Abstract
Tumour cells become addicted to the expression of initiating oncogenes like Ras, such that loss of oncogene expression in established tumours leads to tumour regression1. HRas, NRas, or KRas are mutated to remain in the active GTP-bound oncogenic state in many cancers2. While oncogenic Ras activates a number of proteins to initiate human tumour growth, only PI3K, through activation of the kinase AKT (PKB), must remain activated by oncogenic Ras to maintain this growth3. Here we show that blocking phosphorylation of the AKT substrate, eNOS, inhibits tumour initiation and maintenance. Moreover, eNOS enhances the nitrosylation and activation of endogenous wildtype Ras proteins, which is required for throughout tumorigenesis. We suggest that activation of the PI3K-AKT-eNOS-(wildtype)Ras pathway by oncogenic Ras in cancer cells is required to initiate and maintain tumour growth.
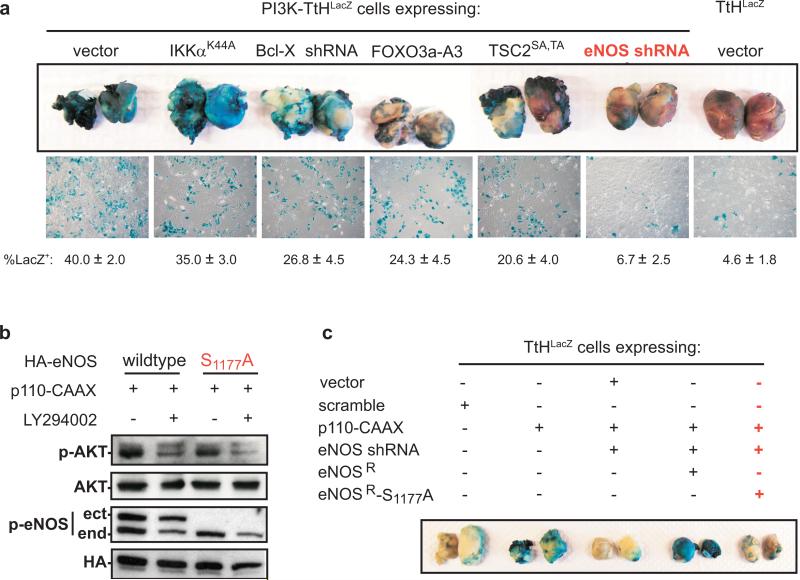
The reduction of Ras oncogene dependence to activation of AKT appears to be a consequence of redundant signalling provided by the established tumour microenvironment. Indeed, activation of AKT fosters tumourigenic growth of otherwise non-tumourigenic cells, provided such cells are mixed with tumour cells to establish the tumour microenvironment3. We exploited this cell-mixing assay to interrogate the signalling pathway downstream of AKT required for tumour maintenance. Although AKT can phosphorylate a number of proteins4, we focused on BAD, FOXO, IKKα, TSC2, and endothelial nitric oxide synthase (eNOS or NOS3), as the consequence of AKT phosphorylation of these proteins is not redundant with the functions of the oncoproteins expressed in cells used in the cell-mixing assay of tumour maintenance (Ref 3-5, Supp. Fig. 1). Non-tumourigenic PI3K-TtHLacZ cells, derived from normal human kidney cells transformed by oncoproteins T/t-Ags and immortalized by hTERT (hereafter termed TtH cells), expressing p110-CAAX (to activate the PI3K-AKT pathway) and LacZ (to demark the cells in the tumour), had Bcl-XL shRNA, eNOS shRNA, or as reported by others6-8, dominant-acting FOXO3a-A3 or TSC2SA,TA (mutated at AKT phosphorylation sites), or IKKαK44A (kinase-inactive) proteins expressed to suppress the effects of AKT on these individual pathways (Supp. Fig. 1). Knockdown of Bcl-XL and eNOS, ectopic expression and nuclear localization of FOXO3a-A36, ectopic expression of TSC2SA,TA leading to mTOR repression, as assessed by decreased S6k phosphorylation7, and ectopic expression of IKKαK44A leading to repression of NF-κB, as assessed by nuclear exclusion of p658, were validated (Supp. Fig. 2). These five cell lines were mixed with tumourigenic HRasG12V transformed TtH cells (termed RasG12V-TtH cells) to establish a tumour microenvironment, injected into mice, and assayed for their contribution to the resultant tumour mass by treating tumours or derived tumour cells with X-gal to stain LacZ+ cells blue. Positive control vector PI3K-TtHLacZ cells extensively populated tumours, whereas negative control vector TtHLacZ cells contributed little to the tumour mass, as evidenced by the prominent or weak blue staining, respectively. Expression of IKKαK44A had little effect, Bcl-XL shRNA, FOXO3a-A3, and TSC2SA,TA had a mild effect, but eNOS shRNA had the greatest effect on reducing the contribution of PI3K-TtHLacZ (blue) cells in tumours (Fig. 1a). To then test if AKT phosphorylation, and not just expression of eNOS, is required for tumour maintenance, AKT was validated to phosphorylate S1177 of eNOS9, as evidenced by a loss of S1177 phosphorylation of endogenous eNOS upon pharmacological inhibition of AKT signalling with LY294002 or by mutating S1177 of ectopic eNOS to alanine in PI3K-TtHLacZ cells (Fig. 1b). PI3K-TtHLacZ cells in which eNOS was knocked down were then engineered to express eNOSR in the wildtype or S1177A mutant configuration (Supp. Fig. 3) and assayed for tumour maintenance by the aforementioned cell-mixing assay. Control PI3K-TtHLacZ cells populated tumours, which was greatly reduced upon knockdown of eNOS, as evidenced by the reduction in blue staining. This loss was rescued by wildtype, but not S1177A mutant eNOSR (Fig. 1c). Thus, activation of the PI3K-AKT-eNOS pathway promotes tumour maintenance.
Figure 1.
AKT promotes tumour maintenance by phosphorylation of eNOS. a, PI3K-TtHLacZ or c, TtHLacZ cells expressing indicated constructs were mixed with RasG12V-TtH cells, injected into mice, and tumours or recultured tumour cells were stained with X-gal. n=5, mean±s.e.m. b, Protein levels of phosphorylated AKT (p-AKT), phopshorylated eNOS (p-eNOS, ect: ectopic, end: endogenous), HA-eNOS (HA), and AKT in PI3K-TtHLacZ cells expressing wildtype or S1177A HA-eNOS treated with DMSO or LY294002.
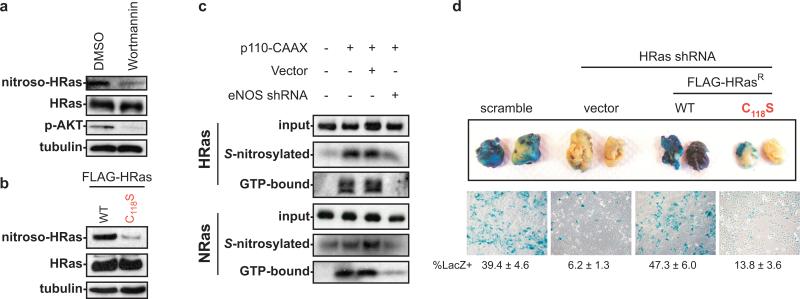
eNOS has been detected in tumour cells10, and catalyzes the synthesis of nitric oxide (NO), which can facilitate S-nitrosylation of the thiol group of cysteines in proteins11, such as that of C118 of HRas, which enhances the dissociation of guanine nucleotides thereby increasing GTP-bound HRas12. Wildtype Ras proteins can be required for activation of the MAPK pathway by oncogenic Ras13, and membrane-targeting of RasGAP, which inhibits wildtype but not oncogenic Ras, reverts oncogenic Ras transformation of NIH3T3 cells14, suggesting that wildtype Ras proteins may facilitate oncogenic signalling. Collectively, we speculated that AKT activation of eNOS maintains tumour growth in the absence of oncogenic Ras by activating wildtype Ras through S-nitrosylation of C118. To test this, activated AKT in PI3K-TtH was shown to foster HRas nitrosylation through eNOS, as the majority of HRas nitrosylation was lost by treatment with the PI3K inhibitor wortmannin (Fig. 2a), by mutating C118 in HRas to Serine, a minor change that exchanges the sulphur atom for oxygen but nevertheless blocks nitrosylation12 (Fig 2b), or by knocking down eNOS (Fig. 2c), and as HRas nitrosylation was elevated upon activation of AKT (Fig. 2c). Reduction of HRas nitrosylation by eNOS shRNA also reduced levels of active GTP-bound HRas (Fig. 2c). Since TtH cells express HRas and NRas, but not KRas (not shown), and C118 is conserved amongst all Ras proteins, we tested and confirmed that activated AKT in PI3K-TtH cells also led to elevated levels of nitrosylated and GTP-bound endogenous NRas, which were reduced upon knockdown of eNOS (Fig. 2c). Thus, AKT activation of eNOS promotes nitrosylation and activation of wildtype Ras proteins. To then assess the biological consequence of S-nitrosylation of wildtype HRas in tumour maintenance, we tested whether replacing endogenous wildtype HRas with the nitrosylation-resistant C118S mutant version reduced tumour maintenance. HRas was knocked down by shRNA in PI3K-TtHLacZ cells and complemented with vector encoding HRas resistant to RNAi (HRasR) in the wildtype configuration or the C118S mutant configuration that reduced GTP loading, or as a control, no transgene (Supp. Fig. 4). These cell lines, or as controls cells expressing either a scramble control sequence or HRas shRNA alone, were mixed with RasG12V-TtH cells, injected into mice, and the resultant tumours assayed for the presence of blue LacZ+ cells as a measure of tumour maintenance. Knockdown of wildtype HRas reduced the ability of PI3K-AKT signalling to foster tumour maintenance, as evidenced by a sixfold reduction of PI3K-TtHLacZ (blue) cells in the tumours. This effect was reversed upon expressing wildtype, but less so with the C118S mutant version of HRasR (Fig. 2d). Thus, activation of the PI3K-AKT-eNOS pathway promotes tumour maintenance by S-nitrosylation and activation of wildtype Ras (Fig. 4g).
Figure 2.
eNOS activates wildtype HRas to promote tumour maintenance. Protein levels of S-nitrosylated (nitroso), GTP-bound, total, or input HRas or NRas, phosphorylated AKT (p-AKT), or as a loading control tubulin in a, PI3K-TtHLacZ cells treated with DMSO or wortmannin, b, PI3K-TtHLacZ cells transfected with wildtype or C118S HRas or c, TtHLacZ cells expressing the indicated combinations of p110-CAAX, vector or eNOS shRNA. d, RasG12V-TtH cells were mixed with PI3K-TtHLacZ cells expressing the indicated constructs, injected into mice, and tumours or recultured tumour cells were stained with X-gal to visualize PI3K-TtHLacZ cells. n=5, mean±s.e.m.
Figure 4.
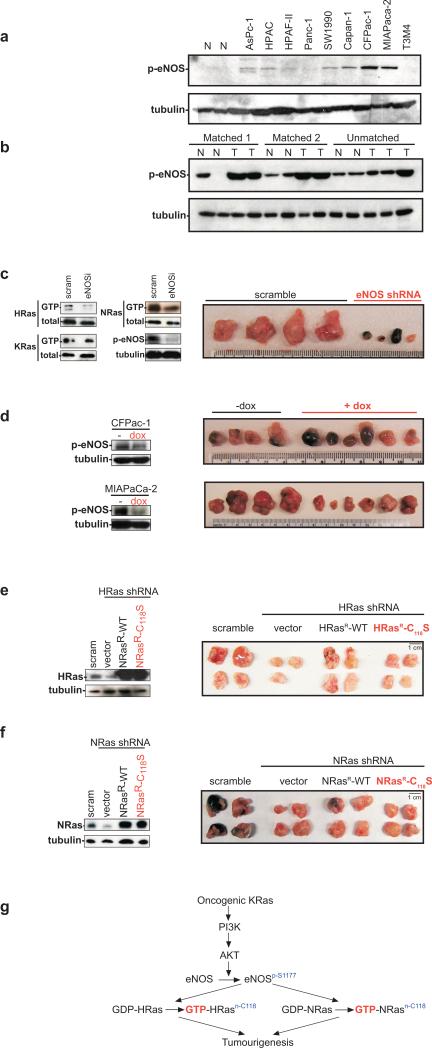
eNOS activation fosters cancer cell growth through activation of endogenous Ras. Protein levels of phosphorylated eNOS (p-eNOS) or as a loading control, tubulin, in pancreatic a, cancer lines, b, tumour and normal tissue. Protein levels of GTP-bound or total HRas, NRas, or KRas, p-eNOS or tubulin, and excised tumours of c, CFPac-1 cells expressing eNOS or scramble shRNA, d, CFPac-1 and MIAPaCa-2 cells expressing dox-inducible shRNA +/− dox, and CFPac-1 cells expressing e, HRas or f, NRas shRNA plus a vector or an RNAi-resistant wildtype or C118S NRas or HRas, or a scramble sequence. g, Proposed signalling.
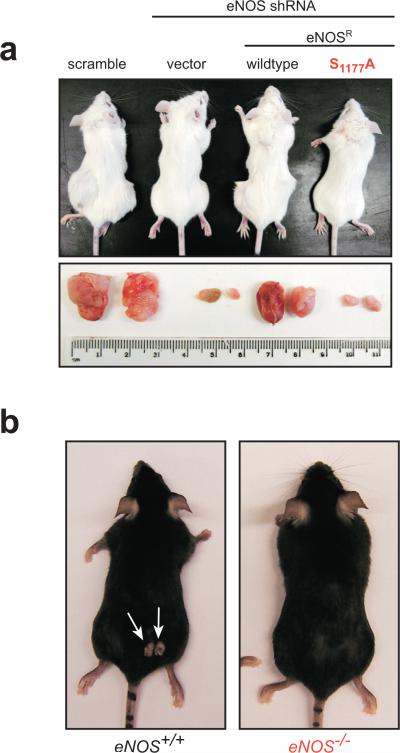
As oncogenic Ras must activate the PI3K-AKT pathway to both initiate and maintain tumour growth3, we tested whether AKT-mediated activation of eNOS was also required for the establishment of tumours. A scramble control or eNOS shRNA was introduced into tumourigenic RasG12V-TtH cells, and knockdown of eNOS complemented by RNAi-resistant eNOS (eNOSR) in the wildtype configuration or the S1177A mutant configuration resistant to S1177 phosphorylation (Supp. Fig. 5). These four cell lines were injected into mice and tumour growth monitored over time. Scramble control cells rapidly formed tumours, whereas tumour growth was almost abolished upon knockdown of eNOS. This loss of tumour growth was rescued by the wildtype, but not the S1177A version of eNOSR (Fig. 3a, Supp. Fig. 6), indicating that S1177 phosphorylation of eNOS is required for tumour initiation and maintenance. These results were validated in a spontaneous cancer model. Chemical carcinogen DMBA followed by TPA were topically applied to eNOS+/+ and eNOS−/− mice to induce skin papillomas characterized by Ras oncogenic mutations15, with the end result being an approximate threefold drop in the number of tumours per eNOS−/− mouse (Fig. 3b, Supp. Fig. 7). Thus, independent models of cancer demonstrate eNOS is required for tumorigenesis.
Figure 3.
eNOS activation is required for tumour growth. Representative mice and/or tumours a, following injection with RasG12V-TtH cells expressing indicated constructs or b, after treatment with DMBA/TPA to induce skin tumours on mice of indicated genotype (week 20).
To test whether eNOS mediates oncogenic Ras signalling in a cancer associated with the most commonly mutated Ras family member, KRas2, the amount of activated (S1177 phosphorylated) eNOS was first assayed in cancer cell lines and tumour specimens isolated from patients diagnosed with pancreatic cancer. Compared to normal tissue specimens, CFPac-1, MIAPaCa-2, and Capan-1 cells exhibited the highest level of S1177 phosphorylation of eNOS (Fig. 4a). Activated KRas and S1177 phosphorylated eNOS were also elevated in the tumour specimens compared to matched and unmatched normal tissue controls (Fig. 4b and ref. 16), with the caveat that biopsies also contain stromal tissue that could contribute to detected eNOS phosphorylation. Second, knockdown of eNOS was shown to reduce tumour growth by 50 fold in CFPac-1 cells and tumour size of MIAPaCa-2 cells by 80% (Fig. 4c, Supp. Figs. 8,9). As a control, knockdown of eNOS in cell lines AsPc-1 and SW1990 that exhibited poor eNOS activation had no obvious effect on tumour growth (not shown). Third, using a more direct assay for tumour maintenance, in vivo doxycycline (dox)-induced shRNA knockdown of eNOS in CFPac-1 and MIAPaCa-2 cells after tumours were established inhibited tumour growth, as evidenced by reduced size and/or gross necrosis of tumours excised at the termination of the experiment (Fig. 4d). In some mice, this eventually led to tumour regression (not shown). Thus, eNOS is required to both initiate and maintain tumour growth of these human pancreatic cancer cells. Fourth, to test if eNOS promoted tumour growth through nitrosylation of Ras in pancreatic cancer cells, we determined which Ras family members were inactivated by eNOS shRNA in CFPac-1 and MIAPaCa-2 cells. Not surprisingly, GTP-bound KRas was unchanged upon knockdown of eNOS (Fig. 4c, Supp. Fig. 9), as K-Ras is mutated to remain active in these two cell lines17,18. Consistent with this, oncogenic KRas harbouring the C118S mutation remained tumourigenic (Supp. Fig. 10), pointing towards wildtype Ras proteins as the target of eNOS signalling. GTP-bound endogenous wildtype HRas and NRas were reduced upon shRNA knockdown of eNOS, however the wildtype allele of KRas is deleted in the MIAPaCa-2 cells18 and a number of oncogenic KRas-positive cell lines18 and tumour tissues19, suggesting that wildtype HRas and NRas, but not KRas, are the targets of eNOS signalling in pancreatic cancer cells (Fig. 4c, Supp. Fig. 9). To then test if activation of HRas or NRas by eNOS is required for pancreatic tumour growth, HRas or NRas were knocked down by shRNA in CFPac-1 and/or MIAPaCa-2 cells and complemented with an HRas or NRas engineered to be resistant to RNAi (HRasR, NRasR) in the wildtype or the C118S mutant configuration, and resultant cells assayed for tumour growth in mice. Positive control scramble treated CFPac-1 and/or MIAPaCa-2 cells readily formed tumours in mice, whereas this growth was reduced when endogenous HRas, and to a lesser degree NRas, was knocked down. This loss of tumour growth was rescued by expressing the appropriate wildtype HRas or NRas, but not the C118S nitrosylation mutants (Fig. 4e,f, Supp. Figs. 11-13). Similar results were found when the cells were assayed for transformed growth in vitro, suggestive of a tumour cell autonomous defect when wildtype Ras proteins cannot be nitrosylated (Supp. Fig. 14). Thus, oncogenic KRas-driven pancreatic cancer tumour growth was mediated by eNOS nitrosylation of endogenous wildtype HRas and NRas (Fig. 4g).
In summary, we demonstrate that the continual need for PI3K-AKT signalling during initiation and maintenance of oncogenic Ras-driven tumour growth is due, at least in part, to activation of eNOS through phosphorylation of S1177, which in turn leads to S-nitrosylation at C118 and correspondingly activation of the other wildtype Ras family members, perhaps as a means to diversify the Ras signal beyond that of oncogenic Ras (Fig. 4g). In agreement, the wildtype counterpart of oncogenic Ras is not required for tumorigenesis (Supp. Fig. 15), and is even deleted in some tumours19, whereas wildtype HRas and NRas are required for oncogenic KRas-driven tumour growth, and appear to have non-redundant activies20. Effects of eNOS on tumourigenesis have been largely attributed to its activity in endothelial cells10. Our results now suggest a key role for tumour-expressed eNOS in the tumourigenic process. As eNOS plays multiple roles in tumourigenesis10, and delivery of a peptide fragment of the protein cavtratin, which can inhibit eNOS, displays anti-tumour activity21, we speculate that inhibition of eNOS, perhaps in combination with inhibition of wildtype Ras protein function or processing2, could have therapeutic value in the treatment of oncogenic Ras-driven human cancers such as pancreatic.
METHODS SUMMARY
TtH and the pancreatic cancer cell lines22 were stably infected retroviruses encoding the indicated shRNAs, transgenes, or no insert as described23, and appropriate expression verified by immunoblot or RT-PCR. Detection of GTP-bound or nitrosylated Ras was performed as described24. One or a mixture of two cell lines were injected into the flanks of immunocompromised mice to assay for tumour growth, and where indicated, excised and assayed for LacZ-positive cells, as described3. Induction of shRNA in vivo by dox3 and DMBA/TPA treatments25 were performed as described. All animal work was approved by IACUC, all tissue samples were provided under an approved IRB protocol.
FULL METHODS
Plasmids
pBabepuro, neo, bleo, and hygro were used as control vectors3. HA-IKKαK44A cDNA26, FOXO3a-A3 cDNA27 engineered with an N-terminal HA tag, FLAG-TSC2S393A,T1462A cDNA7 (termed here as TSC2SA,TA), eNOS cDNA engineered with a C-terminal HA tag and to be resistant to shRNA by introducing the three silent mutations G1821→A, T1827→C and G1830→A alone (eNOSR) or in conjunction with the mutation A3519GC→GCC that altered S1177 to A (S1177A eNOSR), and wildtype FLAG-epitope tagged HRas or NRas cDNAs engineered to be resistant to shRNA by introducing the silent mutations in the region targeted by RNAi (FLAG-HRasR; FLAG-NRasR) alone or in conjunction with the mutation T342GT→TCT (C118S FLAG-HRasR, C118S FLAG-NRasR) that altered C118 to S, were subcloned into one of the aforementioned pBabe vectors. Bcl-XL shRNA (5’-AGCGTAGACAAGGAGATGC), eNOS shRNA (5’-AAGAGTTATAAGATCCGCTTC), HRas shRNA (5’-GGCAAGAGTGCGCTGACCATC), NRas shRNA (5’-CAAGAAGAGTACAGTGCCATG) or an eNOS scramble control (5’-AAGCGTTAAAAGATCCGCTTC) sequences were cloned into pSUPER-PURO-RETRO (Oligoengine). The plasmid system for dox-inducible shRNA3 was adapted to encode eNOS shRNA.
Cell lines
TtH and the pancreatic cancer cell lines were previously described22. Derived lines were generated by stable infection with the indicated combinations of amphotrophic retroviruses generated from the aforementioned pBabe plasmids, as previously described23.
Cell treatments
Cells were treated with LY294002 (Cell Signaling Technologies) or Wortmannin (Sigma) at a final concentration of 20 μM or 10 nM, respectively, for 1 hr prior to analysis.
Immunoblotting
HA-IKKαK44A, FOXO3a-A3-HA, HA-eNOS or variants thereof, FLAG-TSC2SA,TA, endogenous Bcl-XL, p70 S6 kinase, T389 phosphorylated p70 S6 kinase, HRas, KRas or NRas, S1177 phosphorylated eNOS (both to detect activated eNOS and assess eNOS expression), S473 phosphorylated AKT, actin, p65, and tubulin were detected by immunoblotting with αHA (Roche), αFLAG (Sigma), αBcl-xL, αp70 S6 Kinase, αThr389 Phospho-p70 S6 Kinase, αSer1177 Phospho-eNOS, αSer473 Phospho-AKT (Cell Signalling Technology), αHRas, αKRas, αNRas, αactin (Santa Cruz), αp65 (Rockland), and αtubulin (Sigma) antibodies, respectively.
RT-PCR
eNOS and GAPDH mRNA was RT-PCR amplified with the primers 5’-CAGTGTCCAACATGCTGCTGGAAATTG and 5’-TAAAGGTCTTCTTCCTGGTGATGC, and the primers 5’-ACCACAGTCCATGCCATCAC and 5’-TCCACCACCCTGTTGCTGTA, respectively.
GTP and nitrosylated Ras
GTP-bound or nitrosylated Ras were captured as previously described24 and immunoblotted with either an αFLAG (Sigma) or an αHRas, αKRas or αNRas (Santa Cruz) antibody to detect FLAG-H-Ras or endogenous H, N, or KRas proteins, respectively.
Soft agar
Soft agar assays were done in triplicate and twice independently as previously described5.
Tumour growth
As previously described3, the tested cell line (tumour initiation) or a mixture of two cell lines (cell mixing assay for tumour maintenance) were injected subcutaneously into 4 flanks of SCID/Beige mice. For tumour initiation experiments, tumours were removed and photographed when control tumours reached maximum volume. For cell mixing assays, the 4 tumours were removed when they reached maximum volume, human cells derived from the 2 tumours by re-culture in selective media (G418), and the 2 other whole tumours were treated with X-gal to stain LacZ+ cells blue and photographed. CFPac-1 and MIAPaCa-2 cells engineered to contain a dox-inducible eNOS shRNA3 were injected into both flanks of 5 SCID/Beige mice, tumours were permitted to reach a diameter of 0.6 cm, after which 3 mice were provided with doxycycline in their diet and 2 mice left untreated for 11 days (CFPac-1 cells) or 13 days (MIAPaCa-2 cells), after which tumours were removed and photographed. DMBA/TPA treatments were performed as previously described25 on 15 eNOS+/+ C57BL/6J and 15 eNOS−/− C57BL/6J (B6.129P2-Nos3tm1Unc/J) mice28 (Jackson Laboratory). All animal work was approved by DUMC IACUC.
Tumour and normal human specimens
Flash frozen tissue samples were provided devoid of all identifying information under a DUMC approved IRB protocol.
Supplementary Material
Acknowledgements
We thank J.S. Stamler for human eNOS, L.C. Cantley for TSC2S393A,T1462A, A. Baldwin for IKKαK44A, K. Walsh for FOXO3a cDNAs, A.D. Proia for tissue specimens, and X-.F. Wang, T.-P. Yao, A.M. Pendergast, C.J. Der, A.D. Cox, and M.A. Hollingsworth for discussions and C. Ring for technical assistance. This research is supported by NIH grants CA94184 and CA123031. C.M.C. is a Leukemia and Lymphoma Society Scholar, D.F.K. is a Leukemia and Lymphoma Society Fellow, K.-H.L. and B.B.A. are DoD Breast Cancer Research Predoctoral Scholars.
Footnotes
Supplementary Information accompanies the paper at www.nature.com/nature
REFERENCES
- 1.Giuriato S, et al. Conditional animal models: a strategy to define when oncogenes will be effective targets to treat cancer. Semin. Cancer Biol. 2004;14:3–11. doi: 10.1016/j.semcancer.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 3.Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 5.Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]; Yeh E, et al. A signalling pathway controlling Myc degredation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 6.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 7.Manning BD, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 8.Regnier CH, et al. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 9.Michell BJ, et al. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]; Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 10.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 11.Hess DT, et al. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 12.Lander HM, et al. Redox regulation of cell signalling. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M, Wolfman A. Ha-ras and N-ras regulate MAPK activity by distinct mechanisms in vivo. Oncogene. 1998;16:1417–1428. doi: 10.1038/sj.onc.1201653. [DOI] [PubMed] [Google Scholar]
- 14.Huang DC, Marshall CJ, Hancock JF. Plasma membrane-targeted ras GTPase-activating protein is a potent suppressor of p21ras function. Mol. Cell. Biol. 1993;13:2420–2431. doi: 10.1128/mcb.13.4.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintanilla M, et al. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 16.Lim KH, et al. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr. Biol. 2006;16:2385–2394. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Moore PS, et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 18.Kita K, et al. Growth inhibition of human pancreatic cancer cell lines by anti-sense oligonucleotides specific to mutated K-ras genes. Int. J. Cancer. 1999;80:553–558. doi: 10.1002/(sici)1097-0215(19990209)80:4<553::aid-ijc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Wan J, et al. Loss of heterozygosity of Kras2 gene on 12p12−13 in Chinese colon carcinoma patients. World J. Gastroenterol. 2006;12:1033–1037. doi: 10.3748/wjg.v12.i7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li J, et al. LOH of chromosome 12p correlates with Kras2 mutation in non-small cell lung cancer. Oncogene. 2003;22:1243–1246. doi: 10.1038/sj.onc.1206192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh C, Subrahmanyam R, Ren R. Oncogenic NRAS, KRAS, and HRAS Exhibit Different Leukemogenic Potentials in Mice. Cancer Res. 2007;67:7139–7146. doi: 10.1158/0008-5472.CAN-07-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]; Esteban LM, et al. Targeted genomic disruption of H-ras and N-ras, individually or in combination, reveals the dispensability of both loci for mouse growth and development. Mol. Cell. Biol. 2001;21:1444–1452. doi: 10.1128/MCB.21.5.1444-1452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Johnson L, et al. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11:2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fotiadou PP, et al. Wild-Type NRas and KRas Perform Distinct Functions during Transformation. Mol. Cell. Biol. 2007;27:6742–6755. doi: 10.1128/MCB.00234-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratton JP, et al. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 22.Lim KH, et al. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–545. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 23.O'Hayer KM, Counter CM. A genetically defined normal somatic human cell system to study ras oncogenesis in vitro and in vivo. Methods Enzymol. 2006;407:637–647. doi: 10.1016/S0076-6879(05)07050-3. [DOI] [PubMed] [Google Scholar]
- 24.de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]; Jaffrey SR, et al. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 25.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woronicz JD, et al. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 27.Hu MC, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 28.Shesely EG, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Woronicz JD, et al. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 3.Hu MC, et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 4.Manning BD, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Lim KH, et al. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–545. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 6.O'Hayer KM, Counter CM. A genetically defined normal somatic human cell system to study ras oncogenesis in vitro and in vivo. Methods Enzymol. 2006;407:637–647. doi: 10.1016/S0076-6879(05)07050-3. [DOI] [PubMed] [Google Scholar]
- 7.de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 8.Jaffrey SR, et al. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 9.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shesely EG, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.