versus KD animals using primers corresponding to the indicated genes (see Supplementary Table 3). The data are normalised against β2 microglobulin expression and the wild-type values set as 1.
Next, we decided to investigate whether the failure of c-myb knockdown KSL cells to repopulate can be explained by an altered transcriptional program. We selected a variety of genes that have been related with HSC function. Semi-quantitative (Figure 6C) or quantitative (Figure 6D) RT–PCR revealed that three genes known to be crucial in stem cells, namely ikaros, c-mpl and flt-3, were expressed at a much-reduced level in the mutant cells. In addition, among the family of cell-cycle inhibitors we observed that p21, p27 and p57 mRNAs were present in lower amounts in the c-myb knockdown KSL cells, correlating with their loss of quiescence and enhanced proliferation. Also correlating with an increased proliferative, yet still immature state, was an observed increase in the level of expression of GATA-2 mRNA.
Kit+CD11low/med cells have the capacity to transfer the c-myb knockdown myeloproliferative phenotype
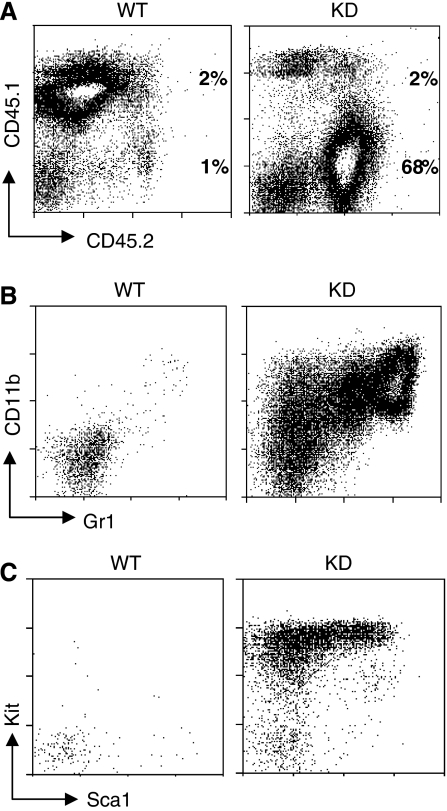
Collectively, the phenotypic and functional data for the c-myb knockdown KSL cells indicate that they have lost all capacity for stem cell behaviour, in which case the question remains as to the nature of the cells that are capable of transferring and perpetuating the mutant phenotype. In order to address this, we carried out similar transplantations, subdividing the bone marrow into four different fractions: Kit−Lin−, Kit−Lin+, Kit+Lin− and Kit+Lin+ cells (Supplementary Figure 4). Successful engraftment and perpetuation of the c-myb knockdown myeloproliferative phenotype was achieved with the Kit+Lin+ fraction (Figure 7A). As observed in both c-myb knockdown animals and mice engrafted with total c-myb-knockdown bone marrow, the bone marrow of animals transplanted with Kit+Lin+ cells contained an expanded population of CD11blow/medGr1−/low cells (Figure 7B). Surprisingly, although c-myb knockdown KSL cells failed to engraft, mutant Kit+Lin+ cells gave rise to a population of cells in the bone marrow of engrafted animals that were Kit+Sca+ and had lost lineage markers, thus being phenotypically KSL-like (Figure 7C).
Figure 7.
Engrafting cells from c-myb knockdown bone marrow are in a lineage+ fraction that is expanded relative to the wild type. Irradiated hosts were injected with WT or KD subpopulations sorted on the basis of Kit and Lin expression (see Materials and Methods for the numbers used) together with 1 × 106 CD45.1/CD45.2 WT competitor cells (for each cell type n=3). (A) Flow cytometric profiles of bone marrow taken from animals engrafted with Kit+Lin+ cells seven weeks after transplantation identifying host, competitor and donor cell populations based on CD45.1/CD45.2 expression. The percentages of the wild-type competitor and test donor populations are indicated. The ratios of wild-type competitor to test donor for all animals transplanted gave means and s.d. of 0.89±0.55 and 27±13 for WT and KD test donors, respectively. Staining for CD11b/Gr1 (B) and KSL cells (C) in bone marrow of test donor origin (CD45.2+) from animals engrafted with Kit+Lin+ cells 7 weeks after transplantation.
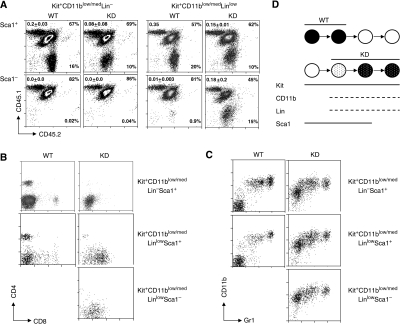
As the Kit+Lin+ fraction is a heterogenous population, we decided to define more precisely which cells act as the stem cell component of the myeloproliferative phenotype. On the basis of the predominance of Kit+CD11blow/med in the bone marrow of c-myb knockdown animals and recipients transplanted with c-myb knockdown cells, we wanted to determine whether these or a specific subfraction were responsible for the transplantable phenotype. We first examined the heterogeneity of the Kit+CD11blow/med population with respect to other lineage-associated antigens. This showed that both wild-type and c-myb knockdown Kit+CD11blow/med cells co-expressed low to medium levels of a range of myeloid and lymphoid markers (Supplementary Figure 5A), the only significant difference being that the wild-type cells could be subdivided into two populations on the basis of low/medium or high Gr1 expression whereas c-myb knockdown Kit+CD11blow/med cells consistently expressed low/medium levels of the antigen. Both the wild-type and c-myb knockdown Kit+CD11blow/med populations were found to be heterogenous for Sca1 expression, although the distribution of Sca1+ and Sca1− cells was different, there being proportionately about twice as many Sca1− cells in the case of the c-myb knockdown (Supplementary Figure 5B). Taking into account the results of this analysis, we sorted Kit+CD11blow/med cells into Lin− and Linlow fractions and then further subdivided these on the basis of Sca1 expression (Supplementary Figure 6). For each of the sorted subpopulations, 500 cells (CD45.2) were injected together with 1 × 106 wild-type (CD45.1/CD45.2) total bone-marrow cells into lethally irradiated hosts (CD45.1). Peripheral blood was collected and analysed after 8 weeks in order to determine the relative efficiency of engraftment. Both wild-type and c-myb knockdown Kit+CD11blow/medLin−Sca1+ cells resulted in efficient engraftment, whereas the Kit+CD11blow/medLin−Sca1− cells did not (Figure 8A). Likewise, wild-type and c-myb knockdown Kit+CD11blow/medLinlowSca1+ cells engrafted and wild-type Kit+CD11blow/medLinlowSca1− failed to. However, the equivalent c-myb knockdown Sca1− population successfully engrafted (Figure 8A). Staining of peripheral blood test donor-derived cells (CD45.2) for markers of lymphoid and myeloid lineages indicated that wild-type Kit+CD11blow/medLin−/lowSca1+ cells gave rise to CD4+ and CD8+ T-cells and CD11b+Gr1med/high myelomonocytic cells (Figure 8B and C), presumably because inclusion of low-level expression of Lin antigens (including CD11b) retains some degree of overlap with the KSL population. A similar analysis of the recipients of c-myb knockdown cells showed a subtle difference in the contribution of different subpopulations to the myeloproliferative phenotype. Hence, Kit+CD11blow/medLin−Sca1+ cells gave rise to small numbers of peripheral blood CD4+ cells and a majority of CD11blow/medGr1low/med myelomonocytic cells characteristic of the c-myb knockdown phenotype (Figure 8B and C). By contrast, c-myb knockdown Kit+CD11blow/medLinlow cells, whether Sca1+ or Sca1−, gave rise to cells in the peripheral blood that were also characteristically CD11blow/medGr1low/med but also largely co-expressed CD8 at a level somewhat lower than normally seen on mature T-cells (Figure 8B and C).
Figure 8.
Engrafting cells from c-myb knockdown bone marrow express low to medium levels of CD11b. Irradiated hosts were injected with 500 WT or KD Kit+CD11blow/med cell subpopulations that had been sorted on the basis of Lin (CD5, CD8a, B220, Gr-1, Ter119) and Sca1 expression together with 1 × 106 CD45.1/CD45.2 WT competitor cells (for each cell type n=3). (A) Flow cytometric profiles of bone marrow taken from animals engrafted with Kit+CD11low/med cells 8 weeks after transplantation identifying host, competitor and donor cell populations based on CD45.1/CD45.2 expression. The percentages of the wild-type competitor and test donor populations are indicated. The means and s.d. of the ratios of wild-type competitor to test donor for all animals transplanted are indicated in the top left-hand corner of each profile (note: only one animal receiving transplantation of WT Kit+CD11blow/medLinlowSca1+ cells survived to be analysed). Staining for CD4/CD8 (B) and CD11b/Gr1 (C) in bone marrow of test donor origin (CD45.2+) from animals engrafted with Kit+CD11low/med cells 8 weeks after transplantation. (D) Schematic representation of the engraftment achieved with fractionated WT and KD populations. The antigens expressed are indicated and the shading depicts whether transplanted cells gave full reconstitution (black), no reconstitution (white) or reconstitution of the myeloproliferative phenotype with either a limited myelomonocytic profile (white dotted) or the characteristic KD phenotype involving co-expression of lymphoid and myelomonocytic markers (black dotted).
Collectively, the transplantation studies on sorted bone marrow cells indicated that normal lymphoid and myeloid engraftment potential of wild-type cells can be shown for the expected KSL population and also for cells that express low levels of lineage-associated antigens, including CD11b. By contrast, reduced c-Myb expression in the knockdown mutant abolishes the capacity of KSL cells to engraft but at the same time confers myeloproliferative stem-cell characteristics upon a spectrum of Kit+ cells that express low to medium levels of myeloid and lymphoid antigens, but that can be positive or negative for the stem-cell antigen Sca1 (Figure 8D).
Discussion
Here we have shown that reduction of c-Myb expression leads to a myeloproliferative phenotype, with an overabundance of platelets and monocytes in peripheral blood and an increase in megakaryocytes and a distinct myeloid CD11blow/medGr1low/med population in the bone marrow. In combination with the absence of myelofibrosis and presence of spenomegaly, the phenotype overall resembles the MPD essential thrombocythemia (ET). Most interestingly, the phenotype is transplantable and seems to have an underlying stem-cell component that can be defined as Kit+CD11blow/medLin−/low, with no dependence on Sca1 expression. Human MPDs are believed to arise clonally at the level of HSC/multilineage progenitors (Campbell and Green, 2006) and the results of our transplantation experiments were consistent with a stem-cell component maintaining the aberrant myeloproliferative phenotype. As the myeloproliferative phenotype is clearly a direct consequence of the c-myb knockdown mutation, this means that this does not represent a model for the evolution of MPD from stem cells or progenitors but, however, it does provide a basis to study the mechanisms MPD maintenance and possibly even for investigation into factors that can promote its progression towards leukaemia.
Similar to the situation observed in mice carrying the M3030V mutation in c-Myb (Sandberg et al, 2005) our knockdown mutant animals showed an increase in the number of phenotypically KSL cells showing increased proliferation. However, although we, like Sandberg et al (2005), were able to show the capacity of c-myb knockdown bone marrow to be transplanted into irradiated hosts, we recognised some features that indicated that normal stem-cell properties had been lost. First, repopulation by c-myb knockdown cells required co-transplanted wild-type cells in order for all lineages to be successfully reconstituted. Second, we observed early lethality of recipient animals after secondary transplant. No lethality in primary recipients was found even 10 months post-transplantation, invalidating the explanation of a rapid death because of a development of a leukaemia-like disease. We consider that the most likely explanation for the death of these animals is the greatly reduced proportion of HSC of competitor wild-type origin in total bone marrow of the primary transplanted animals due of the extensive overproliferation of c-myb knockdown Kit+ cells. Third, and most significantly, phenotypically KSL cells derived from our c-myb knockdown animals showed no capacity to reconstitute irradiated animals. This result contrasts with the conclusions of Sandberg et al. (2005); however, they did not specifically isolate KSL cells for use in their assay but instead used the total bone marrow. The simplest explanation for these differences is that the c-myb M303V mutation is effectively a less potent hypomorph relative to our knockdown allele, either because it only partially disrupts a specific aspect of c-Myb function or because the presumed loss of interaction with p300/CBP has a limited effect on all, or affects only a subset of c-Myb targets.
A possible explanation, at the molecular level, of why c-myb knockdown KSL cells do not behave as normal KSL came from our preliminary studies of gene expression. We found that the mutant KSL cells have an altered transcriptional program. For example, the changes in the expression of crucial genes such as ikaros (Lopez et al, 2002; Yoshida et al, 2006), c-mpl and flt-3 might explain why c-myb knockdown KSL cells commit towards myelo-megakaryocytic lineages, whereas the low levels of the cell-cycle inhibitors p21, p27 and p57 could explain the shift of KSL HSCs towards an aberrant proliferative progenitor state. Indeed, p27 downregulation has been described to increase haematopoietic progenitor proliferation and p27-deficient HSCs generate progenitors that eventually dominate blood cell production (Cheng et al, 2000).
In order to determine which specific cell population is responsible for the transplantable myeloproliferative phenotype, we subdivided the bone marrow and carried out competitive repopulation experiments. We found that the cells capable of transferring the c-myb knockdown myeloproliferative phenotype were contained in the Kit+Lin+ fraction, suggesting that when c-Myb expression is lower either cells with stem cell-like properties derive from normal stem cells or more mature cells acquire stem cell properties. The fact that we observe lymphoid cells in the transplanted animals, albeit at low levels or only partly differentiated, argues that a modified multipotential stem cell underlies the phenotype. Further subfractionation of bone marrow Kit+ cells allowed us to define the transplantable component as Kit+CD11blow/medLin−/lowSca1−/+. The presence of Sca1 on one of the active subfractions also supports the notion that the transplantable c-myb knockdown cells derive from immature cells. CD11b is known to be expressed on foetal liver HSCs (Morrison et al, 1995), and this might suggest that c-Myb is required for the developmental transition to adult HSC, which are CD11b−, and, furthermore, that the cells giving rise to the myeloproliferative phenotype are in fact foetal liver HSCs. However, we were unable to detect expression of cells co-expressing AA4.1 and CD11b or of Sox17 RNA (data not shown), both of which have been shown to be characteristic of foetal HSCs (Kim et al, 2007). Interestingly, it was reported recently that a Kit+CD11b+ population acquires stem-cell characteristics in AML arising as the result of point mutations in C/EBPα (Kirstetter et al, 2008).
Surprisingly, although c-myb knockdown cells that are phenotypically KSL-like do not act as stem cells, Kit+CD11blow/medLin−/low cells that can transfer the phenotype generated a proliferating population that equates with KSL on the basis of surface antigen expression. Contamination of KSL cells in the transplanted Kit+CD11blow/medLin−/low fraction cannot be an explanation for the presence of KSL cells after the transplantation as we have shown that c-myb knockdown KSL cells are not able to reconstitute. At present we can only speculate on the nature of these KSL-like cells ultimately deriving from the Kit+CD11blow/medLin−/lowcells. As the properties of the c-myb knockdown KSL-like cells are so distinct from those of wild-type KSL, we favour the view that the Kit+CD11blow/medLin−/low cells are able to give rise to a population that express the same markers as KSL cells, but in all other regards are not true HSCs.
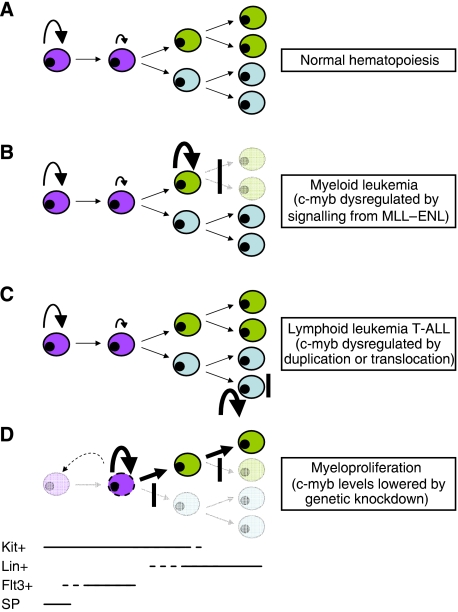
In summary, we have shown that when c-Myb is tipped towards an abnormally low level of expression, then a myeloproliferative phenotype with skewed lineage differentiation can result (Figure 8). This adds to other recently described instances where the loss of normal control of c-Myb expression can lead to haematological disorders. It is becoming clear that miRNAs can regulate c-Myb levels and might underlie both normal and disease-related control of haematopoiesis (García and Frampton, 2008). The miRNA miR-150 has been shown recently to affect, presumably also through an influence of c-Myb, both normal and MPD-related lineage choice in the erythroid/megakaryocytic axis (Bruchova et al, 2007; Lu et al, 2008). Another instance of loss of normal control of c-Myb expression that leads to haematological disruption seems to underlie T-ALL in which the c-myb gene has been duplicated or translocated to the TCRβ locus (Clappier et al, 2007; Lahortiga et al, 2007). A similar specific alteration to c-myb expression caused by new signalling from a disease-associated translocation also seems to be a crucial component of the leukaemogenic process in MLL–ENL induced AML, potentially through the intermediate action of HoxA9 and Meis1 (Hess et al, 2006). Furthermore, in each of these situations, the cells arising from altered c-Myb expression have properties that are stem cell-like (Figure 9).
Figure 9.
Correct control of c-Myb levels is required to maintain the haematopoietic hierarchy. The diagram summarises the ways in which disrupted loss of normal (A) control of c-Myb expression has been shown to lead to expansion of cells with stem-cell characteristics at different points in the haematopoietic hierarchy, resulting in (B) myeloid and (C) lymphoid leukaemia or, as described here, myeloproliferation (D). The highly simplified representation of haematopoiesis is divided on the basis of cells being in the KSL stem cell compartment (purple) or committed towards myeloid (green) or lymphoid (blue) differentiation. The approximate range of expression of crucial markers is indicated at the bottom (SP, side population). Circular arrows are used to depict self-renewal, the extent of which is related to the size. Vertical bars indicate blocks to further differentiation. The dotted arrow and cell in (D) represent a putative new pathway of dedifferentiation seen in the c-myb knockdown.
We hypothesise that c-Myb is a crucial component in a network of regulatory factors that control the balance between self-renewal and differentiation in normal HSCs and more mature cell types, and that its role in this network is crucially dependent on the fine tuning of its expression, both in terms of overall level and the specific timing, and when this control is compromised haematological defects, both leukaemic and myeloproliferative, can result.
Materials and methods
Mice and genotyping
All animal experiments were carried out under an animal project licence in accordance with UK legislation. The c-myb knockdown mutation corresponds to the c-mybloxP allele as described earlier (Emambokus et al, 2003). Following their original derivation, c-myb knockdown mice were backcrossed against the C57/BL6 strain for at least 10 generations and were thereafter maintained on this genetic background. Mice were genotyped by PCR analysis. Mice between 4–6 weeks of age were used as a source of bone marrow cells for analysis or transplantation.
Blood counts
Adult mice were bled in ACD solution (citric acid 6.8 mM, trisodium citrate 11.2 mM, glucose 24 mM) and blood counts were obtained with an ABX Pentra 60 (ABX Diagnostics) automatic blood counter. Five pairs of age-matched animals were analysed. The mean and standard error of the mean (s.e.m.) were calculated.
Bone marrow sections
Histological analysis of paraffin sections of femurs was carried out using standard methods. Hematoxylin and eosin-stained sections were analysed under bright-field microscopy.
Culture of primary cells
Sorted KSL cells were plated in Iscove's modified Eagle's medium (IMDM) containing 10% horse serum, SCF (20 ng/ml), FL (10 ng/ml) and TPO (5 ng/ml). To assess individual cell proliferation and differentiation capacity, 500 sorted KSL cells were plated in 1.5 ml 1% methylcellulose medium (Methocult M3434, Stem Cell Technologies, Vancouver) containing TPO (25 ng/ml). Colony numbers were assessed after 6 days. All cultures were incubated at 37 °C in a fully humidified 5% CO2 in air atmosphere.
Peripheral blood cells were first treated with ACK buffer to deplete red cells before culturing in RPMI, 10% FCS, 5 ng/ml IL-3, IL-6, GM-CSF and 50 ng/ml SCF.
Competitive repopulation assays of HSC function
Transplantations to assess competitive repopulation potential were carried out by injecting 5 × 105 competitor wild-type ACK-treated bone marrow cells (B6 × B6:SJL F1, CD45.1/CD45.2) together with 2.5 × 106 donor cells from either wild-type or c-myb knockdown mice (B6, CD45.2/CD45.2) into the tail vein of host animals (B6:SJL, CD45.1/CD45.1) that had been lethally irradiated (975 Gy). Peripheral blood was analysed every 4 weeks following transplantation using antibodies to distinguish CD45.1 and CD45.2 and a range of lineage-specific markers. For secondary transplantation experiments, 2 × 106 ACK-treated total bone marrow from animals showing successful engraftment at 24 weeks were injected into lethally irradiated B6:SJL hosts. After transplantation, the animals were scored for engraftment by immunofluorescent flow cytometry as above.
Transplantations involving specific populations of cells employed 106 wild-type competitor cells and the following numbers of sorted cells: Kit+Lin+, 1.7 × 105; Kit−Lin+, 3 × 105; Kit+Lin−, 2 × 104; Kit−Lin−, 2 × 104; KSL, 500 or 2 × 103; Kit+CD11blow/medLin−/lowSca−/+, 500.
Antibodies, flow cytometry and cell sorting
Single-cell suspensions of bone marrow were prepared by standard techniques. Red cells were depleted, when required, by selective lysis and non-specific binding of antibodies to Fc receptors was prevented by use of anti-CD16/CD32 Fc block (BD Pharmingen). The lineage cocktail contained the following antibodies: CD5, CD8a, Gr-1, CD11b, Ter119 and B220. In experiments where the expression of CD11b was assayed, this antibody was removed form the lineage cocktail. Antibodies used are described in Supplementary Table 1. Stained cells were analysed on a CyAn flow cytometer using Summit software (Dako).
For cell sorting, red cells were ACK-lysed and after staining with the required conjugated monoclonal antibodies, samples were filtered through a 70-μm strainer and sorted on a Cytomation MoFlo machine. Live cells were selected by forward/side scatter gating and doublet discrimination.
Side population and immunofluorescence
The procedures for SP and immunofluorescent staining were carried out as described earlier (Goodell et al, 1996).
Semi-quantitative RT–PCR determination of mRNA levels
Total RNA from sorted KSL cells was isolated using TRIzol (Invitrogen) and contaminating genomic DNA removed by treatment with RNAse-free DNAseI (Pharmacia). Single-stranded cDNA was prepared using SuperScript III reverse transcriptase (Invitrogen) with first-strand synthesis primed using oligo (dT). PCR was carried out using ReddyMix PCR master mix (ABgene) in 50 μl containing appropriate concentration of gene-specific oligonucleotides, designed to span at least one intron (see Supplementary Table 2). HPRT was used as a control to standardise samples. For a given PCR reaction, samples were removed at intervals of three cycles to enable comparison of the linear phases of amplification. PCR products were analysed by agarose gel electrophoresis. The experiment was carried out in duplicates.
Quantitative RT–PCR determination of mRNA levels
Real-time PCR was carried out using TaqMan PCR master mix with 1 μl of predesigned TaqMan PCR primers (Applied Biosystems, see Supplementary Table 3). 1 μl of cDNA dilutions was used in a 20-μl reaction. The reactions were carried out in a Stratagene MX3000P machine. At least two separate reactions with three replicates per sample were run. Relative gene expression was calculated as 2−ΔΔCt values with β2 microglobulin used as a control.
Supplementary Material
Supplementary Table 1–3
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure Legends
Acknowledgments
We thank David Walton for technical assistance, Ana Maria Gonzalez for help in preparing the bone marrow sections, Roger Bird for assistance with cell sorting and Claudia Roberts for interpretation of the hematological phenotype. We are also grateful to Marella de Bruijn for critically reading the paper. This work was supported by the Wellcome Trust and Leukaemia Research.
References
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE (2005) Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential: a revised road map for adult blood lineage commitment. Cell 121: 295–306 [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197 [DOI] [PubMed] [Google Scholar]
- Allen RD III, Bender TP, Siu G (1999) c-Myb is essential for early T cell development. Genes Dev 13: 1073–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender TP, Kremer CS, Kraus M, Buch T, Rajewsky K (2004) Critical functions for c-Myb at three checkpoints during thymocyte development. Nat Immunol 5: 721–729 [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE (1997) Human acute myeloid leukaemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730–737 [DOI] [PubMed] [Google Scholar]
- Bruchova H, Yoon D, Agarwal AM, Mendell J, Prchal JT (2007) Regulated expression of microRNAs in normal and polycythemia vera erythropoiesis. Exp Hematol 35: 1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Green AR (2006) The myeloproliferative disorders. N Engl J Med 355: 2452–2466 [DOI] [PubMed] [Google Scholar]
- Carpinelli MR, Hilton DJ, Metcalf D, Antonchuk JL, Hyland CD, Mifsud SL, Di Rago L, Hilton AA, Willson TA, Roberts AW, Ramsay RG, Nicola NA, Alexander WS (2004) Suppressor screen in Mpl-/- mice: c-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling. Proc Natl Acad Sci USA 101: 6553–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT (2000) Stem cell repopulation efficiency but not pool size is governed by p27(kip1). Nat Med 6: 1235–1240 [DOI] [PubMed] [Google Scholar]
- Clappier E, Cuccuini W, Kalota A, Crinquette A, Cayuela JM, Dik WA, Langerak AW, Montpellier B, Nadel B, Walrafen P, Delattre O, Aurias A, Leblanc T, Dombret H, Gewirtz AM, Baruchel A, Sigaux F, Soulier J (2007) The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukaemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood 110: 1251–1261 [DOI] [PubMed] [Google Scholar]
- Cox CV, Martin HM, Kearns PR, Virgo P, Evely RS, Blair A (2007) Characterization of a progenitor cell population in childhood T-cell acute lymphoblastic leukaemia. Blood 109: 674–682 [DOI] [PubMed] [Google Scholar]
- Dick JE (2003) Stem cells: self-renewal writ in blood. Nature 423: 231–233 [DOI] [PubMed] [Google Scholar]
- Emambokus N, Vegiopoulos A, Harman B, Jenkinson E, Anderson G, Frampton J (2003) Progression through key stages of haemopoiesis is dependent on distinct threshold levels of c-Myb. EMBO J 22: 4478–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J, Leutz A, Gibson T, Graf T (1989) DNA-binding domain ancestry. Nature 342: 134. [DOI] [PubMed] [Google Scholar]
- García P, Frampton J (2008) Hematopoietic lineage commitment: miRNAs add specificity to a widely expressed transcription factor. Dev Cell 14: 843–853 [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JL, Bittner CB, Zeisig DT, Bach C, Fuchs U, Borkhardt A, Frampton J, Slany RK (2006) c-Myb is an essential downstream target for homeobox-mediated transformation of hematopoietic cells. Blood 108: 297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntly BJ, Gilliland DG (2005) Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer 5: 311–321 [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL (2004) Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med 351: 657–667 [DOI] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ (2007) Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130: 470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstetter P, Schuster MB, Bereshchenko O, Moore S, Dvinge H, Kurz E, Theilgaard-Mönch K, Månsson R, Pedersen TA, Pabst T, Jacobsen SE, Bertone P, Tenen DG, Nerlov C (2008) Modeling of C/EBPalpha mutant acute myeloid leukaemia reveals a common expression signature of committed myeloid leukaemia-initiating cells. Cancer Cell 13: 299–310 [DOI] [PubMed] [Google Scholar]
- Lahortiga I, De Keersmaecker K, Van Vlierberghe P, Graux C, Cauwelier B, Lambert F, Mentens N, Beverloo HB, Pieters R, Speleman F, Odero MD, Bauters M, Froyen G, Marynen P, Vandenberghe P, Wlodarska I, Meijerink JP, Cools J (2007) Duplication of the MYB oncogene in T cell acute lymphoblastic leukaemia. Nat Genet 39: 593–595 [DOI] [PubMed] [Google Scholar]
- Lessard J, Faubert A, Sauvageau G (2004) Genetic programs regulating HSC specification, maintenance and expansion. Oncogene 23: 7199–7209 [DOI] [PubMed] [Google Scholar]
- Lipsick JS, Wang DM (1999) Transformation by v-Myb. Oncogene 18: 3047–3055 [DOI] [PubMed] [Google Scholar]
- Lopez RA, Schoetz S, DeAngelis K, O'Neill D, Bank A (2002) Multiple hematopoietic defects and delayed globin switching in Ikaros null mice. Proc Natl Acad Sci USA 99: 602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Guo S, Ebert BL, Zhang H, Peng X, Bosco J, Pretz J, Schlanger R, Wang JY, Mak RH, Dombkowski DM, Preffer FI, Scadden DT, Golub TR (2008) microRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Dev Cell 14: 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ (2004) Characterization of clonogenic multiple myeloma cells. Blood 103: 2332–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL (1995) The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci USA 92: 10302–10306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, Pietryga DW, Scott WJ Jr, Potter SS (1991) A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 65: 677–689 [DOI] [PubMed] [Google Scholar]
- Mukai HY, Motohashi H, Ohneda O, Suzuki N, Nagano M, Yamamoto M (2006) Transgene insertion in proximity to the c-myb gene disrupts erythroid-megakaryocytic lineage bifurcation. Mol Cell Biol 26: 7953–7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh IH, Reddy EP (1999) The myb gene family in cell growth, differentiation and apoptosis. Oncogene 18: 3017–3033 [DOI] [PubMed] [Google Scholar]
- Pohlmann SJ, Slayton WB, Spangrude GJ (2001) Stem cell populations: purification and behavior. In Hematopoiesis—A Developmental Approach Oxford, Zon LI (ed), pp 35–47. UK: Oxford University Press [Google Scholar]
- Rosenbauer F, Koschmieder S, Steidl U, Tenen DG (2005) Effect of transcription-factor concentrations on leukemic stem cells. Blood 106: 1519–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg ML, Sutton SE, Pletcher MT, Wiltshire T, Tarantino LM, Hogenesch JB, Cooke MP (2005) c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev Cell 8: 153–166 [DOI] [PubMed] [Google Scholar]
- Sumner R, Crawford A, Mucenski M, Frampton J (2000) Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene 19: 3335–3342 [DOI] [PubMed] [Google Scholar]
- Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ (1990) Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci USA 87: 8736–8740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MD, Kremer CS, Ravichandran KS, Rajewsky K, Bender TP (2005) c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity 23: 275–286 [DOI] [PubMed] [Google Scholar]
- Vegiopoulos AP, Garcia P, Emambokus N, Frampton J (2006) Coordination of erythropoiesis by the transcription factor c-Myb. Blood 107: 4703–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K (2007) MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 131: 146–159 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yao-Ming Ng S, Zuniga-Pflucker JC, Georgopoulos K (2006) Early hematopoietic lineage restrictions directed by Ikaros. Nat Immunol 7: 382–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1–3
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure Legends