Abstract
Regulators of G protein signaling (RGS) proteins accelerate the intrinsic GTPase activity of certain Gα subunits and thereby modulate a number of G protein-dependent signaling cascades. Currently, little is known about the regulation of RGS proteins themselves. We identified a short-lived RGS protein, RGS7, that is rapidly degraded through the proteasome pathway. The degradation of RGS7 is inhibited by interaction with a C-terminal domain of polycystin, the protein encoded by PKD1, a gene involved in autosomal-dominant polycystic kidney disease. Furthermore, membranous expression of C-terminal polycystin relocalized RGS7. Our results indicate that rapid degradation and interaction with integral membrane proteins are potential means of regulating RGS proteins.
Keywords: protein–protein interaction, yeast two-hybrid system, polycystic kidney disease
The recently discovered RGS family consists of negative Regulators of G protein Signaling characterized by an RGS domain of ≈120 aa (reviewed in refs. 1–5) that allow members to accelerate the intrinsic GTPase activity of Gαi and Gαq subunits and thereby terminate downstream G protein-linked signaling pathways (6–14). RGS proteins catalytically interact with their respective Gα subunits; a single molecule binds and inactivates multiple GTP-bound Gα subunits. Thus, tight regulatory mechanisms are required to curtail this enzymatic activity (reviewed in ref. 2). Some RGS proteins are transcriptionally regulated, transiently expressed only after cellular activation (reviewed in refs. 3 and 15). The function of other RGS proteins may depend on subcellular localization (16–18). Several investigators postulate that posttranslational modification of Gα target proteins may modulate the enzymatic activity of RGS proteins. For example, palmitoylation or protein kinase C-dependent phosphorylation of certain Gα subunits decreases their affinities for RGS proteins; these activated Gα subunits are thereby resistant to the GTPase activity of RGS proteins (19, 20).
Many regulatory proteins are short-lived intracellular proteins that contain motifs subject to ubiquitin-dependent proteasome degradation (reviewed in ref. 21). A partially conserved 9-aa destruction box is located ≈40–50 residues from the N terminus of several cell-cycle regulators. Other proteins follow the N-end rule, in which rapid degradation is triggered by basic or bulky-hydrophobic N-terminal amino acid residues (reviewed in ref. 22). The presence of PEST sequences, regions enriched in proline, glutamate, serine, and threonine residues, can also target proteins for degradation (reviewed in ref. 23). We found that RGS7 is a short-lived protein that is rapidly degraded by the ubiquitin–proteasome pathway. It contains two putative PEST sequences that flank a potential coiled-coil domain. Utilizing the yeast two-hybrid system, this region of RGS7 was found to interact with the C-terminal domain of polycystin. PKD1, the gene encoding for polycystin, is mutated in ≈85% of patients with autosomal-dominant polycystic kidney disease, a common hereditary disease (1:1,000) with progressive epithelial cyst formation that accounts for 8–10% of end-stage renal disease (24). Affected individuals commonly develop kidney and liver cysts and nearly half require renal replacement therapy by age 60. Polycystin is a glycoprotein with multiple transmembrane domains and a C-terminal cytoplasmic tail of 226 aa (25, 26). The N-terminal extracellular region of over 2,500 aa contains several domains with significant homology to membrane proteins involved in cell–cell/matrix interactions. However, the precise function of polycystin remains unknown. We found that the C-terminal domain of polycystin prolonged the half-life of RGS7 and altered its subcellular localization. The regulation of an RGS family member by polycystin may represent a prototypic model of a more prevalent mechanism for regulating G protein signaling by integral membrane proteins.
MATERIALS AND METHODS
Plasmids.
The C-terminal cytoplasmic domain of wild-type PKD1 was amplified by PCR from KG8 (M.C. Schneider, Brigham and Women’s Hospital, Boston). DNA fragments for PKD1 and RGS7 deletion constructs were obtained by PCR-directed introduction of in-frame stop codons. For the yeast two-hybrid analysis, the fragments were cloned into pLEX, a derivative of plex202, to generate a LexA fusion protein, or into pCGA, a derivative of pcgatrp2, containing a bacterial transcriptional activator under the control of the galactose-inducible promoter GAL1 (E.K. and B. Seed, unpublished data). The construct Pkd1.1 contains the C-terminal 226 aa of polycystin from E4078 to T4303, Pkd1.2 contains amino acids E4078–L4266, Pkd1.3 contains E4078–A4232, Pkd1.4 contains S4192–T4303, Pkd1.5 contains R4228–L4266, and Pkd1.6 contains E4078–S4169. The CD16.7 and sIg.7 fusions have been described (27). The Flag-tagged RGS7 constructs contain the Flag-His-tag sequence DRKLATMLDYKDDDDKHHHHHHHHH; the complete coding region of RGS7 was fused to the Flag-His tag to yield F.RGS7. RGS7.1 represents L50–Y469; RGS7.2, Q170–Y469; RGS7.3, Q170–R437; RGS7.4, Q170–W330; RGS7.5, Q170–T247; RGS7.6, P253–Y469; RGS7.7, M220–W330; and RGS7.8, E315–Y469. A plasmid directing the expression of green fluorescent protein was used to monitor transfection efficiency. Sequence identity of the PCR constructs was confirmed by restriction analysis and DNA sequencing. F.ERE consists of the 5′ and 3′ ends of EGL-10, which flank the PstI–NcoI fragment (Q157–N304) of RGS7, creating a Flag-tagged EGL-10/RGS7/EGL-10 fusion protein.
Two-Hybrid Assay.
The yeast screen of a B cell expression cDNA library was performed as described (28, 29). For assessing the interactions of polycystin and hRGS7, a pLEX plasmid expressing the appropriate fragments of polycystin was transformed into the yeast strain EGY48/pRB1840 bearing the lacZ reporter plasmid JK103 under the control of LexA binding sites. The bait-expressing clones were subsequently transformed with pCGA-RGS7 deletion plasmids. Interaction between bait- and prey-encoding fusion proteins was determined by β-galactosidase production and leucine prototrophy of yeast grown in the presence of galactose. The RGS7 clone initially identified by a yeast two-hybrid screen contains a 135-bp insertion that changes the C terminus at amino acid 454 of the B cell-derived RGS7 (RGS7b) (GenBank accession no. AF090117). A full-length human RGS7 clone was isolated from a fetal brain Uni-ZAP XR library (Stratagene) and subcloned into pCR3 (Invitrogen) (GenBank accession no. AF090116).
In Vitro Binding Assay.
[35S]Methionine-labeled RGS7 and RGS7.8 were generated by using the Promega TNT system following the instructions of the manufacturer, incubated with 2 μg of glutathione S-transferase (GST)–Pkd1.4, GST–Pkd1.6, or GST–FIT, an unrelated control protein of equal length (E.K. and B. Seed, unpublished data), and immobilized on glutathione–Sepharose in the presence of 450 μl of reaction buffer [50 mM potassium phosphate, pH 7.5/150 mM KCl/1 mM MgCl2/10% (vol/vol) glycerol/1% Triton X-100/protease inhibitors]. In selected experiments, unlabeled RGS7 was added as indicated. The reaction mix was incubated for 2 h, washed three times in reaction buffer, and separated on a 10% SDS acrylamide gel. Radiolabeled protein was detected by using autoradiography.
In Vivo Coimmunoprecipitation.
293T cells were transiently transfected with 10 μg of plasmid DNA by using the calcium phosphate method. After incubation for 24 h, cells were washed twice with PBS and then lysed in 1 ml of 1% Triton X-100 buffer containing 150 mM NaCl/10 mM Tris⋅HCl, pH 7.5/1 mM EDTA/1 mM EGTA/protease inhibitors. Cell lysates containing equal amounts of total protein were incubated for 1 h at 4°C with 50 μl of 30% protein G-Sepharose beads (Amersham Pharmacia). The beads were washed extensively with lysis buffer, and bound proteins were fractionated by SDS/10% PAGE. Western blot analysis was performed with anti-Flag M2 (10 μg/ml) (IBI Kodak) followed by incubation with horseradish peroxidase-coupled sheep anti-mouse Ig (Dako). Immobilized antibodies were detected by using chemiluminescence (Pierce).
Ubiquitination Assay.
293T cells seeded in 10-cm plates were transiently transfected with the indicated constructs. After incubation for 24 h, cells were incubated with MG132 or media alone for 3 h, lysed in 6 M guanidine⋅HCl/0.1 M Na2HPO4/NaH2PO4, pH 8.0/5 mM imidazole, and sonicated for 1 min. After centrifugation for 15 min at 4°C, the His-tagged RGS7 conjugates were precipitated from the cleared lysate with nickel-nitrilotriacetic acid (NTA) agarose (Qiagen, Chatsworth, CA) for 2 h at RT. Complexes were washed with 8 M urea/0.1 M Na2HPO4/NaH2PO4, pH 6.3/0.01 M Tris, pH 8.0, and resuspended in sample buffer. Proteins were fractionated on SDS/PAGE, electroblotted to a poly(vinylidene difluoride) membrane, and labeled with rabbit polyclonal anti-HA serum (Santa Cruz Biotechnology) or anti-Flag M2 (IBI Kodak), followed by incubation with horseradish peroxidase-coupled anti-rabbit (Amersham Pharmacia) or anti-mouse Ig (Dako). Immobilized antibodies were detected by using chemiluminescence (Pierce).
RESULTS
RGS7 Interacts with the C-Terminal Domain of Polycystin in Yeast.
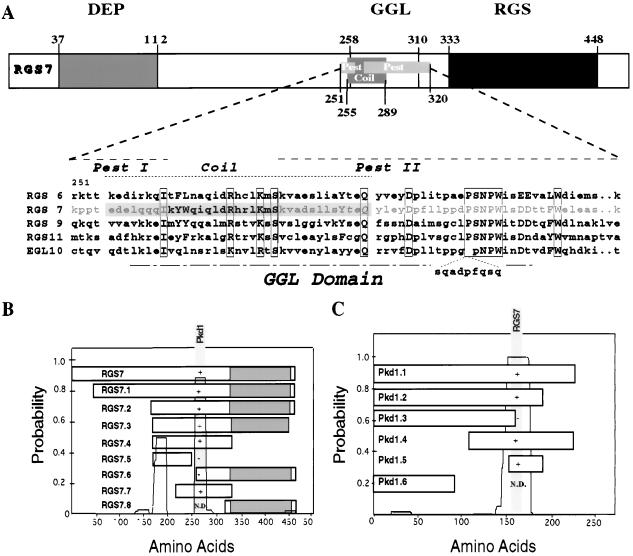
A yeast two-hybrid screen, performed with the C-terminal cytoplasmic domain of polycystin, identified RGS7, a member of a recently described gene family involved in G protein signaling, as a polycystin-binding protein. Isolation of a full-length human RGS7 clone revealed that RGS7 shares with the mammalian RGS6, RGS9, RGS11, and the Caenorhabditis elegans RGS protein EGL-10, a common domain architecture containing the N-terminal dishevelled/Egl-10/pleckstrin (DEP) homology domain (30), a G protein γ subunit-like (GGL) domain (31), and a C-terminal RGS domain (Fig. 1). RGS7 is most similar in sequence to RGS6 (84% similarity and 70% identity), but is closest in evolution to EGL-10 (70% similarity and 53% identity) by analysis using Genetics Computer Group programs (bestfit, pileup, and distances). RGS7 diverges from other RGS proteins in its GGL domain, which contains a putative coiled-coil domain that is flanked by two potential PEST sequences (Fig. 1A). Sequential 5′ and 3′ deletions revealed that it is this divergent domain that mediates binding of RGS7 to polycystin (Fig. 1B) (23). Progressive deletion of the C-terminal cytoplasmic domain of polycystin revealed that binding of RGS7 depends on the last 70 aa of polycystin, the deletion of which completely abrogated the interaction of polycystin and RGS7. Further mapping determined that the coiled-coil structure of polycystin is required for binding to RGS7 (Fig. 1C).
Figure 1.
Sequence analysis of human RGS7 and interaction with polycystin in yeast. (A) RGS7 shares a common domain structure with the mammalian RGS6, RGS9, RGS11, and EGL-10. Depicted in detail is the region unique to RGS7, containing two PEST sequences and a potential coiled-coil structure. The similarity and identity of the RGS7 GGL domain versus that of RGS6, 9, 11, and EGL-10 were 81% and 55%, 60% and 37%, 58% and 33%, and 59% and 40%, respectively. (B) Mutational analysis of RGS7–polycystin interactions in yeast. The presence of coiled-coil structures is depicted as a probability plot by using coils Version 2.1 from the Swiss Institute for Experimental Cancer Research, window 28. Interactions between RGS7 and polycystin were tested in the yeast two-hybrid system, with interaction (+) indicated by β-galactosidase production and leucine prototrophy. Interaction between RGS7 and polycystin is mediated by a region of RGS7 that contains a putative coiled-coil structure (shaded region). (C) Deletion of the C-terminal 70 aa of polycystin (Pkd1.3) abolishes binding to RGS7. Pkd1.1 represents the C-terminal 226 aa of polycystin. The minimal interacting domain, Pkd1.5, caused leucine prototrophy without β-galactosidase production, indicative of a weak interaction. The putative binding domain within polycystin contains a coiled-coil structure, L4215–R4249. N.D., interaction not tested.
Interaction Between RGS7 and Polycystin in Vitro and in Vivo.
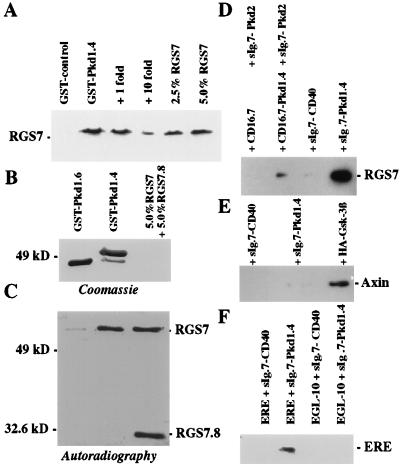
In vitro-transcribed and -translated RGS7 specifically bound to a GST fusion protein containing the C-terminal 112 aa of polycystin (GST–Pkd1.4), but not to an unrelated control GST fusion protein, or a GST–polycystin fusion protein (GST–Pkd1.6) lacking the coiled-coil domain of polycystin (Fig. 2 A and B). RGS7.8, a derivative of RGS7 lacking the PKD1-interacting domain, did not bind to either GST–Pkd1.4 or GST–Pkd1.6 under the same conditions. Approximately 5% of the radiolabeled RGS7 used in the in vitro binding assay was retained by the immobilized GST–Pkd1.4 (Fig. 2 B and C); similar values have been reported for the in vitro interaction between polycystin 1 and polycystin 2 (32). To demonstrate the interaction of polycystin and RGS7 in vivo, we transiently cotransfected the human embryonic kidney cell line 293T with expression plasmids encoding a Flag-tagged RGS7 and a heterologous membrane protein containing the last 112 aa of polycystin (PKD1.4). RGS7 coimmunoprecipitated with the polycystin fusion protein, but not with PKD2 or a control protein (Fig. 2D). Moreover, the presence of polycystin enabled RGS7 to be coimmunoprecipitated with the cytoplasmic tail of PKD2. These results suggest that RGS7, polycystin, and PKD2 form a ternary complex in vivo and that RGS7 and PKD2 interact with separate sites on polycystin. In contrast, a control RGS protein with a coiled-coil domain, axin, did not interact with the polycystin fusion protein, but immunoprecipitated glycogen synthase kinase-3β (GSK-3B) (Fig. 2E). As an additional control, EGL-10, a C. elegans homologue lacking a coiled-coil structure, did not interact with the C-terminus of polycystin. However, a Flag-tagged chimera of EGL-10 containing the polycystin-binding domain of RGS7 (F.ERE) coprecipitated with sIg.7-Pkd1.4 but not with sIg.7-CD40 (Fig. 2F).
Figure 2.
RGS7 associates with polycystin in vitro and in vivo. (A) In vitro binding of RGS7 to C-terminal polycystin. The amount of [35S]methionine-labeled RGS7 that bound to the last 112 aa of polycystin fused to GST (GST–Pkd1.4) or to GST–FIT, an unrelated control peptide of equal length, was compared with 2.5% and 5% 35S-labeled RGS7 input protein. The interaction between polycystin and [35S]methionine-labeled RGS7 was blocked by increasing concentrations of unlabeled RGS7. (B and C) In vitro interaction of RGS7 and RGS7.8 with GST–Pkd1.4 and GST–Pkd1.6, respectively. The GST fusion proteins were immobilized to glutathione–Sepharose and incubated with a mixture of equal amounts of RGS7 and RGS7.8 (5% input proteins). RGS7 binds to GST–Pkd1.4, but not to GST–Pkd1.6; no binding to either GST fusion was detectable for RGS7.8 (lacking the PKD1-interacting domain) compared with 5% input proteins. (D) In vivo coimmunoprecipitation of F.RGS7 with C-terminal polycystin. The fragment of polycystin containing the last 112 aa (Pkd1.4) was fused to sIg.7, a construct containing the leader sequence of human CD5 followed by the CH2 and CH3 domain of human IgG1 and the transmembrane region of human CD7. The control plasmid, sIg.7-CD40 contains the cytoplasmic domain of CD40 following the end of the transmembrane region of CD7. For interaction between polycystin and RGS7, 293T cells were transiently transfected with F.RGS7 and either sIg.7-CD40 (control) or sIg.7-Pkd1.4. Lysates were immunoprecipitated with protein G and blotted with anti-Flag M2 mAb. To demonstrate that RGS7 binds the polycystin-PKD2 complex, 293T cells were transiently transfected with F.RGS7 and sIg.7-PKD2, an IgG-fusion protein containing the C-terminal cytoplasmic domain of PKD2 (27), and either CD16.7 or CD16.7-Pkd1.4 (27). Lysates were immunoprecipitated with protein G and blotted with anti-Flag M2 mAb. (E) The coiled-coil RGS protein axin binds GSK-3β, but does not interact with polycystin. sIg.7-CD40, sIg.7-Pkd1.4, or HA-tagged GSK-3β were transfected with myc-tagged axin and immunoprecipitated with protein G or a combination of anti-HA mAb and protein G. HA–GSK-3β, but neither sIg.7-CD40 nor sIg.7-Pkd1.4, precipitated axin. (F) EGL-10, a C. elegans homologue lacking a coiled-coil structure, does not interact with the C terminus of polycystin. However, a Flag-tagged chimera of EGL-10 containing the polycystin-binding domain of RGS7 (F.ERE), coprecipitated with sIg.7-Pkd1.4 but not with sIg.7-CD40.
RGS7 Is Degraded by the Ubiquitin–Proteasome Pathway.
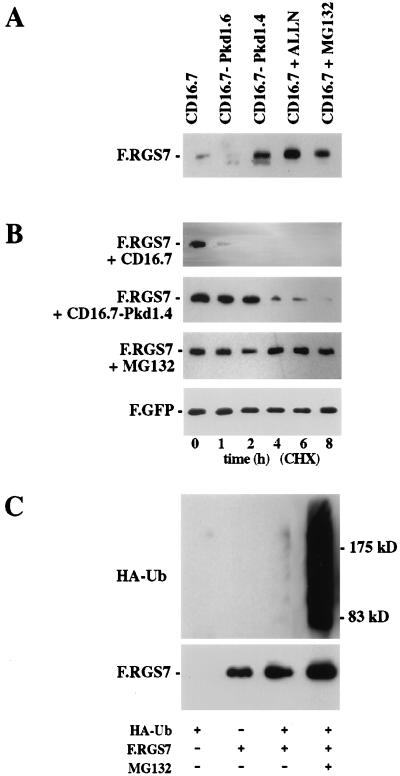
RGS7 was poorly expressed following transient expression in HEK 293T cells; however, its expression was markedly augmented by treatment with the proteasome inhibitors ALLN and MG132 (Fig. 3A). RGS7 levels were similarly elevated by coexpression of the RGS7-interacting domain of polycystin in the fusion protein CD16.7-Pkd1.4, containing the C-terminal 112 aa of polycystin. In contrast, the fusion protein CD16.7–Pkd1.6 containing the N-terminal 92 aa of the polycystin cytoplasmic tail failed to augment RGS7 protein levels (Fig. 3A). To demonstrate that this effect was mediated through the stabilization of RGS7 rather than an increase in protein synthesis, we examined the effect of MG132 and the C-terminal domain of polycystin on the half-life of RGS7 while blocking protein synthesis. In the presence of the protein synthesis inhibitor cycloheximide, the half-life of RGS7 was increased in the presence of CD16.7–Pkd1.4 or MG132, a proteasome inhibitor (Fig. 3B). CD16.7, a construct with a stop codon shortly after the transmembrane domain, had no detectable effect on RGS7 stability. Ubiquitination of RGS7 was clearly detectable in the presence of the proteasome inhibitor MG132 and hemagglutinin (HA)-tagged ubiquitin (Fig. 3C), demonstrating that RGS7 is subjected to ubiquitin-dependent degradation by the proteasome complex.
Figure 3.
Effect of polycystin on RGS7 protein levels. (A) RGS7 protein expression is augmented by the presence of polycystin or proteasome inhibitors. 293T cells were transfected with F.RGS7 and cotransfected with CD16.7-Pkd1.4 or controls (CD16.7 and CD16.7-Pkd1.6). After 24 hours, cells were incubated for 3 hours in medium alone (lanes 1–3), or with N-Acetyl-Leu-Leu-Norleucinal (ALLN) (40 μmol) or MG132 (40 μmol). Equal amounts of protein were separated by using SDS/PAGE, and Flag-tagged proteins were detected by Western blot analysis. (B) RGS7 protein half-life is increased in the presence of polycystin or a proteasome inhibitor. 293T cells were cotransfected with F.RGS7 and CD16.7.Pkd1.4 or CD16.7 (control). After 24 hours, cells were incubated 3 hours in medium alone or with MG132 (40 μmol). Cells were harvested at the time points indicated after the addition of cycloheximide (40 μg/ml), and whole cell lysates were immunoblotted with anti-Flag M2 to detect F.RGS7. Equal transfection efficiency and amount of loaded protein was monitored by a Flag-tagged green fluorescent protein construct (data shown for F.RGS7 + CD16.7). (C) Ubiquitination of RGS7 is increased in the presence of a proteasome inhibitor. HEK 293T cells were transfected with F.RGS7 and HA–ubiquitin as indicated. Flag/His-tagged RGS7 was purified by nickel-NTA chromatography from lysates of transfected cells, run on a SDS/PAGE gel, and transferred to nitrocellulose membrane. RGS7-ubiquitin conjugates were detected with anti-HA antibodies and F.RGS7 detected with the anti-Flag M2 antibody.
The C-Terminal Domain of Polycystin Alters the Subcellular Distribution of RGS7.
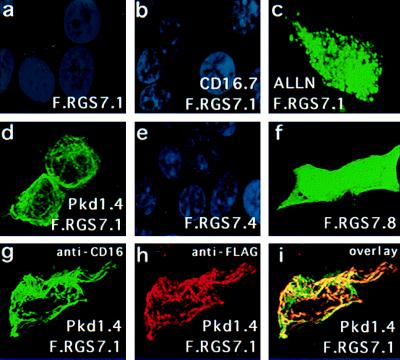
We examined whether the presence of polycystin could influence the subcellular localization of RGS7. The C-terminal 112 aa of polycystin (CD16.7–Pkd1.4) is well expressed and assumes a cage-like structure that is readily detected on the surface of nonpermeabilized HEK 293T cells (and other cell lines; ref. 27 and data not shown). Coexpression of the C terminus of polycystin greatly altered the subcellular localization of RGS7 to a cage-like pattern that colocalized with polycystin (Fig. 4). This cage-like pattern distinctly differed from the cellular staining patterns observed for RGS7 in the presence of ALLN, and for RGS7.8, a truncation lacking the PKD1-binding domain (Fig. 4). Thus, PKD1 appears to both stabilize RGS7 and target its subcellular localization.
Figure 4.
Colocalization of RGS7 with polycystin in 293T cells. Cells were transiently transfected with expression plasmids encoding F.RGS7.1 (a–d and g–i), F.RGS7.4 (e), F.RGS7.8 (f), CD16.7 (b), and CD16.7-Pkd1.4 (d and g–i). Shown are confocal images (Bio-Rad) after labeling of RGS7 with anti-Flag M2 and FITC-conjugated goat anti-mouse IgG (a–f). Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI). Cells were transfected with RGS7.1 alone (a) or with the control plasmid CD16.7 (b) and were virtually negative other than the DAPI-labeled nuclei. Addition of ALLN at 40 μmol for 6 h resulted in significant augmentation of RGS7 protein levels and a punctate staining pattern (c). Cells cotransfected with F.RGS7.1 and CD16.7-Pkd1.4 showed a marked augmentation of F.RGS7.1 protein levels and a membrane-associated cage-like staining pattern (d). Deletion of the RGS domain (F.RGS7.4) did not elevate expression levels of RGS7 (e). Deletion of the N-terminal domain, including the PKD1-binding domain, resulted in increased and diffuse cytoplasmic expression of the RGS7 truncation (F.RGS7.8) (f). (g–i) Colocalization of RGS7 and polycystin in 293T cells transfected with F.RGS7.1 and CD16.7-Pkd1.4. CD16.7-Pkd1.4 was labeled with FITC-conjugated anti-CD16 mAb (g). F.RGS7 was labeled with anti-Flag M2 followed by rhodamine-conjugated anti-mouse IgG (h). An overlay shows the colocalization of RGS7 and polycystin expression (i). Identical results were obtained with full-length RGS7, indicating that the N-terminal domain does not affect the polycystin-mediated relocalization of RGS7 (data not shown).
DISCUSSION
RGS7 belongs to a rapidly growing family of proteins of at least 20 members. Canonical RGS proteins bind and accelerate the intrinsic GTPase activity of Gαi and Gαq subunits (33, 34). Biological effects of RGS proteins on cellular signaling arise from their capacity to inactivate Gαi or G αq subunits. Consequently, RGS proteins interfere with multiple G protein-coupled programs including calcium mobilization (14, 35), activation of phospholipase C (10, 36), synthesis of inositol triphosphate (36–39), mGluR1a- and mGluR5a-mediated activation of chloride and potassium currents (40), and G protein-coupled inwardly rectifying K+ channels (41) RGS proteins also regulate more distal G protein-linked pathways such as mitogen-activated protein kinase cascades in mammalian cells and yeast (37, 39, 42–44) and lymphocyte migration in response to chemokines (45). The capacity of RGS proteins to diminish the magnitude and duration of Gαi- and Gαq-dependent signaling pathways mandates tight regulation of their potentially rampant catalytic activity.
Functional specificity as well as temporal activity of RGS proteins appears to derive from patterns of expression, transcriptional regulation, and subcellular localization. Whereas some RGS proteins are quite ubiquitously expressed, others are tissue- and time-specific. Transcriptional regulation of other RGS members results in transient expression only during cellular activation (reviewed in refs. 2 and 3). In addition, posttranslational modification of Gα subunits decreases the ability of RGS proteins to bind and inactivate them (reviewed in ref. 2). Our results demonstrate that RGS7 protein levels are posttranslationally regulated through rapid degradation by the ubiquitin-dependent proteasome pathway. A short half-life is a common feature of many regulatory proteins. RGS7 contains a putative coiled-coil structure flanked by two potential PEST sequences (23) that habitually contain minimal consensus phosphorylation sites necessary for degradation. Regulatory proteins that are targeted for ubiquitination and subsequent degradation by phosphorylation of recognition sequences include yeast and mammalian G1 cyclins, the NF-κB inhibitor Iκ-Bα, β-catenin, and the transcription factor STAT1 (reviewed in ref. 21) Because polycystin binds to a PEST sequence-containing domain of RGS7, it may directly impede the phosphorylation-dependent ubiquitination and subsequent degradation of RGS7 and thereby modulate its functional activity. Alternatively, polycystin may relocalize RGS7 to a subcellular compartment away from ubiquitin-protein ligases responsible for recognition and targeting of RGS7 to the proteasome. Further experiments should address whether the phosphorylation pattern of RGS7 is altered by the presence of polycystin.
Polycystin binds to the RGS7 GGL domain adjacent to the RGS domain. The demonstrated binding of RGS GGL domains to Gβ5 and the overlapping tissue distribution of RGS7, polycystin, and Gβ5 in adult brain and kidney suggests that polycystin may modulate other functions of RGS7 (31). The RGS7 GGL domain is unique in containing a putative PEST–coiled-coil–PEST motif that interacts with polycystin. Although polycystin, PKD2, and RGS7 appear to form a heterotrimeric complex, further analysis is required to determine whether polycystin facilitates or competes with the interaction between RGS7 and Gβ5.
The C-terminal domain of polycystin clearly modulates the stability of RGS7; however, the role of RGS7 in the function of polycystin remains unclear. Mutations of polycystin, the protein encoded by PKD1, are responsible for the majority of autosomal-dominant polycystic kidney disease, a common genetic disease accounting for 8–10% of end-stage renal disease worldwide. On a cellular level, human autosomal dominant polycystic kidney disease epithelial cells show characteristics of nondifferentiated cells such as increased proliferation, abnormal polarity, altered growth factor responsiveness, and aberrant expression of certain protooncogenes. G protein-mediated signaling appears to influence cyst formation by modulating cellular proliferation, transepithelial fluid secretion, and differentiation through effectors such as adenylate cyclase and mitogen-activated protein kinase pathways (reviewed in ref. 46). RGS7 is highly expressed in the developing tubular structures of the kidney (data not shown), a pattern of expression correlating with that of polycystin. Defining the functional relationship between polycystin and RGS7 will require further studies to monitor their interaction during renal development, to determine how extracellular events that regulate the conformation of polycystin govern the release or binding of RGS7, and to examine RGS7 expression and function in polycystin-deficient mice.
Acknowledgments
We thank B. Seed for several constructs and expression cassettes, S. Y. Sokol for the myc-tagged axin construct, and M. R. Koelle and H. R. Horvitz for the egl-10 cDNA. E.K. was supported by Public Health Service Grant MH01147, and T.B. by the Deutsche Forschungsgemeinschaft (DFG). This work was supported by NIH-RO1 DK51060 (V.P.S.), NIH-RO1 DK52897 (G.W.), and the Polycystic Kidney Research Foundation (G.W.).
ABBREVIATIONS
- GGL
Gγ-like
- RGS
regulator of G protein signaling
- HA
hemagglutinin
- GST
glutathione S-transferase
- GSK-3β
glycogen synthase kinase-3β
Footnotes
References
- 1.Arshavsky V Y, Pugh E N., Jr Neuron. 1998;20:11–14. doi: 10.1016/s0896-6273(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 2.Berman D M, Gilman A G. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- 3.Kehrl J H. Immunity. 1998;8:1–10. doi: 10.1016/s1074-7613(00)80453-7. [DOI] [PubMed] [Google Scholar]
- 4.Koelle M R. Curr Opin Cell Biol. 1997;9:143–147. doi: 10.1016/s0955-0674(97)80055-5. [DOI] [PubMed] [Google Scholar]
- 5.Dohlman H G, Thorner J. J Biol Chem. 1997;272:3871–3874. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- 6.Popov S, Yu K, Kozasa T, Wilkie T M. Proc Natl Acad Sci USA. 1997;94:7216–7220. doi: 10.1073/pnas.94.14.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman D M, Wilkie T M, Gilman A G. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 8.Natochin M, Granovsky A E, Artemyev N O. J Biol Chem. 1997;272:17444–17449. doi: 10.1074/jbc.272.28.17444. [DOI] [PubMed] [Google Scholar]
- 9.Nekrasova E R, Berman D M, Rustandi R R, Hamm H E, Gilman A G, Arshavsky V Y. Biochemistry. 1997;36:7638–7643. doi: 10.1021/bi970427r. [DOI] [PubMed] [Google Scholar]
- 10.Hepler J R, Berman D M, Gilman A G, Kozasa T. Proc Natl Acad Sci USA. 1997;94:428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt T W, Fields T A, Casey P J, Peralta E G. Nature (London) 1996;383:175–177. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- 12.Watson N, Linder M E, Druey K M, Kehrl J H, Blumer K J. Nature (London) 1996;383:172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- 13.He W, Cowan C W, Wensel T G. Neuron. 1998;20:95–102. doi: 10.1016/s0896-6273(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 14.Shuey D J, Betty M, Jones P G, Khawaja X Z, Cockett M I. J Neurochem. 1998;70:1964–1972. doi: 10.1046/j.1471-4159.1998.70051964.x. [DOI] [PubMed] [Google Scholar]
- 15.Dohlman H G, Song J, Apanovitch D M, DiBello P R, Gillen K M. Semin Cell Dev Biol. 1998;9:135–141. doi: 10.1006/scdb.1998.0218. [DOI] [PubMed] [Google Scholar]
- 16.De Vries L, Elenko E, Hubler L, Jones T L, Farquhar M G. Proc Natl Acad Sci USA. 1996;93:15203–15208. doi: 10.1073/pnas.93.26.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vries L, Elenko E, McCaffery J M, Fischer T, Hubler L, McQuistan T, Watson N, Farquhar M G. Mol Biol Cell. 1998;9:1123–1134. doi: 10.1091/mbc.9.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Druey K M, Sullivan B M, Brown D, Fischer E R, Watson N, Blumer K J, Gerfen C R, Scheschonka A, Kehrl J H. J Biol Chem. 1998;273:18405–18410. doi: 10.1074/jbc.273.29.18405. [DOI] [PubMed] [Google Scholar]
- 19.Tu Y, Wang J, Ross E M. Science. 1997;278:1132–1135. doi: 10.1126/science.278.5340.1132. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Ducret A, Tu Y, Kozasa T, Aebersold R, Ross E M. J Biol Chem. 1998;273:26014–26025. doi: 10.1074/jbc.273.40.26014. [DOI] [PubMed] [Google Scholar]
- 21.Hershko A, Ciechanover A. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 22.Varshavsky A. Proc Natl Acad Sci USA. 1996;93:12142–12149. doi: 10.1073/pnas.93.22.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rechsteiner M. Semin Cell Biol. 1990;1:433–440. [PubMed] [Google Scholar]
- 24.Reeders S T, Breuning M H, Davies K E, Nicholls R D, Jarman A P, Higgs D R, Pearson P L, Weatherall D J. Nature (London) 1985;317:542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- 25.Hughes J, Ward C J, Peral B, Aspinwall R, Clark K, San Millan J L, Gamble V, Harris P C. Nat Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 26.Anonymous. Cell. 1995;81:289–298. [Google Scholar]
- 27.Tsiokas L, Kim E, Arnould T, Sukhatme V P, Walz G. Proc Natl Acad Sci USA. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyuris J, Golemis E, Chertkov H, Brent R. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 29.Stanger B, Leder P, Lee T, Kim E, Seed B. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 30.Ponting C P, Bork P. Trends Biochem Sci. 1996;21:245–246. [PubMed] [Google Scholar]
- 31.Snow B E, Krumins A M, Brothers G M, Lee S F, Wall M A, Chung S, Mangion J, Arya S, Gilman A G, Siderovski D P. Proc Natl Acad Sci USA. 1998;95:13307–13312. doi: 10.1073/pnas.95.22.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian F, Germino F J, Cai Y, Zhang X, Somlo S, Germino G G. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 33.Berman D M, Kozasa T, Gilman A G. J Biol Chem. 1996;271:27209–27212. doi: 10.1074/jbc.271.44.27209. [DOI] [PubMed] [Google Scholar]
- 34.Tesmer J J, Berman D M, Gilman A G, Sprang S R. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 35.DiBello P R, Garrison T R, Apanovitch D M, Hoffman G, Shuey D J, Mason K, Cockett M I, Dohlman H G. J Biol Chem. 1998;273:5780–5784. doi: 10.1074/jbc.273.10.5780. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee T K, Eapen A K, Fisher R A. J Biol Chem. 1997;272:15481–15487. doi: 10.1074/jbc.272.24.15481. [DOI] [PubMed] [Google Scholar]
- 37.Yan Y, Chi P P, Bourne H R. J Biol Chem. 1997;272:11924–11927. doi: 10.1074/jbc.272.18.11924. [DOI] [PubMed] [Google Scholar]
- 38.Huang C, Hepler J R, Gilman A G, Mumby S M. Proc Natl Acad Sci USA. 1997;94:6159–6163. doi: 10.1073/pnas.94.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neill J D, Duck L W, Sellers J C, Musgrove L C, Scheschonka A, Druey K M, Kehrl J H. Endocrinology. 1997;138:843–846. doi: 10.1210/endo.138.2.5034. [DOI] [PubMed] [Google Scholar]
- 40.Saugstad J A, Marino M J, Folk J A, Hepler J R, Conn P J. J Neurosci. 1998;18:905–913. doi: 10.1523/JNEUROSCI.18-03-00905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. Nature (London) 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- 42.Druey K M, Blumer K J, Kang V H, Kehrl J H. Nature (London) 1996;379:742–746. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- 43.Dohlman H G, Song J, Ma D, Courchesne W E, Thorner J. Mol Cell Biol. 1996;16:5194–5209. doi: 10.1128/mcb.16.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C, Zheng B, Han J, Lin S C. J Biol Chem. 1997;272:8679–8685. doi: 10.1074/jbc.272.13.8679. [DOI] [PubMed] [Google Scholar]
- 45.Bowman E P, Campbell J J, Druey K M, Scheschonka A, Kehrl J H, Butcher E C. J Biol Chem. 1998;273:28040–28048. doi: 10.1074/jbc.273.43.28040. [DOI] [PubMed] [Google Scholar]
- 46.Grantham J J. Am J Kid Dis. 1996;28:788–803. doi: 10.1016/s0272-6386(96)90378-9. [DOI] [PubMed] [Google Scholar]