Abstract
The tumor suppressor serine-threonine kinase LKB1 is mutated in Peutz-Jeghers syndrome (PJS) and in epithelial cancers, including hormone-sensitive organs such as breast, ovaries, testes, and prostate. Clinical studies in breast cancer patients show low LKB1 expression is related to poor prognosis, whereas in PJS, the risk of breast cancer is similar to the risk from germline mutations in breast cancer (BRCA) 1/BRCA2. In this study, we investigate the role of LKB1 in estrogen receptor α (ERα) signaling. We demonstrate for the first time that LKB1 binds to ERα in the cell nucleus in which it is recruited to the promoter of ERα-responsive genes. Furthermore, LKB1 catalytic activity enhances ERα transactivation compared with LKB1 catalytically deficient mutants. The significance of our discovery is that we demonstrate for the first time a novel functional link between LKB1 and ERα. Our discovery places LKB1 in a coactivator role for ERα signaling, broadening the scientific scope of this tumor suppressor kinase and laying the groundwork for the use of LKB1 as a target for the development of new therapies against breast cancer.
INTRODUCTION
Clinical evidence suggests a role for the multitasking tumor suppressor kinase LKB1 (Marignani, 2005) in the development of breast carcinoma (Esteller et al., 2000; Shen et al., 2002; Fenton et al., 2006). Early evidence in the literature suggests that a loss of LKB1 expression leads to papillary breast carcinoma (Esteller et al., 2000). More recently, a study that evaluated the expression of LKB1 in 85 cases of breast cancers, the authors found that in a subset of high-grade in situ and invasive mammary carcinomas, the expression of LKB1 was completely lost as determined by immunohistochemistry (Fenton et al., 2006). In a separate study, 116 patients with confirmed breast carcinoma were evaluated for LKB1 protein expression, of which expression was low in approximately one third of the patients compared with control population of women. The authors of this study concluded that low expression of LKB1 correlated with higher histological grade, tumor size, and presence of lymph node status (Shen et al., 2002). Both studies conclude that LKB1 expression profile may serve as a prognostic marker for breast carcinoma; however, further investigation is warranted (Shen et al., 2002; Fenton et al., 2006). It should be noted that neither study determined whether LKB1 was mutated in the patients. These recent findings suggest a role for LKB1 in breast cancer however the molecular mechanisms by which this occurs is not fully understood.
Mutations in LKB1 are responsible for Peutz-Jeghers syndrome (PJS) (Hemminki et al., 1997; Ylikorkala et al., 1999; Marignani, 2005), an autosomal-dominant disease characterized by mucocutaneous pigmentation and benign gastrointestinal hamartomatous polyps (Hemminki, 1999). PJS patients are at a high risk of developing various cancers such as those of the gastrointestinal tract, breast, ovary, cervix, testis, lung, and pancreas (Avizienyte et al., 1999; Hruban et al., 1999; Wang et al., 1999; Esteller et al., 2000; Sanchez-Cespedes et al., 2002; Lim et al., 2003; Carretero et al., 2004; Nakanishi et al., 2004). PJS patients have a 54% greater risk of developing breast cancer than the general population (Giardiello et al., 2000), as well as a reported loss of heterozygosity of LKB1 in breast cancer (Bignell et al., 1998; Chen et al., 2000; Nakanishi et al., 2004; Yang et al., 2004). LKB1 contains a nuclear localization sequence that allows for LKB1 localization within the nuclear compartment of the cell (Nezu et al., 1999; Tiainen et al., 2002; Dorfman and Macara, 2008). Because a predominant number of mutations occur within the LKB1 kinase domain, the tumor suppressor function of LKB1 has been linked to its catalytic activity (Tiainen et al., 1999; Ylikorkala et al., 1999; Boudeau et al., 2003). More recently, we showed that LKB1 catalytic-deficient mutants exhibit oncogenic property, such that they are able to enhance the expression of the oncogene cyclin D1 in cells that lack expression cyclin D1-negative regulators p53 or p21WAF/CIP1 (Scott et al., 2007).
Aberrant ERα signaling is a major contributor to the occurrence of ERα-positive breast cancer (Sommer and Fuqua, 2001). ERα is a member of the nuclear receptor (NR) family of transcription factors characterized by similar structural domains. There are two isoforms of ERs; ERα (Walter et al., 1985) and ERβ (Kuiper et al., 1996). ERα regulates genes such as cathepsin D, trefoil factor (pS2), c-myc, and cyclin D1 (reviewed in O'Lone et al., 2004). Whereas ERα interacts with the estrogen response elements (EREs) in the promoters of the first two genes (classical pathway), it indirectly regulates the latter genes by interaction with DNA-bound transcription factors (nonclassical pathway). In general, upon stimulation with 17-β-estradiol (E2), ERα dimerizes and translocate to the nucleus in which the dimer interacts with DNA and coregulator proteins to regulate gene transcription. Because alterations in LKB1 expression have been linked to breast cancer, we investigated the molecular mechanism by which LKB1 regulates ERα-mediated signaling.
MATERIALS AND METHODS
Cell Culture and Transfection
MCF7 human breast cancer cells (American Type Culture Collection, Manassas, VA), G361 melanoma cells (gift from Dr. Alessi, Medical Research Council, Protein Phosphorylation Unit, University of Dundee), and human embryonic kidney (HEK) 293 were maintained in DMEM supplemented with 8% fetal bovine serum (Invitrogen, Carlsbad, CA). Cells were transfected with expression plasmids, as indicated, using Lipofectamine Plus reagent (Invitrogen).
Glutathione Transferase (GST) Pull-Down Assay
ERα expression plasmid was transcribed and translated (TnT) in vitro by using T7-coupled reticulocyte lysate and wheat germ extract (Promega, Madison, WI), in the presence of [35S]methionine. Labeled proteins were incubated with purified recombinant fusion proteins, GST-LKB1 and GST, on glutathione-Sepharose beads for 4 h at 4°C as described previously (Marignani et al., 2001). The samples were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and visualized by autoradiography.
Western Blotting and Antibodies
Cells were harvested in lysis buffer as described previously (Scott et al., 2007). Lysates were prepared for SDS-PAGE, and proteins were separated and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). ERα (HC-20), LKB1 (Ley-37D), Lamin B1 (H-90), p300 (H-272), BRCA1 (D-9), and actin (I-19) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-ERα (AER314) was from NeoMarkers (Fremont, CA). Brg1, MP-activated protein kinase (AMPK), and pAMPK(T172) antibodies were from Cell Signaling Technology (Danvers, MA).
Immunoprecipitation Assay
MCF7 cells were maintained in tissue culture dishes (10 cm) in phenol-free (PF)-DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 5% charcoal-stripped fetal bovine serum (csFBS) and transfected with FLAG-LKB1, ERα expression plasmids. Cells were left untreated or treated with E2 (100 nM) for 45 min followed by lysis. LKB1 was immunoprecipitated (IP) from 500 μg of precleared total cell lysate (TCL) by using anti-LKB1.
Nuclear Cytoplasmic Fractionation
MCF7 cells were trypsinized followed by centrifugation (700 × g), cells were resuspended in hypotonic buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.1% NP-40, 1 mM NaF, and 4 μg/ml protease inhibitors), incubated on ice, and centrifuged (700 × g) to separate the cytoplasmic fraction. To obtain the nuclear fraction, cells were homogenized in nuclear lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.2% NP-40, 0.04% SDS, 0.1% sodium deoxycholate, 50 mM NaF, 1 mM Na3VO4, and 4 μg/ml protease inhibitors), and ultracentrifuged (125,000 × g) to remove cellular debris.
Reporter Assay
Reporter assays were performed using Dual Luciferase kit (Promega), and promoter activity was analyzed using an Auto Lumat Plus luminometer (Berthold Technologies, Bad Wildbad, Germany) as described previously (Scott et al., 2007). Briefly, MCF7 or G361 cells were transfected with the following plasmids: LKB1 (50 ng), D194A/R304W (50 ng) LKB1 NR mutants (50 ng), pRL-tk (0.5 ng), ERE-luc (gift from Dr. Myles Brown, Harvard University, Boston, MA)/cyclin D1-luc (50 ng), ERα/ERβ (Addgene plasmid 11356) (10 ng)/androgen receptor (AR; 10 ng), glucocorticoid receptor (GR; 50 ng), mouse mammary tumor virus luciferase (MMTV-luc; 50 ng) (gifts from Dr. Steven Balk, Harvard University), p300 (50 ng) (Addgene plasmid 10718; Addgene, Cambridge, MA), BRCA1, and p53 (indicated concentrations). Six hours after transfection, phenol-free DMEM 5% serum-stripped FBS (ssFBS) was used to replace the media. Twenty-four hours after transfection, cells were treated with E2 (10 nM), 4-hydroxy tamoxifen (4-OHT; 1 uM), dexamethasone (100 nM), or testosterone (100 nM) (Sigma-Aldrich). Control cells were left untreated. Cells were washed in phosphate-buffered saline (PBS) and harvested after 24 h in passive lysis buffer according to manufacturer's protocol (Promega).
Small Interfering RNA (siRNA) Transfection
MCF7 cells were transfected with LKB1 siRNA (100 nM; Target sequences 1, GCUCUUACGGCAAGGUGAA; 2, UGAAAGGGAUGCUUGAGUA; and 3 GAAGAAGGAAAUUCAACUA; Dharmacon, RNA Technologies, Lafayette, CO) by using DharmaFECT transfection reagent.
Flow Cytometry.
MCF7 maintained in PF-DMEM 5% csFBS for 72 h before transfection with LKB1 siRNA and myr-green fluorescent protein (GFP)-cytomegalovirus (CMV) as described previously (Scott et al., 2007). Twenty-four hours after transfection, cells were incubated in PF-DMEM, 3% ssFBS for 24 h before treatment with vehicle or E2 (100 nM) for 24 h and then harvested and prepared for flow cytometry. Propidium iodide was purchased from Sigma-Aldrich. Fluorescence-activated cell sorting (FACS) analysis was conducted on an FACSCalibur flow cytometer (Beckman Coulter, Fullerton, CA) with data acquired using CellQuest software. Cell cycle profiles were determined using ModFit LT.
Immunofluorescence
Cells were prepared for fluorescent microscopy as described previously (Marignani and Carpenter, 2001). Cells were incubated in primary antibody for 1 h, followed by incubation with appropriate secondary antibodies: Oregon green and rhodopsin (Invitrogen). Nuclear staining was accomplished using 4,6-diamidino-2-phenylindole (DAPI) for 2 min. Images were obtained using an Eclipse TE 2000-E inverted research microscope (Nikon, Tokyo, Japan), mounted with a Q-Imaging charge-coupled device camera and acquired using the Simple PCI software.
In Vitro Kinase Assay
LKBtide assays were conducted as described previously (Marignani et al., 2007). Briefly, HEK293 cells were transfected with the following expression plasmids: LKB1pEBG-2T, R304WpEBG-2T, and pEBG-2T vector alone together with MO25α-MYC-CMV and STRADα-FLAG-CMV. Phosphotransferase activity of LKB1 toward the LKBtide peptide (150 μM) (Millipore, Billerica, MA) was determined using protein complex (0.5 μg) in the presence of 0.1 mM [γ-32P]ATP (PerkinElmer Life and Analytical Sciences, Boston, MA) at 30°C for 15 min. Kinase assays in vitro were performed as described previously (Marignani et al., 2001). ERα, AMPK, p300, BRCA1, and Brg1 were immunoprecipitated with appropriate antibodies from MCF7 cells for 3 h followed by incubation with purified LKB1/Stradα/Mo25 complexes in the presence of [γ-32P]ATP. Purified recombinant human AMPK was from Cell Signaling Technology. Phosphorylation was visualized by autoradiography.
RNA Isolation and Polymerase Chain Reaction (PCR)
RNA was isolated using TRIzol reagent (Invitrogen) and reverse transcribed as per manufacturer's protocol (Ambion, Austin, TX). PCR was conducted at 95°C for 15 min, 24 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 1.5 min, and a final step of 72°C for 5 min (iCycler; Bio-Rad Laboratories, Hercules, CA) on cDNA by using primers listed on Supplemental Table 1.
Chromatin Immunoprecipitation
G361 and MCF7 cells were grown in DMEM supplemented with 8% FBS or PF-DMEM with 5% charcoal-stripped FBS, respectively. MCF7 cells were left untreated or treated with 100 nM E2 for 45 min. G361 cells were transfected with ERα, LKB1, and R304W expression plasmids. Chromatin immunoprecipitation (ChIP) assays were conducted as described previously (Scott et al., 2007). Immunoprecipitation was performed at 4°C overnight using anti-LKB1 or anti-mouse surface immunoglobulin G (mIgG) (control) antibodies. PCR was performed using primers for the promoters of pS2, c-myc, and cathepsin D as listed in Supplemental Table 1.
RESULTS
LKB1 Interacts with ERα
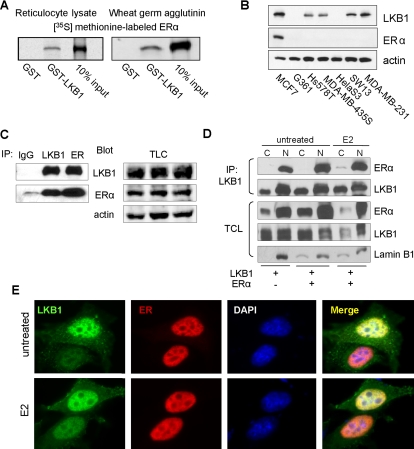
We previously identified the first binding partner for LKB1, the SWI/SNF chromatin remodeling protein Brg1 (Marignani et al., 2001). LKB1 interacts with the helicase domain of Brg1, facilitating the ATPase activity of this chromatin remodeler (Marignani et al., 2001). Given that Brg1 is necessary for ERα-mediated gene transactivation (DiRenzo et al., 2000) and LKB1 enhances Brg1-ATPase activity, we investigated whether LKB1 interacts with the nuclear hormone receptor ERα. Here, we TnT ERα expression plasmid in vitro in the presence of [35S]methionine by using rabbit reticulocyte lysate and wheat germ agglutinin followed by GST pull-down assays (Figure 1A). We observed that recombinant GST-LKB1 protein bound to ERα, whereas control recombinant GST protein did not (Figure 1A). The interaction was confirmed in MCF7 cells that express both endogenous LKB1 and ERα (Figure 1B). Here, endogenous LKB1 and ER were IP using anti-LKB1 and anti-ERα antibodies followed by Western blot analysis to confirm coimmunoprecipitation (Figure 1C).
Figure 1.
LKB1 binds to ERα. (A) Left, GST pull-down assays were conducted using 35S-ERα incubated with GST and GST-LKB1 (50 pmol) fusion proteins and visualized by autoradiography. (B) Western blot analysis of LKB1 and ERα expression in different cancer cell lines was confirmed by western blot analysis using antibodies specific for LKB1, ERα, and actin. (C) Anti-LKB1, -ERα, and IgG (control) antibodies were used to IP endogenous proteins from MCF7 cells, followed by Western blot analysis for IP LKB1, ERα, and TCL in which actin was used as loading control. (D) MCF cells were transfected with pcDNA3.1, ERα and LKB1 expression plasmids. Cells were treated with E2 (100 nM) followed by cellular fractionation: nuclear (N) and cytoplasmic (C). LKB1 was IP using anti-LKB1 antibody, followed by Western blot analysis in which membranes were probed for LKB1, ERα, and lamin B1 (nuclear protein marker). (E) Representative immunofluorescent images of MCF7 cells left untreated or treated with E2 (100 nM), fixed, and incubated with anti-LKB1 (green) and anti-ERα (red) antibodies and appropriate secondary antibodies. Nuclei were stained with DAPI. Results are representative of three experiments.
Nuclear Localization of LKB1–ERα Interaction
To determine the cellular localization of LKB1 and ERα interaction, MCF7 cells were transfected with LKB1 and ERα expression plasmids. Cells were treated with E2 (100 nM) for 45 min before harvesting. Nuclear and cytoplasmic fractions were prepared, followed by immunoprecipitation of equal amounts of protein from each fraction with anti-LKB1 antibody. We observed that the interaction between LKB1 and ERα was restricted to the nuclear fraction (Figure 1D). To further verify these observations, immunofluorescence microscopy was conducted in MCF7 cells left untreated or treated with E2. We observe the nuclear colocalization of endogenous LKB1 and ERα (Figure 1E). These results confirm our Western blot analysis that shows coimmunoprecipitation of LKB1 and ERα occurs in the nuclear fraction (Figure 1D).
LKB1 Functions as a Coactivator of ERα
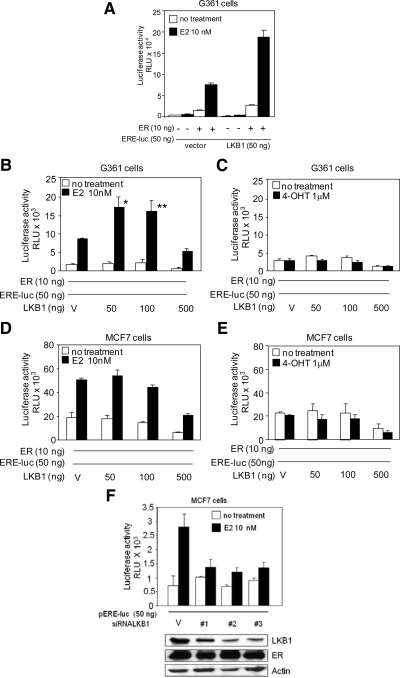
Because LKB1 interacts with the ERα in the nuclear compartment of the cell, we investigated the possibility that LKB1 played a role in ERα-mediated gene transactivation by performing reporter assays. Because we were interested in whether the catalytic activity of LKB1 was required for this function, we used LKB1 null cells to facilitate in the interpretations of results in the absence of endogenous LKB1. In addition, previous work by others have shown that ectopic expression of ERα in cells that express endogenous ERα, such as MCF7, may lead to aberrant ERα signaling because the endogenous protein may function as a dominant-negative (Bocquel et al., 1989; Webb et al., 1992). Hence, we surveyed known ERα-negative breast cancer cell lines for LKB1 expression. In contrast to previous reports (Shen et al., 2002), we observed LKB1 expression in MDA-MB-435S melanoma-derived cell line and MDA-MB-231 breast cancer cells (Figure 1B). Therefore, we conducted ERE-luciferase reporter gene assays in G361 melanoma cell line that do not express either LKB1 or ERα. G361 cells were transfected with LKB1 and ERα expression plasmids pERE-luc and pRL-tk, (Figure 2A). In the presence of LKB1, ERα-mediated transactivation was enhanced in response to E2 treatment, compared with ERα alone and LKB1 alone. To determine whether increasing concentrations of LKB1 altered ERα-mediated transactivation of a reporter gene, G361 and MCF7 cells were transfected with increasing concentrations of LKB1 (50, 100, and 500 ng), followed by ERE-luc reporter assays. In G361 cells, optimal transactivation was observed at 50 ng LKB1, as squelching of the reporter gene, as described previously by Lee et al. (2005), occurred at higher concentrations (Figure 2B). In contrast, overexpression of LKB1 in MCF7 cells did not significantly alter ERα-mediated gene transactivation in response to E2 treatment (Figure 2D). In the presence of 4-OHT, a selective estrogen receptor modulator of ERα, as expected, transactivation of the reporter was abrogated both in the presence of ERα alone and ERα plus LKB1 (Figure 2, C and E). Knockdown of LKB1 expression in MCF7 cells using three separate siRNA duplexes suppressed ERα-mediated transcriptional activity (Figure 2F).
Figure 2.
LKB1 enhances ERα activity in the presence of 17-β estradiol. (A) G361 cells were transfected with LKB1, ERα, ERE-luc, and pRL-tk expression plasmids or vector (V), left untreated or treated with E2 as described in Materials and Methods, followed by reporter assays. Data representative of two experiments in triplicate mean ± SD. (B) G361 cells were transfected with ERα, ERE-luc, and pRL-tk expression plasmids, and increasing concentrations of LKB1 expression plasmid, left untreated, or treated with E2 or (C) 4-OHT as described in Materials and Methods followed by reporter assays. Results are three experiments in triplicate. Data are mean ± SEM. *p < 0.02 compared with 0 ng; **p < 0.05 compared with 0 ng. (D and E) MCF7 cells were transfected and treated as described in B and C. Results are three experiments in triplicate. Data are mean ± SEM. (F) MCF7 cells were transfected with three different siRNA-LKB1 duplexes, ERE-luc, pRL-tk, and treated with E2 as described in Materials and Methods followed, by reporter assays. Western blot analysis confirms abrogation of LKB1 expression by using anti-LKB1, -ERα, and -actin antibodies. Results are representative of two experiments in triplicate mean ± SD.
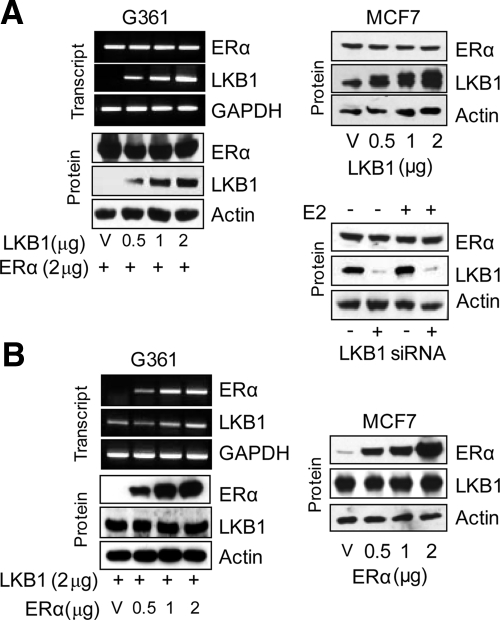
To investigate whether enhanced ERα-mediated transactivation was attributed to the introduction of LKB1 and not to changes in ERα expression levels due to the introduction of LKB1, G361 and MCF7 cells were transfected with increasing concentrations of LKB1 expression plasmid (0, 0.5, 1.5, and 2 μg), while simultaneously maintaining the concentration of ERα expression plasmid (2 μg) constant in G361 cells. Increased expression of LKB1 did not alter the expression of ectopic (G361 cells) or endogenous ERα (MCF7) as determined by reverse transcription (RT)-PCR and Western blot analysis (Figure 3A). Furthermore, knockdown of LKB1 expression in MCF7 cells by siRNA did not affect ERα expression (Figure 3A, bottom right). In addition, the introduction of increasing concentrations of ERα expression plasmid (0, 0.5, 1.5, and 2 μg), while simultaneously maintaining the concentration of LKB1 expression plasmid (2 μg) constant in G361 cells did not alter LKB1 expression of ectopic LKB1 in G361 or endogenous LKB1 in MCF7 cells (Figure 3B).
Figure 3.
ERα expression is not altered by LKB1. (A) Left, G361 cells were transfected with expression plasmids or vector (V) as indicated. LKB1 and ERα expression were determined by PCR and Western blot analysis. Top, right, Western blot analysis of MCF7 cells transfected with increasing concentrations of LKB1. Bottom, right, Western blot analysis of LKB1 and ERα expression in MCF7 cell transfected with LKB1 siRNA-#2 duplex (100 nM). (B) Cells were transfected with expression plasmids as indicated. LKB1 and ERα expression were determined by PCR and Western blot analysis. Results are representative of three experiments.
LKB1 Synergizes with p300
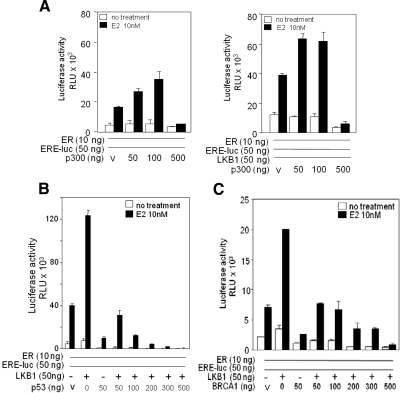
To study the effect of LKB1 on ERα activity in the presence of a known ERα coactivator protein p300 (Hanstein et al., 1996), we conducted ERE-luc reporter assays as described above. We first tested whether expression of p300 in G361 cells maintained coactivator function of ERα-ERE-luc (Figure 4A, left). When p300 and LKB1 expression plasmids were introduced simultaneously, we observe a synergistic effect, suggesting cooperation between LKB1 and p300 in enhancing ERα activity (Figure 4A, right). However, coactivator function of LKB1 in the presence of known corepressor proteins p53 and BRCA1 (Yu et al., 1997; Fan et al., 1999) was suppressed in a concentration-dependent manner (Figure 4, B and C).
Figure 4.
LKB1 and p300 synergize to enhance ERα activity. (A) Left, G361 cells were transfected with increasing concentrations of p300 expression plasmids as indicated; ERα, ERE-luc, and pRL-tk, or vector (V); left untreated or treated with E2 for 24 h followed by reporter gene assays. (A) Right, G361 cells were transfected with increasing concentrations of p300 expression plasmids, a constant concentration of LKB1 as indicated; ERα, ERE-luc, and pRL-tk or V; left untreated or treated with E2 for 24 h followed by reporter gene assays. Results are two experiments in triplicate. Data are mean ± SD (B) G361 cells were transfected with pRL-tk, ERα, ERE-luc, and increasing concentrations of p53, or LKB1 expression plasmids, left untreated or treated with E2 for 24 h followed by reporter gene assays. Data representative of three experiments in triplicate. Data are mean ± SD. (C) Transfection conditions similar to B; however, in the presence of increasing concentrations of BRCA1. Data are representative of three experiments in triplicate mean ± SD.
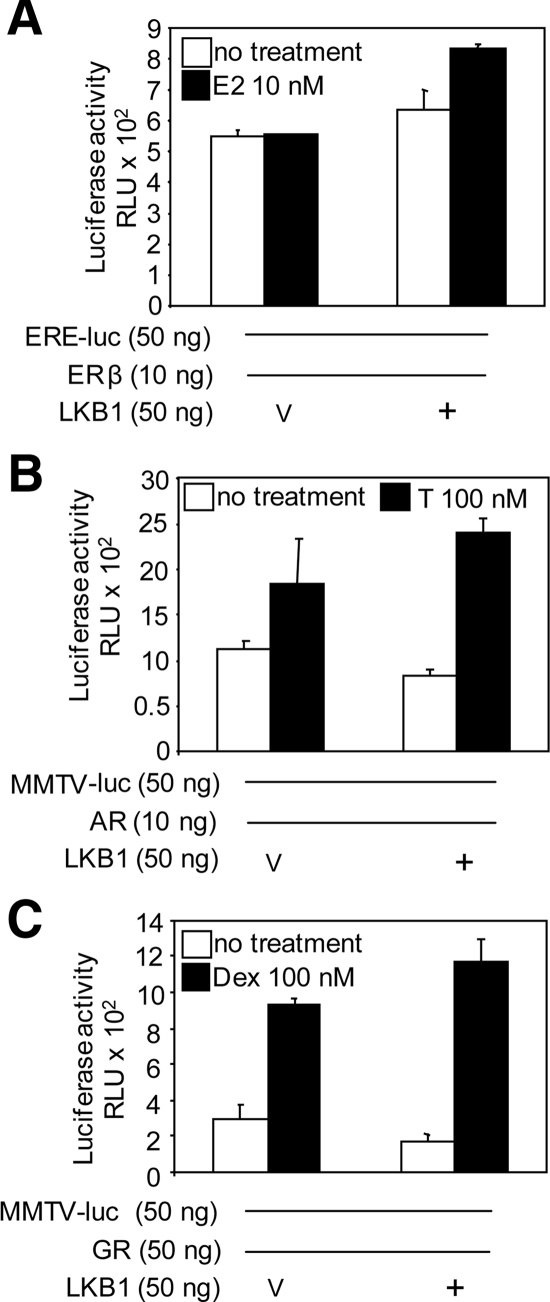
Next, we examined whether the coactivator function of LKB1 applies to other members of the nuclear receptor family such as ERβ, AR, and GR by performing reporter assays. G361 cells were transfected with LKB1, ERβ, AR, or GR expression plasmids, pRL-tk and pERE-luc or MMTV-luc as indicated, left untreated, or treated with E2, testosterone, or dexamethasone (Figure 5). Transactivation of respective reporters by ERβ, GR or AR did not significantly increase in the presence of LKB1.
Figure 5.
LKB1 does not alter ERβ, GR, or AR activity. (A) G361 cells were transfected with LKB1, ERβ, ERE-luc, and pRL-tk expression plasmids or vector (V). Cells were left untreated or treated with E2 (10 nM) for 24 h before harvesting for reporter assay. (B) Cells were transfected with LKB1, AR, MMTV-luc, and pRL-tk expression plasmids or V, followed by testosterone (T; 100 nM) treatment. (C) Cells were transfected with LKB1, GR, MMTV-luc, and pRL-tk expression plasmids or V followed by treatment with dexamethasone (Dex; 100 nM). Results are three experiments in triplicate mean ± SEM.
LKB1 Catalytic Activity Is Required
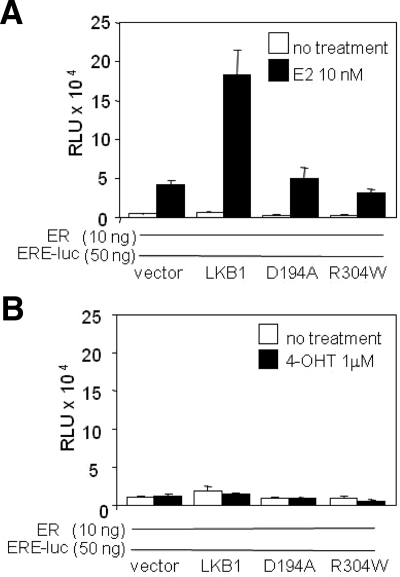
To determine whether LKB1 catalytic activity was necessary for ERα-mediated transactivation of the reporter, reporter assays were conducted using LKB1 catalytic-deficient mutants (LKB1 mutants) D194A and R304W described previously (Marignani et al., 2007; Scott et al., 2007). Both mutants did not alter ERα-mediated transactivation of ERE-luc compared with ERα alone, thereby implying that LKB1 catalytic activity contributes to ERα-mediated transactivation of ERE-luc (Figure 6A). In the presence of 4-OHT transactivation of the reporter was abrogated both in the presence of ERα alone and ERα plus LKB1 or mutants (Figure 6B).
Figure 6.
LKB1 catalytic activity is necessary for ERα transactivation. (A and B) G361 cells were transfected with LKB1, D194A, R304W, ERα, ERE-luc, and pRL-tk expression plasmids or vector (V), left untreated or treated with E2 or 4-OHT for 24 h before harvesting for reporter assay. Results are representative of two experiments in triplicate. Data are mean ± SD.
ERα Is Not a Substrate of LKB1
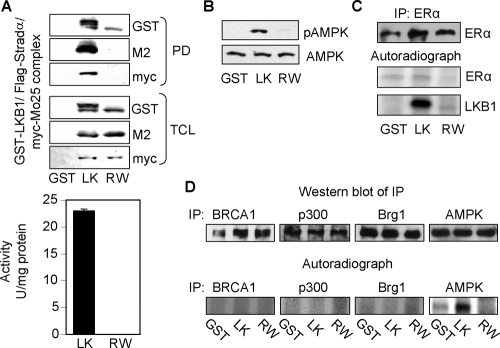
Because we show that LKB1 catalytic activity enhances ERα-mediated transactivation (Figure 2), and LKB1 mutants attenuated ERα-mediated transactivation (Figure 6), we tested whether ERα was a substrate for LKB1. LKB1/Stradα/MO25, R304W/Stradα/MO25 complexes, and GST were prepared from HEK293 cells as described previously (Marignani et al., 2007; Scott et al., 2007) (Figure 7A) and tested for catalytic activity toward the LKBtide peptide (Figure 7A) and purified recombinant human AMPK protein followed by Western blot analysis using anti-phospho-AMPK antibody (pT172) (Figure 7B). Endogenous ERα was immunoprecipitated from MCF7 cells (Figure 7C, top) followed by incubation with purified complexes in the presence of [γ-32P]ATP (Figure 7C, middle). To test whether known coregulator proteins of ERα may be substrates for LKB1, BRCA1, p300, Brg1 (negative control) (Marignani et al., 2001), and AMPK (positive control) (Hawley et al., 2003) were immunoprecipitated from MCF-7 cells by using the appropriate antibodies (Figure 7D, top) followed by incubation with purified LKB1 complex (Figure 7A) followed by kinase assays in vitro (Figure 7D, bottom).
Figure 7.
LKB1 does not phosphorylate ERα. (A) Expression of purified GST-LKB1/FLAG-STRADα/Myc-MO25α complex from HEK293 cells was confirmed by Western blot analysis (top). Confirmation of catalytic activity of complex toward LKBtide peptide (bottom) and (B) purified recombinant human AMPK incubated with complex followed by Western blot analysis using anti-pAMPK(T172) and anti-AMPK for total expression. (C) ERα was IP using anti-ERα antibody from MCF7 cells followed by incubation with purified complex in the presence of [γ-32P]ATP. Top panel confirms IP of ERα by Western blot analysis; bottom panels are corresponding autoradiographs. (D) BRCA1, p300, Brg1 (negative control), and AMPK (positive control) were immunoprecipitated from MCF7 cells by using the appropriate antibodies followed by incubation with complex described in A, in the presence of [γ-32P]ATP. Top panel confirms IP of BRCA1, p300, Brg1, and AMPK by Western blot analysis. Bottom panel represent autoradiographs. Results are representative of three separate experiments. Purified complexes: LK, GST-LKB1/STRADα/Myc-MO25α; RW, GST-R304W/STRADα/Myc-MO25α.
LKB1 Enhances Nonclassical ERα Signaling
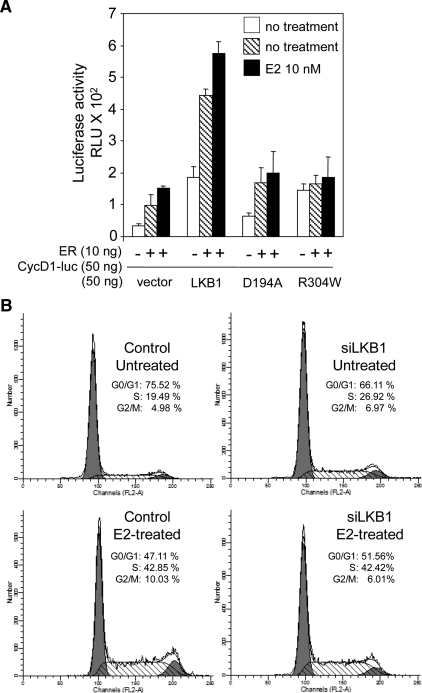
To examine whether LKB1 alters ERα-mediated transcription of genes through the nonclassical pathway, whereby ERα tethers to transcription factors that bind to the promoters of genes, reporter assays were conducted using cyclin D1 luciferase (CD1-luc) (Scott et al., 2007). We observed that ERα enhances CD1-luc in the presence of E2 consistent with a previous report (Sabbah et al., 1999), whereas coexpression of LKB1 and ERα followed by E2 treatment enhanced transactivation of cyclin D1-luc compared with LKB1 alone or ERα alone (Figure 8A). Transactivation of cyclin D1-luc by LKB1 mutants did not differ from ERα alone. Given that G361 cells are wild type for expression of cyclin D1 regulators p53 and p21WAF/CIP, we did not expect LKB1 mutants to function as described previously in DLD1p21−/−p53−/− colorectal cancer (Scott et al., 2007).
Figure 8.
LKB1 enhances nonclassical ERα signaling. (A) G361 cells were transfected with LKB1, D194A and R304W, ERα, cyclin D1-luc and pRL-tk, expression plasmids, or vector. Cells were left untreated or treated with E2 (10 nM) 24 h before harvesting for reporter assay. Results are representative of three experiments in triplicate. Data are mean ± SD. (B) Cells were transfected with siLKB1#2 duplex and GFP-empty vector, left untreated or treated with E2 (100 nM) for 24 h, followed by FACS analysis. DNA profiles for PI/GFP-positive cells were recorded and analyzed. Data as shown are representative of three experiments.
Because we observe that LKB1 enhances ERα-mediated transcription in G361 cells (Figure 2A), we tested whether LKB1 could alter ERα-ligand dependent cell cycle progression (Ikeda and Inoue, 2004). For these studies, LKB1 expression in MCF7 cells was ablated using siLKB1#2 (Figure 2F), followed by treatment with E2 (100 nM) for 24 h, and then flow cytometry to evaluate the cell cycle profile of asynchronous cells. As expected, abrogation of LKB1 expression resulted in an overall increase in the percentage of cells found in S phase for untreated cells with a corresponding reduction in the percentage of cells in G0/G1 phase (Figure 8B), agreeing with previous work that shows the introduction of LKB1 into LKB1 null G361 cells arrests cells in G1 phase of cell cycle (Tiainen et al., 1999). Compared with control E2-treated cells, we did not observe a difference in the percentage of cells in S phase from siLKB1 cells. Similar cell cycle profiles were observed for siLKB1#1 and #3 (data not shown).
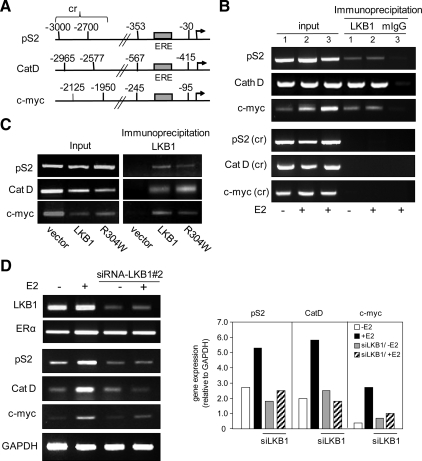
LKB1 Is Recruited to the Promoters of ER-regulated Genes
Because LKB1 enhances ERα transcriptional activity, we examined whether endogenous LKB1 interacts with the promoters of ERα activated genes pS2, cathepsin D (Augereau et al., 1994), and c-myc (Dubik and Shiu, 1988) (Figure 9A). We conducted ChIP in MCF7 cells that were either left untreated or were treated with E2. When endogenous LKB1 was immunoprecipitated using anti-LKB1 antibody, we observed recruitment of LKB1 to the pS2, cathepsin D, and c-myc promoters in both untreated and E2-treated cells, compared with corresponding control regions (cr) (Figure 9B). To determine whether catalytic-deficient mutants were recruited to these promoters, G361 cells were transfected with ERα, LKB1, and R304W expression plasmids or vector control followed by ChIP. Both LKB1 and R304W were recruited to all three gene promoters, compared with the vector control (Figure 9C).
Figure 9.
Recruitment of LKB1 to promoters. (A) Schematic representation of pS2, cathepsin D (Cat D), and c-myc promoters and control regions (cr). (B) ChIP analysis using anti-LKB1 antibody showing recruitment of LKB1 to the promoters of pS2, Cat D, and c-myc in the presence and absence of E2 treatment (100 nM) in MCF-7 cells. Control region (cr) of pS2(−3000 to −2700), Cat D (−2965 to −2577), and c-Myc (−2125 to −1950) (C) G361 cells lacking endogenous LKB1 and ERα expression were transfected with ERα, plus LKB1 or R304W expression plasmids followed by ChIP using anti-LKB1 antibody. (D) MCF7 cells were transfected with siLKB1#2 duplex, left untreated or treated with E2 (100 nM). Expression of ERα-responsive genes pS2, Cat D, and c-myc was determined by RT-PCR (left) and expressed relative to GAPDH as determined by densitometry (right). Results are representative of three separate experiments.
Because we observed recruitment of LKB1 to the promoter of ERα-responsive genes, we asked whether LKB1 altered the expression of pS2, cathepsin D, and c-myc. To test this, we depleted the expression of LKB1 in MCF7 cells by siRNALKB1#2 (Figure 2F), followed by RT-PCR (Supplemental Table 1). In cells depleted of ∼75% of LKB1 (Figure 9D, left), we observed a >50% reduction in pS2, cathepsin D, and c-myc expression in response to E2 treatment, compared with cells not depleted of LKB1 expression (Figure 9D, right; RT-PCR expressed relative to glyceraldehyde-3-phosphate dehydrogenase [GAPDH]).
DISCUSSION
We describe a novel function for LKB1, a regulator of ERα signaling. We demonstrate that LKB1 binds to ERα in a ligand-independent manner (Figure 1A) in the nuclear compartment of the cell (Figure 1, B and C). A function of this interaction is that LKB1 enhances ERα-mediated transactivation in a ligand-dependent manner (Figure 2, A–C), because abrogation of LKB1 expression by siRNA resulted in a suppression of ERα activity (Figure 2F). We confirmed by mRNA and protein analysis that the introduction of LKB1 did not alter the overall expression of ERα (Figure 3); thus, the observed enhancement in ERα activity was not due to increased ERα levels but due to the interaction of LKB1 with ERα. Equally important, the introduction of ERα into cells did not alter the overall expression of LKB1 (Figure 3). Our discovery is consistent with a previous report (Dutertre and Smith, 2003) that demonstrates coactivator proteins such as p160 interacting with ERα independently of ligand binding; however, p160-enhanced ERα function was dependent of ligand (Dutertre and Smith, 2003). Furthermore, we show that LKB1 did not alter the transcriptional activity of ERβ, AR, or GR (Figure 5), suggesting that LKB1 is selective for ERα.
Because coregulator proteins function to alter ERα activity, we investigated the effect of LKB1 in the presence of known ERα coregulatory proteins. In the presence of ERα coactivator p300 (Hanstein et al., 1996), LKB1 synergizes ERα-mediated transactivation of ERE-luc (Figure 4A). Synergy between ERα coactivator proteins has been described, such as GRIP1, CARM1, and p300 (Chen et al., 2000), and between SRC1 and CBP (Smith et al., 1996). In the presence of ERα corepressor proteins p53 and BRCA1 (Yu et al., 1997; Fan et al., 1999), the enhanced transactivation we observe with LKB1 and ERα alone is antagonized when either of the ERα corepressor proteins is introduced, suggesting that these corepressor proteins compete with LKB1 for ERα (Figure 4, B and C). Previously, our laboratory discovered a functional interaction between LKB1 and Brg1, whereby the interaction enhanced Brg1 ATPase activity, necessary for chromatin remodeling and therefore gene transactivation (Marignani et al., 2001). Furthermore, LKB1 induces p21WAF/CIP1 (Tiainen et al., 2002), which in turn enhances ERα activity (Fritah et al., 2005). Coregulator proteins function by participating in chromatin remodeling or assist in the assembly of other coregulatory proteins. Because LKB1 exhibits both these functions, it is likely that LKB1 may be part of coactivator complexes with functionally diverse roles that are dependent on the availability of other coregulatory proteins (Szapary et al., 1999).
Catalytically deficient LKB1 mutants D194A and R304W failed to enhance ERα-mediated transactivation, indicating that LKB1 catalytic activity is necessary for this function (Figure 6). Thus, we tested whether LKB1 phosphorylated ERα because previous reports suggest phosphorylation of ERα enhances transcription activity by several mechanisms (Weigel and Moore, 2007). From kinase assays, we determined that LKB1 does not phosphorylate ERα (Figure 7B). Others have reported that alteration in the phosphorylation state of coregulator proteins modulate nuclear receptor activity (Frigo et al., 2006). It is conceivable that LKB1 phosphorylates an ERα coregulator protein, thereby enhancing ERα activity. A previous study suggested that a novel nuclear protein kinase that interacts with AR enhances AR activity by phosphorylating coregulatory proteins and not AR (Moilanen et al., 1998). We demonstrate that LKB1 does not phosphorylate ERα coregulatory proteins p300 and BRCA1, compared with Brg1 (negative control) (Marignani et al., 2001) and AMPK (positive control) (Lizcano et al., 2004; Sakamoto et al., 2004) (Figure 7C), indicating that a plausible substrate of LKB1 may be a coregulatory protein of hormone receptor signaling other than the coregulatory proteins we tested in our system.
In addition to the classical pathway of gene regulation, ERα regulates genes such as cyclin D1 by an alternative mechanism (Sabbah et al., 1999; Castro-Rivera et al., 2001), that is, the nonclassical pathway (Nilsson et al., 2001; Sommer and Fuqua, 2001), whereby ERα associates with transcription factors that bind to DNA. Increases in cyclin D1 expression leads to enhanced cellular proliferation, promoting atypical lobular-alveolar development and contributing to the formation of mammary adenocarcinomas (Wang et al., 1994). Clinical studies show >50% of breast tumors express elevated levels of cyclin D1 (Barnes and Gillett, 1998) and display elevated levels of ERα activity (Shoker et al., 2001). Previously, we showed recruitment of LKB1 to the promoter of cyclin D1 in cells lacking the expression of cyclin D1 negative regulators p53 and p21WAF1/CIP1 (Scott et al., 2007). Our current results demonstrate that LKB1 enhances ERα-mediated transactivation of the cyclin D1 promoter in G361 cells (Figure 8A). Hence, it is possible that recruitment of LKB1 to the promoter of cyclin D1 facilitates the protein–DNA or protein–protein interactions necessary for ERα activity. Previous work by others show that ERα associates with the p160-CBP coactivators, which are in turn recruited by jun/fos to the AP-1 site (Kushner et al., 2000). Perhaps LKB1 may function in a similar manner by recruiting ERα to the cyclin D1 promoter, along with additional coactivator proteins. Interestingly, ablation of LKB1 expression in MCF7 cells by siRNA resulted in an increase in the percentage of cells transitions to S phase of cell cycle compared with control untreated cells, as measure by flow cytometry (Figure 8B). In response to E2 treatment, however, the percentage of cells in S phase of cell cycle did not differ between control and siLKB1 cells (Figure 8B). These data are supported by recent studies conducted in three-dimensional acini cultures of 4-OHT–inducible MCF10A-MycERtm cells. In this study, the reduction of LKB1 expression by short hairpin RNA led to an increase in c-myc–mediated cell proliferation as measured by Ki-67 labeling of acini (Partanen et al., 2007). The authors of this study correlate reduced LKB1 expression with disruption of quiescent 4-OHT–induced MCF10A-MycERtm cell architecture for up to 10 d, confirming cell polarity plays a protective role against apoptosis (Partanen et al., 2007). Together, these data suggest that the tumor suppressor activity of LKB1 is likely distinct from its hormone receptor coactivator function and further highlight the multitasking and therefore multifunctional activities of LKB1 (Marignani, 2005).
We show that LKB1 is recruited to the promoters of ERα-regulated genes independently of E2 treatment, specifically cathepsin D, pS2/TFF1, and c-Myc (Shang and Brown, 2002; Métivier et al., 2003) (Figure 9, B and C). Furthermore, we confirmed by siRNA that LKB1 contributes to the expression of cathepsin D, pS2/TFF1, and c-Myc because abrogation of LKB1 expression attenuated expression of pS2 in the absence of ligand and expression of all three ERα targets—pS2, cathepsin D, and c-myc—in the presence of E2 ligand (Figure 9D). In addition to wild-type LKB1, ChIP assays confirm the recruitment of catalytic deficient mutant R304W to the promoters of cathepsin D, pS2/TFF1, and c-Myc, supporting our position that a plausible substrate of LKB1 may be a coregulatory protein of hormone receptor signaling. Overall, these results suggest that LKB1 through its association with ERα is involved in the expression of downstream targets of ERα and that LKB1 tumor suppressor function, that is, preventing cells from transitioning from G1 phase to S phase of cell cycle (Tiainen et al., 1999) is distinct from its ERα coactivator function.
Our discovery defines a new function for the tumor suppressor serine threonine kinase LKB1, as a coactivator of ERα. It is paradoxical that LKB1, a tumor suppressor, enhances ERα activity, which would likely contribute to breast cancer risk. This said, there are reports in the literature for tumor suppressor proteins functioning as nuclear receptor coactivators. Briefly, despite being the archetypical tumor suppressor, retinoblastoma (Rb) also has the distinction of being a coactivator of nuclear hormone receptors ERα, AR, progesterone receptor (PR), and GR (Singh et al., 1995; Lu and Danielsen, 1998; Yeh et al., 1998; Batsche et al., 2005). Interestingly, Rb is the first example of a tumor suppressor to function in a role as an activator of nuclear hormone receptor signaling. The regulation of the GR by Rb is mediated through Rb association with SWI/SNF chromatin remodeling complex, specifically Brg1(Singh et al., 1995), whereas up-regulation of AR-mediated transcription by Rb is independent of Rb–Brg1 interaction (Lu and Danielsen, 1998). A second tumor suppressor, p21WAF/CIP1, was found to bind to ERα, enhancing ERα-dependent transcription of target genes by forming a ternary complex composed of p21WAF/CIP1, CREB-binding protein, and ERα (Fritah et al., 2005). The tumor suppressor tuberous sclerosis 2 (TSC2) has also been shown to function as a coactivator of nuclear hormone receptors, specifically the retinoid X receptors, peroxisome proliferator receptor (PPAR) α, and vitamin D receptor (VDR) (Henry et al., 1998). In these studies, TSC2 weakly associated with the receptors and up-regulated both PPARα- and VDR-mediated transactivation. However, TSC2 functioned as a corepressor of GR-mediated transcription (Henry et al., 1998). Finally, in addition to binding to LKB1 (Marignani et al., 2001), Brg1 is reported to bind to nuclear hormone receptors including the ERα, AR, GR, and PR (Trotter and Archer, 2008). Interestingly, when bound to ERα, AR, and PR, Brg1 functions as a coactivator protein and when bound to GR, Brg1 functions as a corepressor protein for this receptor (Trotter and Archer, 2008).
In summary, we demonstrate for the first time a functional link between LKB1 and the hormone receptor ERα. Our discovery now places LKB1 in a coactivator role for ERα signaling, broadening the scientific scope of this tumor suppressor kinase and laying the groundwork for further investigations into the role of LKB1 in breast disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. D. Hoskin (Dalhousie University) for providing MDA-MB-231 cells and Dr. J. Berman (Dalhousie University) for BRCA1 plasmid. We thank Drs. Melanie Dobson and Catherine Too for helpful discussions. PM is supported by the Canadian Breast Cancer Foundation-Atlantic Chapter (CBCF-AC). SNS was supported by the Cancer Research Training Program-CIHR/DCRP, CBCF-AC and the Nova Scotia Health Research Foundation.
Abbreviations used:
- 4-OHT
4-hydroxy tamoxifen
- E2
17β-estradiol
- ERα
estrogen receptor α
- ERE
estrogen response element
- PJS
Peutz-Jeghers syndrome
- TnT
transcribed and translated.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-11-1138) on April 15, 2009.
REFERENCES
- Augereau P., Miralles F., Cavailles V., Gaudelet C., Parker M., Rochefort H. Characterization of the proximal estrogen-responsive element of human cathepsin D gene. Mol. Endocrinol. 1994;8:693–703. doi: 10.1210/mend.8.6.7935485. [DOI] [PubMed] [Google Scholar]
- Avizienyte E., et al. LKB1 somatic mutations in sporadic tumors. Am. J. Pathol. 1999;154:677–681. doi: 10.1016/S0002-9440(10)65314-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Gillett C. Cyclin D1 in breast cancer. Breast Cancer Res. Treat. 1998;52:1–15. doi: 10.1023/a:1006103831990. [DOI] [PubMed] [Google Scholar]
- Batsche E., Desroches J., Bilodeau S., Gauthier Y., Drouin J. Rb enhances p160/SRC coactivator-dependent activity of nuclear receptors and hormone responsiveness. J. Biol. Chem. 2005;280:19746–19756. doi: 10.1074/jbc.M413428200. [DOI] [PubMed] [Google Scholar]
- Bignell G. R., Barfoot R., Seal S., Collins N., Warren W., Stratton M. R. Low frequency of somatic mutations in the LKB1/Peutz-Jeghers syndrome gene in sporadic breast cancer. Cancer Res. 1998;58:1384–1386. [PubMed] [Google Scholar]
- Bocquel M. T., Kumar V., Stricker C., Chambon P., Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 1989;17:2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J., Kieloch A., Alessi D. R., Stella A., Guanti G., Resta N. Functional analysis of LKB1/STK11 mutants and two aberrant isoforms found in Peutz-Jeghers syndrome patients. Hum. Mutat. 2003;21:172. doi: 10.1002/humu.9112. [DOI] [PubMed] [Google Scholar]
- Carretero J., Medina P. P., Pio R., Montuenga L. M., Sanchez-Cespedes M. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene. 2004;23:4037–4040. doi: 10.1038/sj.onc.1207502. [DOI] [PubMed] [Google Scholar]
- Castro-Rivera E, Samudio I., Safe S. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J. Biol. Chem. 2001;276:30853–30861. doi: 10.1074/jbc.M103339200. [DOI] [PubMed] [Google Scholar]
- Chen D., Huang S.-M., Stallcup M. R. Synergistic, p160 coactivator-dependent enhancement of estrogen receptor function by CARM1 and p300. J. Biol. Chem. 2000;275:40810–40816. doi: 10.1074/jbc.M005459200. [DOI] [PubMed] [Google Scholar]
- DiRenzo J., Shang Y., Phelan M., Sif S., Myers M., Kingston R., Brown M. BRG-1 is recruited to estrogen-responsive promoters and cooperates with factors involved in histone acetylation. Mol. Cell Biol. 2000;20:7541–7549. doi: 10.1128/mcb.20.20.7541-7549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman J., Macara I. G. STRAD{alpha} Regulates LKB1 Localization by Blocking Access to Importin-{alpha}, and by Association with Crm1 and Exportin-7. Mol. Biol. Cell. 2008;19:1614–1626. doi: 10.1091/mbc.E07-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubik D., Shiu R. P. Transcriptional regulation of c-myc oncogene expression by estrogen in hormone-responsive human breast cancer cells. J. Biol. Chem. 1988;263:12705–12708. [PubMed] [Google Scholar]
- Dutertre M., Smith C. L. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-{alpha}: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol. Endocrinol. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- Esteller M., Avizienyte E., Corn P. G., Lothe R. A., Baylin S. B., Aaltonen L. A., Herman J. G. Epigenetic inactivation of LKB1 in primary tumors associated with the Peutz-Jeghers syndrome. Oncogene. 2000;19:164–168. doi: 10.1038/sj.onc.1203227. [DOI] [PubMed] [Google Scholar]
- Fan S., Wang J. A., Yuan R., Ma Y., Meng Q., Erdos M. R., Pestell R. G., Yuan F., Auborn K. J., Goldberg I. D., Rosen E. M. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science. 1999;284:1354–1356. doi: 10.1126/science.284.5418.1354. [DOI] [PubMed] [Google Scholar]
- Fenton H., Carlile B., Montgomery E., Carraway H., Herman J., Sahin F., Su G., Argani P. LKB1 protein expression in human breast cancer. Appl. Immunohistochem. Mol. Morphol. 2006;14:146–153. doi: 10.1097/01.pai.0000176157.07908.20. [DOI] [PubMed] [Google Scholar]
- Frigo D. E., et al. p38 Mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol. Endocrinol. 2006;20:971–983. doi: 10.1210/me.2004-0075. [DOI] [PubMed] [Google Scholar]
- Fritah A., Saucier C., Mester J., Redeuilh G., Sabbah M. p21WAF1/CIP1 selectively controls the transcriptional activity of estrogen receptor {alpha} Mol. Cell Biol. 2005;25:2419–2430. doi: 10.1128/MCB.25.6.2419-2430.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello F. M., Brensinger J. D., Tersmette A. C., Goodman S. N., Petersen G. M., Booker S. V., Cruz-Correa M., Offerhaus J. A. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- Hanstein B., Eckner R., DiRenzo J., Halachmi S., Liu H., Searcy B., Kurokawa R., Brown M. p300 is a component of an estrogen receptor coactivator*complex. Proc. Natl. Acad. Sci. USA. 1996;93:11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A. The molecular basis and clinical aspects of Peutz-Jeghers syndrome. Cell Mol. Life Sci. 1999;55:735–750. doi: 10.1007/s000180050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A., et al. Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat. Genet. 1997;15:87–90. doi: 10.1038/ng0197-87. [DOI] [PubMed] [Google Scholar]
- Henry K. W., Yuan X., Koszewski N. J., Onda H., Kwiatkowski D. J., Noonan D. J. Tuberous sclerosis gene 2 product modulates transcription mediated by steroid hormone receptor family members. J. Biol. Chem. 1998;273:20535–20539. doi: 10.1074/jbc.273.32.20535. [DOI] [PubMed] [Google Scholar]
- Hruban R. H., Petersen G. M., Goggins M., Tersmette A. C., Offerhaus G. J., Falatko F., Yeo C. J., Kern S. E. Familial pancreatic cancer. Ann. Oncol. 1999;10(suppl 4):69–73. [PubMed] [Google Scholar]
- Ikeda K., Inoue S. Estrogen receptors and their downstream targets in cancer. Arch. Histol. Cytol. 2004;67:435–442. doi: 10.1679/aohc.67.435. [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Enmark E., Pelto-Huikkom M, Nilssonm S, Gustafsson J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner P. J., Agard D. A., Greene G. L., Scanlan T. S., Shiau A. K., Uht R. M., Webb P. Estrogen receptor pathways to AP-1. J. Steroid Biochem. Mol. Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Lee J. E., Kim K., Sacchettini J. C., Smith C. V., Safe S. DRIP150 Coactivation of estrogen receptor {alpha} in ZR-75 breast cancer cells is independent of LXXLL motifs. J. Biol. Chem. 2005;280:8819–8830. doi: 10.1074/jbc.M413184200. [DOI] [PubMed] [Google Scholar]
- Lim W., et al. Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br. J. Cancer. 2003;89:308–313. doi: 10.1038/sj.bjc.6601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano J. M., et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Danielsen M. Differential regulation of androgen and glucocorticoid receptors by retinoblastoma protein. J. Biol. Chem. 1998;273:31528–31533. doi: 10.1074/jbc.273.47.31528. [DOI] [PubMed] [Google Scholar]
- Marignani P. A. LKB1, the multitasking tumour suppressor kinase. J. Clin. Pathol. 2005;58:15–19. doi: 10.1136/jcp.2003.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marignani P. A., Carpenter C. L. Vav2 is required for cell spreading. J. Cell Biol. 2001;154:177–186. doi: 10.1083/jcb.200103134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marignani P. A., Kanai F., Carpenter C. L. LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest. J. Biol. Chem. 2001;276:32415–32418. doi: 10.1074/jbc.C100207200. [DOI] [PubMed] [Google Scholar]
- Marignani P. A., Scott K. D., Bagnulo R., Cannone D., Ferrari E., Stella A., Guanti G., Simone C., Resta N. Novel splice isoforms of STRADalpha differentially affect LKB1 activity, complex assembly and subcellular localization. Cancer Biol. Ther. 2007;6:1627–1631. doi: 10.4161/cbt.6.10.4787. [DOI] [PubMed] [Google Scholar]
- Métivier R., Penot G., Hübner M., Reid G., Brand H., Kos M., Gannon F. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- Moilanen A.-M., Karvonen U., Poukka H., Janne O. A., Palvimo J. J. Activation of androgen receptor function by a novel nuclear protein kinase. Mol. Biol. Cell. 1998;9:2527–2543. doi: 10.1091/mbc.9.9.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi C., Yamaguchi T., Iijima T., Saji S., Toi M., Mori T., Miyaki M. Germline mutation of the LKB1/STK11 gene with loss of the normal allele in an aggressive breast cancer of Peutz-Jeghers syndrome. Oncology. 2004;67:476–479. doi: 10.1159/000082933. [DOI] [PubMed] [Google Scholar]
- Nezu J., Oku A., Shimane M. Loss of cytoplasmic retention ability of mutant LKB1 found in Peutz-Jeghers syndrome patients. Biochem. Biophys. Res. Commun. 1999;261:750–755. doi: 10.1006/bbrc.1999.1047. [DOI] [PubMed] [Google Scholar]
- Nilsson S., Makela S., Treuter E., Tujague M., Thomsen J., Andersson G., Enmark E., Pettersson K., Warner M., Gustafsson J. A. Mechanisms of estrogen action. Physiol. Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- O'Lone R., Frith M. C., Karlsson E. K., Hansen U. Genomic targets of nuclear estrogen receptors. Mol. Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Partanen J. I., Nieminen A. I., Makela T. P., Klefstrom J. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc. Natl. Acad. Sci. USA. 2007;104:14694–14699. doi: 10.1073/pnas.0704677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah M., Courilleau D., Mester J., Redeuilh G. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc. Natl. Acad. Sci. USA. 1999;96:11217–11222. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Goransson O., Hardie D. G., Alessi D. R. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am. J. Physiol. Endocrinol. Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cespedes M., Parrella P., Esteller M., Nomoto S., Trink B., Engles J. M., Westra W. H., Herman J. G., Sidransky D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- Scott K. D., Nath-Sain S., Agnew M. D., Marignani P. A. LKB1 Catalytically Deficient Mutants Enhance Cyclin D1 Expression. Cancer Res. 2007;67:5622–5627. doi: 10.1158/0008-5472.CAN-07-0762. [DOI] [PubMed] [Google Scholar]
- Shang Y., Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- Shen Z., Wen X. F., Lan F., Shen Z. Z., Shao Z. M. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin. Cancer Res. 2002;8:2085–2090. [PubMed] [Google Scholar]
- Shoker B. S., Jarvis C., Davies M.P.A., Iqbal M., Sibson D. R., Sloane J. P. Immunodetectable cyclin D1is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br. J. Cancer. 2001;84:1064–1069. doi: 10.1054/bjoc.2001.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Coe J., Hong W. A role for retinoblastoma protein in potentiating transcriptional activation by the glucocorticoid receptor. Nature. 1995;374:562–565. doi: 10.1038/374562a0. [DOI] [PubMed] [Google Scholar]
- Smith C., Oñate S., Tsai M., O'Malley B. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc. Natl. Acad. Sci. USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S., Fuqua S. A. Estrogen receptor and breast cancer. Semin. Cancer Biol. 2001;11:339–352. doi: 10.1006/scbi.2001.0389. [DOI] [PubMed] [Google Scholar]
- Szapary D., Huang Y., Simons S. S., Jr Opposing effects of corepressor and coactivators in determining the dose-response curve of agonists, and residual agonist activity of antagonists, for glucocorticoid receptor-regulated gene expression. Mol. Endocrinol. 1999;13:2108–2121. doi: 10.1210/mend.13.12.0384. [DOI] [PubMed] [Google Scholar]
- Tiainen M., Vaahtomeri K., Ylikorkala A., Makela T. P. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1) Hum. Mol. Genet. 2002;11:1497–1504. doi: 10.1093/hmg/11.13.1497. [DOI] [PubMed] [Google Scholar]
- Tiainen M., Ylikorkala A., Makela T. P. Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest. Proc. Natl. Acad. Sci. USA. 1999;96:9248–9251. doi: 10.1073/pnas.96.16.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter K., Archer T. K. The BRG1 transcriptional coregulator. Nucl. Recept. Signal. 2008;6:1–12. doi: 10.1621/nrs.06004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Green S., Greene G., Krust A., Bornert J. M., Jeltsch J. M., Staub A., Jensen E., Scrace G., Waterfield M. Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. USA. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. C., Cardiff R. D., Zukerberg L., Lees E., Arnold A., Schmidt E. V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Wang Z. J., Ellis I., Zauber P., Iwama T., Marchese C., Talbot I., Xue W. H., Yan Z. Y., Tomlinson I. Allelic imbalance at the LKB1 (STK11) locus in tumours from patients with Peutz-Jeghers' syndrome provides evidence for a hamartoma-(adenoma)-carcinoma sequence. J. Pathol. 1999;188:9–13. doi: 10.1002/(SICI)1096-9896(199905)188:1<9::AID-PATH326>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Webb P., Lopez G. N., Greene G. L., Baxter J. D., Kushner P. J. The limits of the cellular capacity to mediate an estrogen response. Mol. Endocrinol. 1992;6:157–167. doi: 10.1210/mend.6.2.1569962. [DOI] [PubMed] [Google Scholar]
- Weigel N., Moore N. L. Kinases and protein phosphorylation as regulators of steroid hormone action. Nucl. Recept. Signal. 2007;5:e005. doi: 10.1621/nrs.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. L., Su Y. R., Huang C. S., Yu J. C., Lo Y. L., Wu P. E., Shen C. Y. High-resolution 19p13.2–13.3 allelotyping of breast carcinomas demonstrates frequent loss of heterozygosity. Genes Chromosomes Cancer. 2004;41:250–256. doi: 10.1002/gcc.20080. [DOI] [PubMed] [Google Scholar]
- Yeh S., Miyamoto H., Nishimura K., Kang H., Ludlow J., Hsiao P., Wang C., Su C., Chang C. Retinoblastoma, a tumor suppressor, is a coactivator for the androgen receptor in human prostate cancer DU145 cells. Biochem. Biophys. Res. Commun. 1998;248:361–367. doi: 10.1006/bbrc.1998.8974. [DOI] [PubMed] [Google Scholar]
- Ylikorkala A., et al. Mutations and impaired function of LKB1 in familial and non-familial Peutz-Jeghers syndrome and a sporadic testicular cancer. Hum. Mol. Genet. 1999;8:45–51. doi: 10.1093/hmg/8.1.45. [DOI] [PubMed] [Google Scholar]
- Yu C.-L., Driggers P., Barrera-Hernandez G., Nunez S. B., Segars J. H., Cheng S.-Y. The tumor suppressor p53 is a negative regulator of estrogen receptor signaling pathways. Biochem. Biophys. Res. Commun. 1997;239:617–620. doi: 10.1006/bbrc.1997.7522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.