Abstract
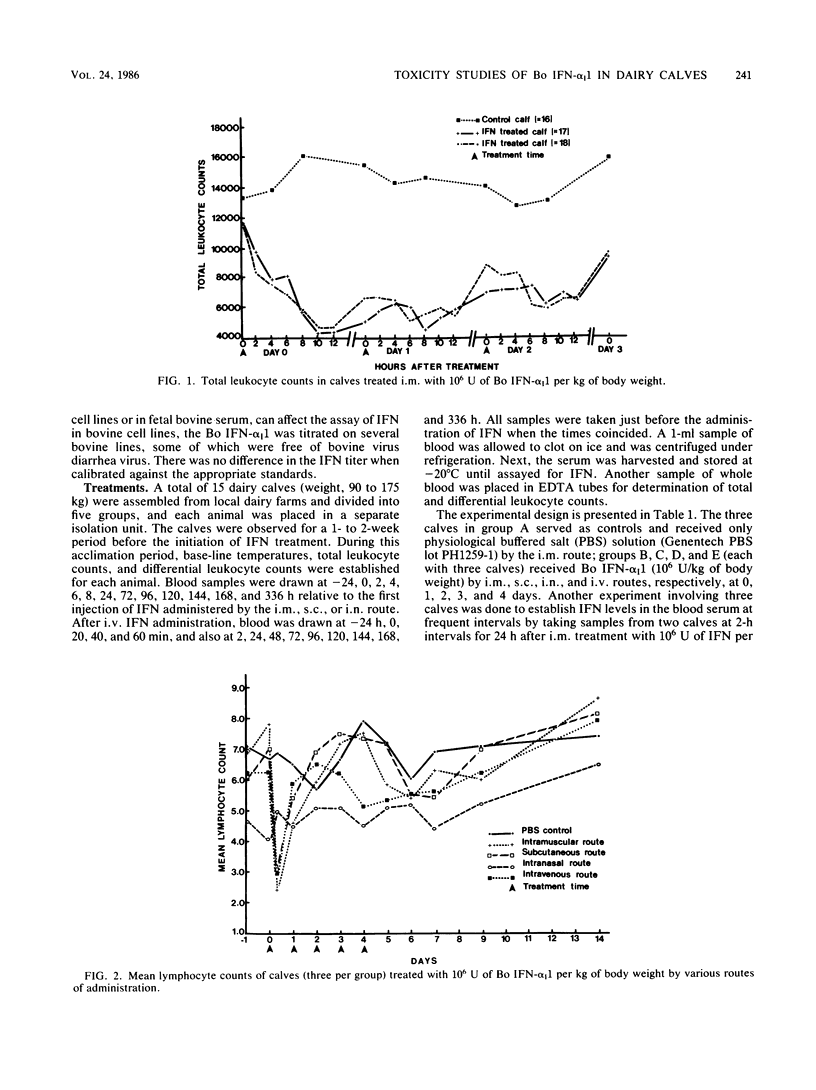
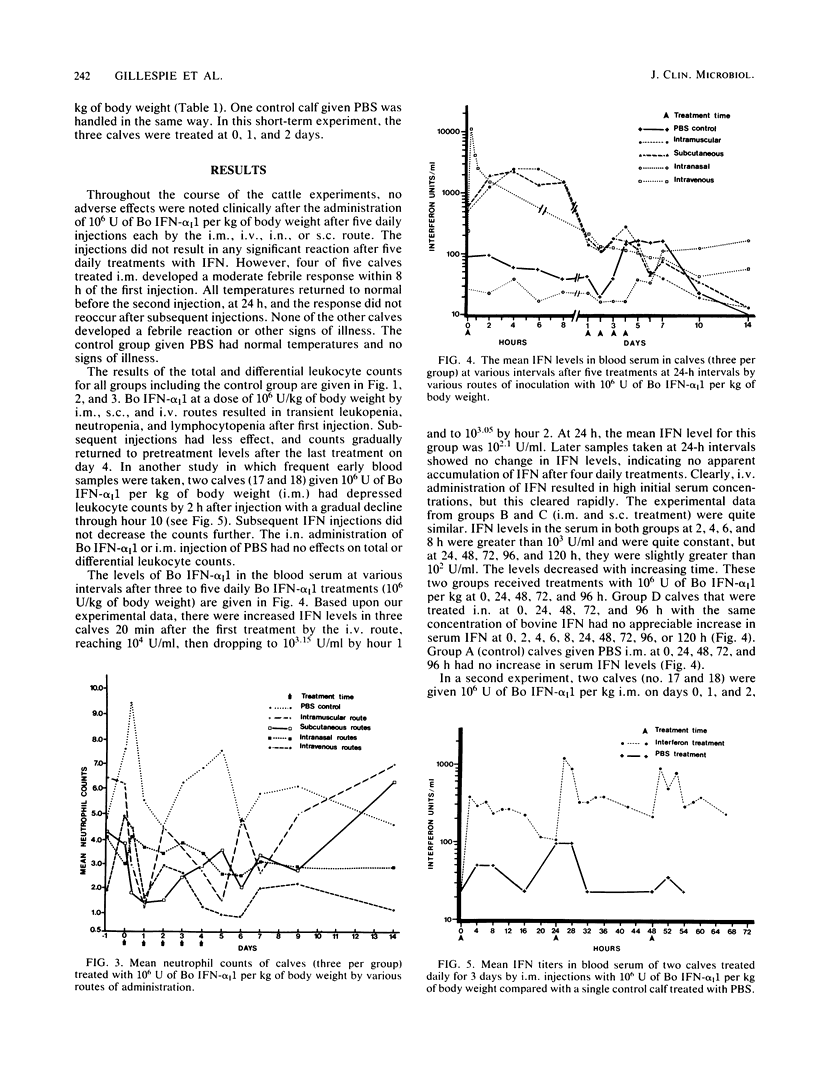
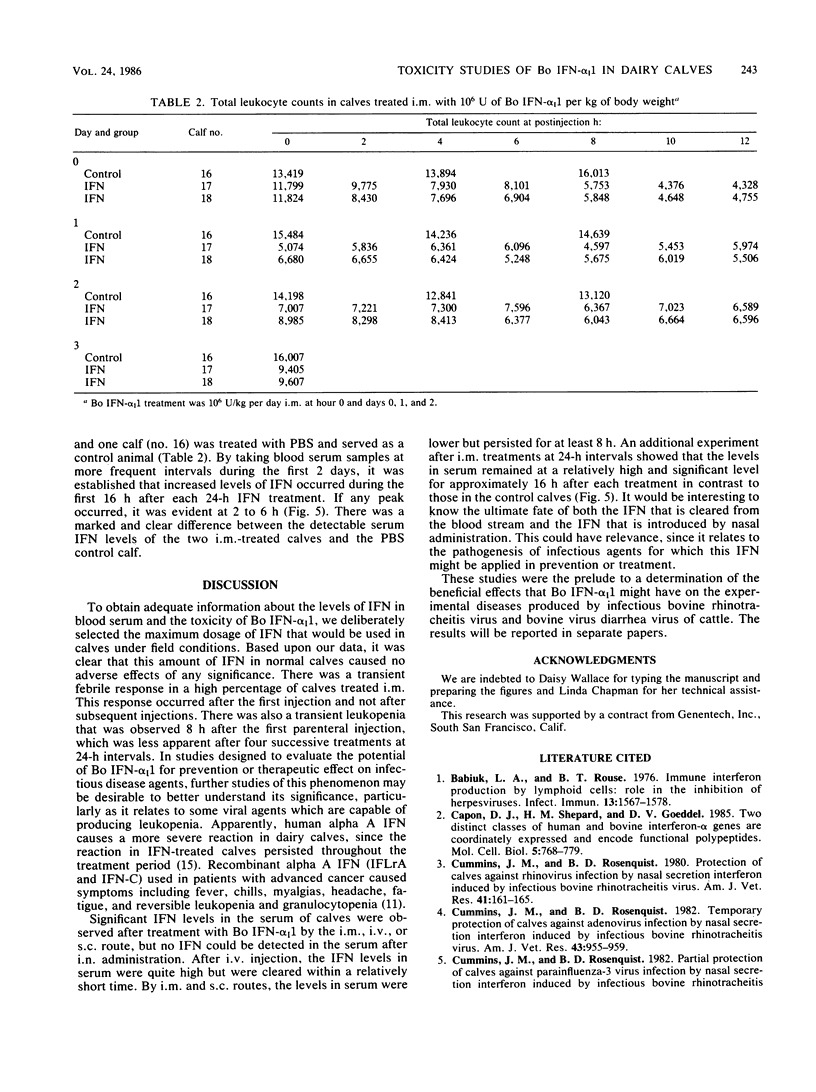
This paper reports information on the levels of interferon (IFN) in the blood serum of dairy calves given 10(6) U of bacteria-derived bovine alpha I1 interferon per kg of body weight by intravenous (i.v.), intramuscular (i.m.), subcutaneous (s.c.), and intranasal (i.n.) routes. Highest levels (10,000 U/ml) in the vesicular stomatitis viral assay system were obtained after i.v. administration and occurred within 30 min of a dose; levels rapidly declined thereafter to a low of 200 to 300 U/ml by 24 h. Serum inhibitory activity against vesicular stomatitis virus in this range is sometimes found in normal dairy calves. Levels after i.m. and s.c. administration were similar: a plateau of 1,000 to 2,000 U/ml between 2 and 8 h after a treatment with a decline to 200 to 300 U/ml by 24 h. Serum IFN was not detected after i.n. dosing or in the control group given physiological buffered saline by the i.m. route. A transitory moderate febrile response, but no other clinical adverse effects, was noted after the first intramuscular dose of IFN, but not after subsequent i.m. doses. No clinical signs were noted after i.v., s.c., or i.n. dosing or in the control calves given physiological buffered saline intramuscularly. After i.v., s.c., and i.m. administration of IFN, leukopenia, neutropenia, and lymphocytopenia were observed; these were most prominent within the first 24 h after the initial dose of IFN.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Rouse B. T. Immune interferon production by lymphoid cells: role in the inhibition of herpesviruses. Infect Immun. 1976 Jun;13(6):1567–1578. doi: 10.1128/iai.13.6.1567-1578.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Shepard H. M., Goeddel D. V. Two distinct families of human and bovine interferon-alpha genes are coordinately expressed and encode functional polypeptides. Mol Cell Biol. 1985 Apr;5(4):768–779. doi: 10.1128/mcb.5.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins J. M., Rosenquist B. D. Protection of calves against rhinovirus infection by nasal secretion interferon induced by infectious bovine rhinotracheitis virus. Am J Vet Res. 1980 Feb;41(2):161–165. [PubMed] [Google Scholar]
- Cummins J. M., Rosenquist B. D. Temporary protection of calves against adenovirus infection by nasal secretion interferon induced by infectious bovine rhinotracheitis virus. Am J Vet Res. 1982 Jun;43(6):955–959. [PubMed] [Google Scholar]
- Diderholm H., Dinter Z. Interference between strains of bovine virus diarrhea virus and their capacity to suppress interferon of a heterologous virus. Proc Soc Exp Biol Med. 1966 Mar;121(3):976–980. doi: 10.3181/00379727-121-30940. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Root S. K. Antiviral activity in interferon-treated bovine tracheal organ cultures. Infect Immun. 1978 Aug;21(2):672–673. doi: 10.1128/iai.21.2.672-673.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R. W., Rosenquist B. D. In vitro interferon production by bovine tissues: induction with infectious bovine rhinotracheitis virus. Am J Vet Res. 1976 Dec;37(12):1497–1502. [PubMed] [Google Scholar]
- Goeddel D. V., Leung D. W., Dull T. J., Gross M., Lawn R. M., McCandliss R., Seeburg P. H., Ullrich A., Yelverton E., Gray P. W. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981 Mar 5;290(5801):20–26. doi: 10.1038/290020a0. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yelverton E., Ullrich A., Heyneker H. L., Miozzari G., Holmes W., Seeburg P. H., Dull T., May L., Stebbing N. Human leukocyte interferon produced by E. coli is biologically active. Nature. 1980 Oct 2;287(5781):411–416. doi: 10.1038/287411a0. [DOI] [PubMed] [Google Scholar]
- Gutterman J. U., Fine S., Quesada J., Horning S. J., Levine J. F., Alexanian R., Bernhardt L., Kramer M., Spiegel H., Colburn W. Recombinant leukocyte A interferon: pharmacokinetics, single-dose tolerance, and biologic effects in cancer patients. Ann Intern Med. 1982 May;96(5):549–556. doi: 10.7326/0003-4819-96-5-549. [DOI] [PubMed] [Google Scholar]
- McClurkin A. W., Pirtle E. C., Coria M. F., Smith R. L. Comparison of low- and high-passage bovine turbinate cells for assay of bovine viral diarrhea virus. Arch Gesamte Virusforsch. 1974;45(3):285–289. doi: 10.1007/BF01249692. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Isackson D. W., Overall J. C., Jr, Glasgow L. A., Brown T. T., Bistner S. I., Gillespie J. H., Scott F. W. Fetal and adult bovine interferon production during bovine viral diarrhea virus infection. Infect Immun. 1976 Sep;14(3):660–666. doi: 10.1128/iai.14.3.660-666.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roney C. S., Rossi C. R., Smith P. C., Lauerman L. C., Spano J. S., Hanrahan L. A., William J. C. Effect of human leukocyte A interferon on prevention of infectious bovine rhinotracheitis virus infection of cattle. Am J Vet Res. 1985 Jun;46(6):1251–1255. [PubMed] [Google Scholar]
- Rosenquist B. D., Loan R. W. Production of circulating interferon in the bovine species. Am J Vet Res. 1969 Aug;30(8):1293–1303. [PubMed] [Google Scholar]
- Rossi C. R., Kiesel G. K., Hoff E. J. Factors affecting the assay of bovine type I interferon on bovine embryonic lung cells. Am J Vet Res. 1980 Apr;41(4):552–556. [PubMed] [Google Scholar]
- Todd J. D., Volenec F. J., Paton I. M. Interferon in nasal secretions and sera of calves after intranasal administration of avirulent infectious bovine rhinotracheitis virus: association of interferon in nasal secretions with early resistance to challenge with virulent virus. Infect Immun. 1972 May;5(5):699–706. doi: 10.1128/iai.5.5.699-706.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


