Abstract
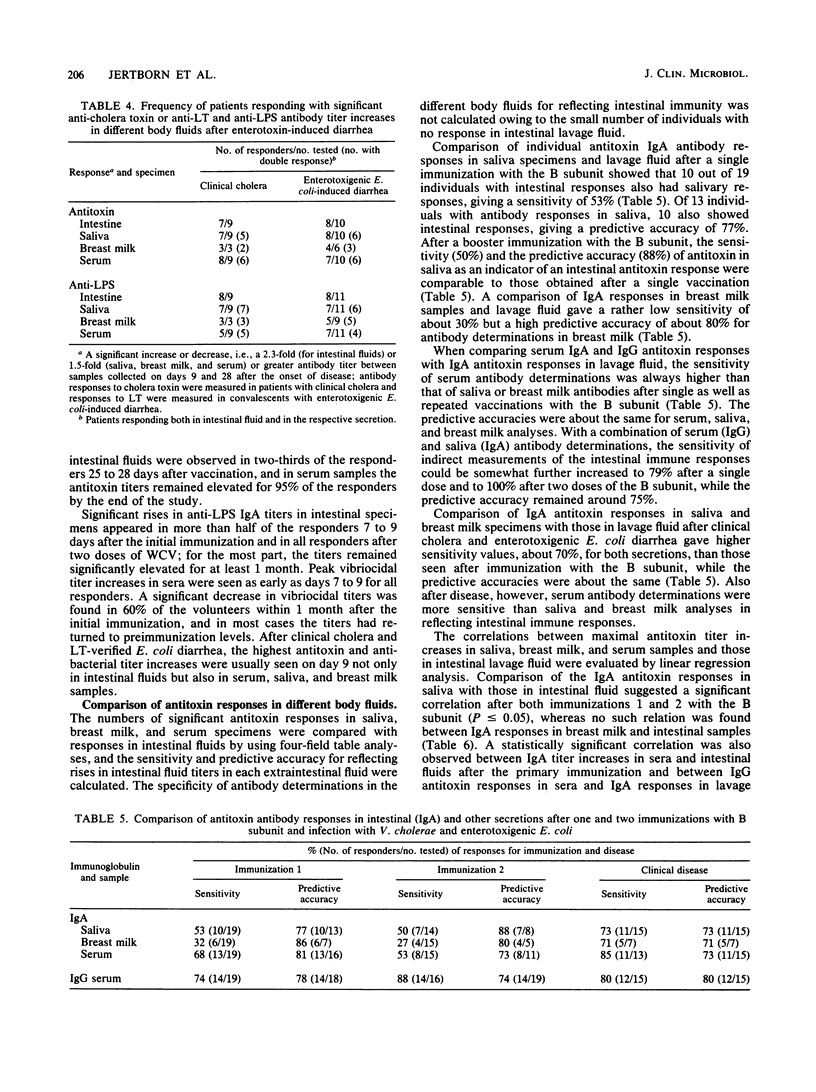
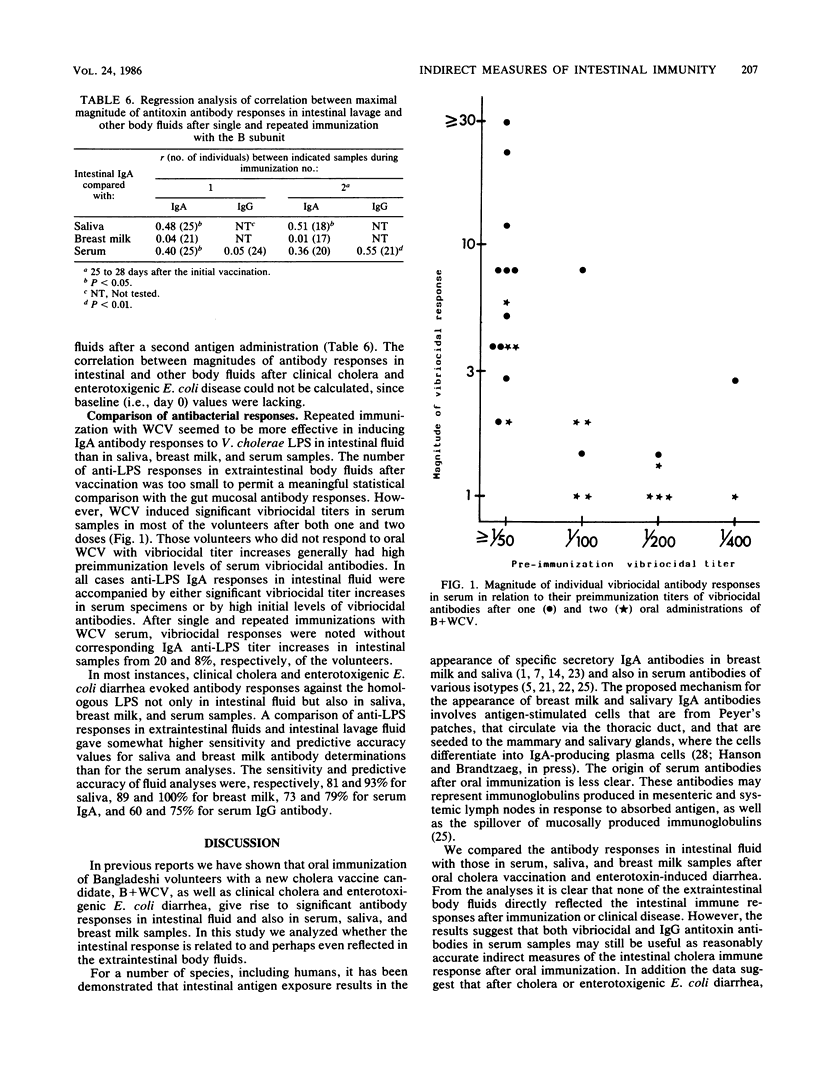
The possibility that antibody responses in serum, saliva, or breast milk samples to oral vaccines or enteric infections may reflect the intestinal immune response was evaluated in Bangladeshi volunteers orally immunized with a cholera B subunit-whole-cell vaccine (B + WCV) and in patients convalescing from enterotoxin-induced diarrheal disease. Two peroral doses of B + WCV induced antitoxin and antibacterial antibody responses in the intestinal fluids of 76 and 92%, respectively, of the volunteers and in serum samples in 90 and 69% of those tested. These responses were comparable to those obtained after cholera or enterotoxigenic Escherichia coli disease. Whereas immunoglobulin A (IgA) antitoxin titer increases in saliva (44%) and breast milk (29%) specimens after vaccination were less frequent than in intestinal fluid (76%), antitoxin responses in saliva and breast milk occurred in 80 to 90% of the patients after disease. Also, antilipopolysaccharide (anti-LPS) titer increases in extraintestinal body fluids were found more frequently after disease than after vaccination. A comparison of the frequency and magnitude of antibody response in different body fluids with those in intestinal lavage fluid revealed no extraintestinal antibody that directly reflected the intestinal immunity. However, comparison of vibriocidal and IgG antitoxin antibodies in serum specimens with antitoxin and anti-LPS IgA responses in intestinal fluids after the vaccination of volunteers showed a sensitivity of 70 to 90% and a predictive accuracy of about 80% for the serum analyses reflecting the intestinal immune responses. Furthermore, antitoxin and anti-LPS antibody responses in saliva and breast milk samples seemed to be useful proxy indicators of a gut mucosal response of these antibodies after enterotoxin-induced diarrheal disease showing sensitivity vales of 70 to 90% and predictive accuracy vales of 70 to 100%.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Curlin G. T., Craig J. P., Subong A., Carpenter C. C. Antitoxic immunity in experimental canine cholera. J Infect Dis. 1970 May;121(5):463–470. doi: 10.1093/infdis/121.5.463. [DOI] [PubMed] [Google Scholar]
- Glass R. I., Svennerholm A. M., Khan M. R., Huda S., Huq M. I., Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985 Feb;151(2):236–242. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- Hall P., Sebag J. Decision-making in clinical practice and medical research: a theroretical analysis of predicators, indicators, and health index. Int J Biomed Comput. 1974 Oct;5(4):301–309. doi: 10.1016/0020-7101(74)90023-3. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Cholera and the immune response. Prog Allergy. 1983;33:106–119. [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Lönnroth I., Fall-Persson M., Markman B., Lundbeck H. Development of improved cholera vaccine based on subunit toxoid. Nature. 1977 Oct 13;269(5629):602–604. doi: 10.1038/269602a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINTYRE O. R., FEELEY J. C. PASSIVE SERUM PROTECTION OF THE INFANT RABBIT AGAINST EXPERIMENTAL CHOLERA. J Infect Dis. 1964 Dec;114:468–475. doi: 10.1093/infdis/114.5.468. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Neogi P. K., Alam M. K. Immunobead enzyme-linked immunosorbent assay for quantitating immunoglobulin A in human secretions and serum. Infect Immun. 1980 Jul;29(1):281–283. doi: 10.1128/iai.29.1.281-283.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B. J., Svennerholm A. M., Gothefors L., Barua D., Huda S., Holmgren J. Local and systemic antibody responses to naturally acquired enterotoxigenic Escherichia coli diarrhea in an endemic area. J Infect Dis. 1986 Mar;153(3):527–534. doi: 10.1093/infdis/153.3.527. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Gothefors L., Sack D. A., Bardhan P. K., Holmgren J. Local and systemic antibody responses and immunological memory in humans after immunization with cholera B subunit by different routes. Bull World Health Organ. 1984;62(6):909–918. [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J., Black R., Levine M., Merson M. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J Infect Dis. 1983 Mar;147(3):514–522. doi: 10.1093/infdis/147.3.514. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J., Hanson L. A., Lindblad B. S., Quereshi F., Rahimtoola R. J. Boosting of secretory IgA antibody responses in man by parenteral cholera vaccination. Scand J Immunol. 1977;6(12):1345–1349. doi: 10.1111/j.1365-3083.1977.tb00376.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Jertborn M., Gothefors L., Karim A. M., Sack D. A., Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Sack D. A., Holmgren J., Bardhan P. K. Intestinal antibody responses after immunisation with cholera B subunit. Lancet. 1982 Feb 6;1(8267):305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- Svennerholm A., Lange S., Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978 Jul;21(1):1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayot J. L., Holmgren J., Svennerholm L., Lindblad M., Tardy M. Receptor-specific large-scale purification of cholera toxin on silica beads derivatized with lysoGM1 ganglioside. Eur J Biochem. 1981 Jan;113(2):249–258. doi: 10.1111/j.1432-1033.1981.tb05060.x. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]


