Abstract
Thyroid stimulating hormone (TSH) is produced by the anterior pituitary and is used to regulate thyroid hormone output, which in turn controls metabolic activity. Currently, the pituitary is believed to be the only source of TSH used by the thyroid. Recent studies in mice from our laboratory have identified a TSH isoform that is expressed in the pituitary, in peripheral blood leukocytes (PBL), and in the thyroid. To determine whether a human TSH splice variant exists that is analogous to the mouse TSH splice variant, and whether the pattern of expression of the splice variant is similar to that observed in mice, PCR amplification of RNAs from pituitary, thyroid, PBL, and bone marrow was done by reverse-transcriptase PCR and quantitative realtime PCR. Human pituitary expressed a TSH isoform that is analogous to the mouse TSH splice variant, consisting of a twenty-seven nucleotide portion of intron 2 and all of exon 3, coding for 71.2% of the native human TSH polypeptide. Of particular interest, the TSH splice variant was expressed at significantly higher levels than the native form or TSH in PBL and the thyroid. The TSH gene also was expressed in the pituitary, thyroid, and PBL, but not the BM, suggesting that the TSH polypeptide in the thyroid and PBL may exist as a dimer with TSH. These findings identify an unknown splice variant of human TSH. They also have implications for immune-endocrine interactions in the thyroid and for understanding autoimmune thyroid disease from a new perspective.
Keywords: splice variant, thyrotropin, intron, leukocytes, thyroid
Introduction
Thyroid stimulating hormone (TSH) is produced by the anterior pituitary and is used to regulate thyroid hormone output. Although it has been known for a quarter of a century that TSH can be produced by hematopoietic cells (Kruger and Blalock, 1986; Kruger et al., 1989; Smith et al., 1983; Wang and Klein, 1994; Wang et al., 1997), the significance of that remains obscure. Whereas some studies point to a role for immune system-derived TSH as a cytokine-like molecule used in hematopoietic cell growth and differentiation (Kelley et al., 2007), recent findings from our laboratory indicate that its primary action may be directed to the thyroid itself (Klein and Wang, 2004). In that vein, we recently identified a novel splice variant isoform of the TSH subunit in mice the first splice variant of TSH to be identified that is preferentially produced by hematopoietic cells and by cells in the thyroid, and is upregulated in the thyroid during systemic virus infection (Vincent et al., 2009). That isoform consists of a twenty-seven nucleotide portion of intron 4 that is contiguous with the coding region of exon 5 of mouse TSH, resulting in a polypeptide that comprises 71.2% of the native TSH molecule. The potential significance of that isoform has recently been discussed (Wang and Koenig, 2009).
The human TSH gene consists of three exons and two introns, with portions of exons 2 and 3 coding for the TSH polypeptide. Analysis of intron 2 revealed a twenty-seven nucleotide sequence starting with an ATG triplet at the 3′ end that is in-frame with exon 3. Thus, we sought: (i) to determine whether a human TSH splice variant exists that is analogous to the mouse TSH splice variant, and (ii) to determine whether the pattern of expression of the splice variant is similar to that observed in mice.
1. Results
2.1 Identification and tissue expression of a human TSH splice variant isoform
Two primer sets were used for analysis of human TSH gene expression. One set (designated native TSH), designed to amplify the complete human TSH open reading frame, consisted of an upstream primer targeted to a region in exon 2 prior to the TSH transcriptional start site, and a downstream primer targeted to a region in exon 3 that began one nucleotide after the stop codon. The second primer set (designated novel TSH) consisted of an upstream primer targeted to a region at the end of intron 2 with the same downstream primer used for native TSH. PCR amplification was done using RNAs from human pituitary, thyroid, PBL, and BM.
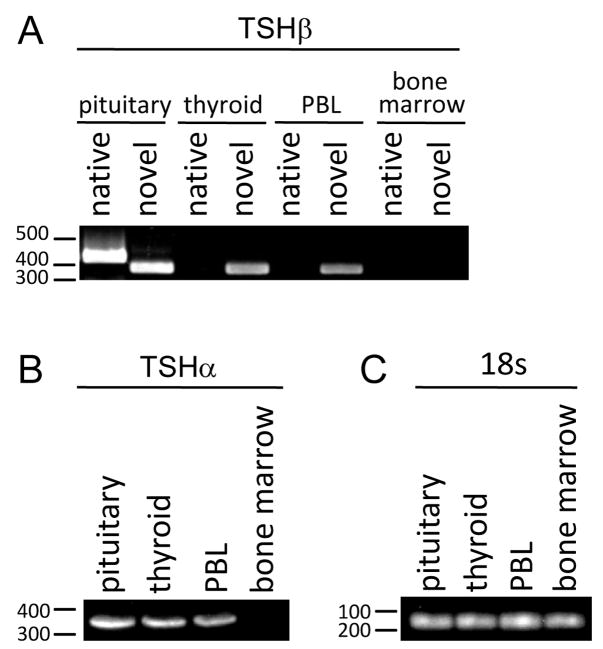
RT-PCR yielded both native and novel TSH PCR products from pituitary RNA, but only novel TSH from thyroid and PBL RNAs (Fig. 1). Neither form of TSH was amplified from BM RNA (Fig. 1A). RT-PCR resulted in a product for TSH from pituitary, thyroid, and PBL, but not BM (Fig. 1B). 18s gene expression was expressed at equivalent levels in all four samples (Fig. 1C).
Fig. 1.
(A) Agarose gel analysis and qRT-PCR of transcript expression of human pituitary, thyroid, PBL, and bone marrow RNAs amplified with native and novel primer sets. PCR-amplified products of both native and novel TSH were detected in pituitary RNA; however, only novel TSH was detected in thyroid and PBL RNA. Neither native nor novel TSH products were detected in BM RNA. (B) The TSH gene was expressed in the pituitary, the thyroid, and PBL, but not BM. (C) 18s gene expression levels were equivalent in all four samples.
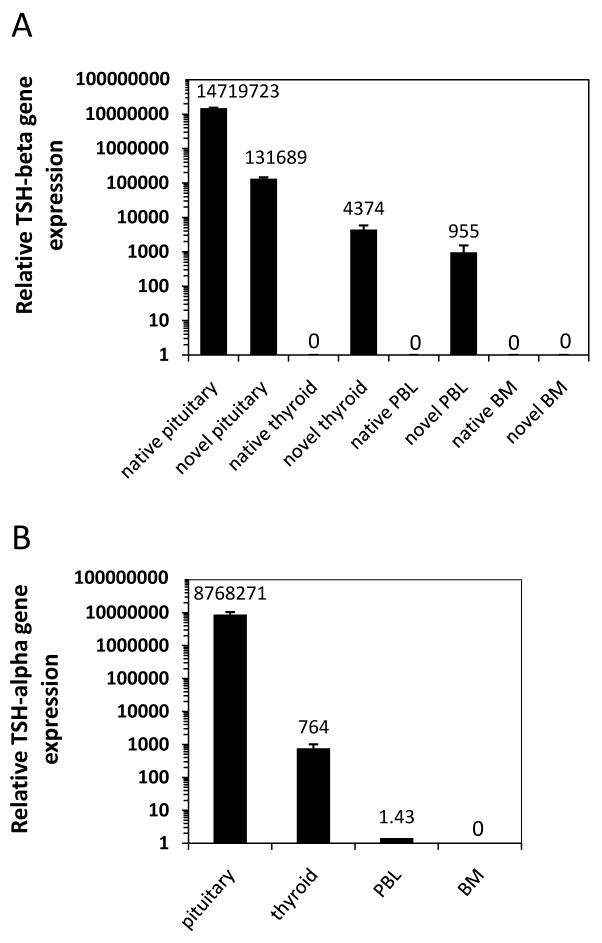
To measure the differences in native vs. novel TSH gene expression, qRT-PCR was conducted. Gene expression values were normalized to 18s values for respective tissues RNAs using the method of Livak and Schmittgen (Livak and Schmittgen, 2001). Although both native and novel TSH forms were expressed in the pituitary, there was a 111-fold preference for native over novel TSH in the pituitary (Fig. 2A). That pattern was reversed in the thyroid and PBL where there was a 4,374-fold preference, and a 955-fold preference, of novel over native TSH gene expression in the thyroid and in PBL, respectively.
Fig. 2.
(A) qRT-PCR analysis revealed high levels of both native and novel TSH transcripts in pituitary RNA. In contrast, only the novel TSH isoform was detected in thyroid and PBL RNAs. Neither native nor novel forms of TSH were detected in BM RNA. (B) qRT-PCR analysis revealed high expression of TSH in the pituitary, modest levels in the thyroid, and low levels in PBL. No TSH gene expression was detected in BM. Data are mean values ± SEM of three replicate samples.
qRT-PCR analysis was done to determine if the TSH gene was expressed in tissues that expressed the TSH splice variant. As seen in Fig. 2B, the pattern of gene expression observed for the TSH splice variant also was present for TSH as seen by high level of expression in the pituitary, modest level of expression in the thyroid, low but detectable expression level in PBL, and undetectable levels of TSH in BM.
2.2 Sequence of the TSH splice variant isoform
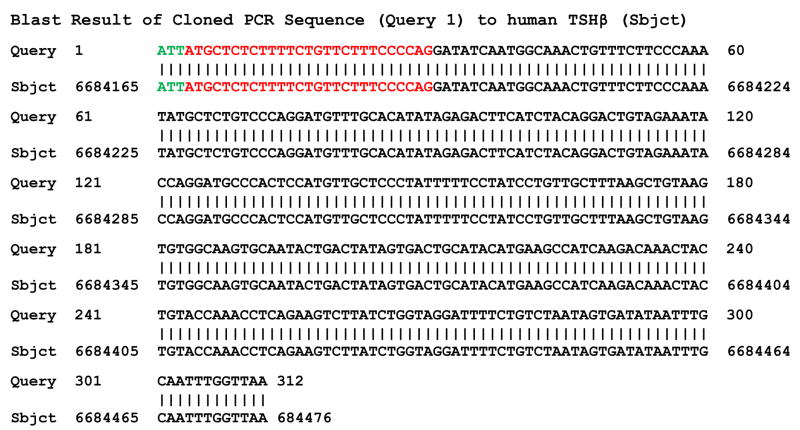
Sequence analysis of the novel TSH PCR product revealed complete homology to human TSH GenBank accession no. NM_000549) Fig. 3), including the twenty-seven nucleotides in intron 2 that precede exon 3. Moreover, seven of the nine amino acids coded for by human TSH intron nucleotides were identical to mouse TSH within that region. Hence, there was a high degree of organizational similarity between the human and the mouse TSH splice variant.
Fig. 3.
Sequence analysis of a PCR-amplified product of novel TSH from thyroid RNA. The sequence was compared by aligning with human TSH. Black nucleotides correspond to exon 3 down to the TAA stop codon. Red nucleotides are the twenty-seven nucleotides in intron 2 that are in-frame with exon 3, and which begin with an ATG start codon. The three green nucleotides correspond to the first three nucleotides of the upstream novel TSH primer.
A comparison of the nucleotide sequence of the human and mouse TSH splice variant is shown in Fig 4A; differences in mouse and human nucleotides are highlighted as underlined black nucleotides. Although there were differences in nucleotides between human exon 3 relative to corresponding exon 5 of the mouse TSH gene (Fig. 4A, red nucleotides), those are known species differences of the TSH gene. Of interest, within the twenty-seven nucleotide region of the putative leader sequence (Fig. 4A, green nucleotides), eight nucleotides differed between the two species. However, this resulted in only two amino acid substitutions, as shown in Fig. 4B at amino acid positions three and four of the leader sequence. Because those substitutions consisted of amino acids that were primarily hydrophobic or uncharged polar, a potential transmembrane function of the human leader sequence remains likely, as was previously predicted for the mouse TSH splice variant molecule (Vincent et al., 2009).
Fig. 4.
(A) Nucleotide sequence alignment of the human (top rows) and mouse (bottom rows) TSH splice variant. Green nucleotides are the putative intron-coded leader sequences. Red nucleotides represent exon 3 and exon 5 of the human and mouse TSH gene, respectively. Underlined black nucleotides show the difference of the mouse relative to the nucleotide in the corresponding human gene. (B) Comparison of amino acids in the putative leader sequence of the human and mouse TSH splice variant, indicating species differences at amino acid positions three and four.
Discussion
We consider these findings to have significant implications for understanding immune-endocrine interactions in a number of ways. The preferential expression of the TSH splice variant in PBL and the thyroid indicates that immune system-derived TSH is significantly different from native TSH. Based on our studies in mice, which demonstrated that TSH-producing myeloid cells migrate to the thyroid where they secrete TSH (Klein and Wang, 2004), the likely source of intrathyroidal TSH in the present study is hematopoietic cells that have trafficked to the thyroid, although we could not formally determine this using the system employed here. The reason for the lack of TSH expression in BM is not evident. One possibility is that the number of cells producing the TSH splice variant in the BM fluctuates according to the need for those cells in the circulation. Therefore, some human BM cells yet may be a source of the TSH splice variant. Additional studies will be required to address this.
These findings also suggest that a role of immune system-derived TSH splice variant may be to intrathyroidally microregulate thyroid hormone output. This would define a heretofore unknown role for the immune system at the organismic level. We have hypothesized that the function of the immune system-derived TSH splice variant polypeptide may be to block or augment the action of pituitary-derived native TSH (Klein, 2006; Klein and Wang, 2004; Vincent et al., 2009). Given that the immune system is especially well-suited to determine the host’s energy and metabolic needs in the face of an ongoing infection or other types of immunological stress, the net effect would be to conserve or re-engage energy-generating processes during and following the immune response.
It is notable that TSH gene expression paralleled that observed for the TSH splice variant, with both being expressed in the pituitary, thyroid, and PBL, but not in the BM. Because gene expression of the splice variant form of TSH (but not native form of TSH) was detected in the thyroid and PBL, these findings suggest, though they do not formally demonstrate, that the TSH splice variant polypeptide may pair with TSH to form a heterodimer. This could occur through the 18-amino acid ‘seatbelt’ region (CNTDNSDCIHEAVRTNYC) (Szkudlinski et al., 2002), which is present in exon 3 of the human TSH splice variant. However, due to a lack of amino acids coded for by exon 2, the human TSH splice variant would lack the CAGYC peptide segment that is used to dimerize with TSH (Hayashizaki et al., 1989). Similarly, the absence of exon 2-encoded amino acids would reduce the overall glycosylation pattern of the TSH splice variant molecule, thus potentially limiting its interaction with TSH (Szkudlinski et al., 2002). Clearly, more work will be needed to determine if the splice variant form of TSH is capable of complexing with TSH.
Finally, These findings may have implications for understanding human autoimmune thyroid disease from a new perspective, particularly if intratyroidal production of the TSH splice variant protein leads to enhanced immunogenicity due to unique physiochemical properties resulting from misfolding, anomalous dimerization, or high output of the splice variant in the thyroid.
2. Methods
4.1 Reverse transcriptase PCR (RT-PCR) and quantitative realtime PCR (qRT-PCR)
Highly pure RNA from human pituitary, bone marrow, peripheral blood leukocytes (PBL), and thyroid tissues were purchased from BioChain Institute (Hayward, CA) from tissues that were obtained with informed consent. Primers used were:
forward TSH native: 5′-AGCATGACTGCTCTCTTTCT-3′
forward TSH novel: 5′-ATTATGCTCTCTTTTCTGTTCTTT-3′
reverse TSH native and novel: 5′-AACCAAATTGCAAATTATATCACTA-3′.
forward TSH: 5′-ATGGATTACTACAGAAAATATGC-3′
reverse TSH: 5′-AGATTTTGTATAATAACAAGTACT-3′
Primers were purchased from IDT Technologies (Coralville; IA). cDNA were made from RNA using an iScript cDNA Synthesis Kit (Bio-Rad; Hercules, CA) with a program of 5 minutes at 25°C, 30 minutes at 42°C, and 5 minutes at 85°C. An iScript kit with SYBR Green (Bio-Rad) was used for qRT-PCR using 20 ng cDNA; a blank sample with RNase-free water was used for primer controls. Amplification was done in 96-well thin-wall plates sealed with optical quality film in a Mini-Opticon (Bio-Rad) using a program of 45 cycles of 95°C for 10s and 55°C for 30s for data collection. A melt curve was performed using a protocol of 1 min at 95°C, 1 min at 55°C, and increasing the temperature in 0.5°C increments for 80 cycles of 10s each. Gene expression values were normalized to 18s values for respective tissue RNAs according to the 2−Ct method of Livak and Schmittgen (Livak and Schmittgen, 2001) using a Gene Expression Macro Version 1.1 program (Bio-Rad). PCR-amplified products were analyzed by electrophoresis through a 1.2% agarose gel run at 60 V for 90 min followed by 10 min stain with 0.3 mg/ml ethidium bromide and a 10 min water de-stain.
4.2 Sequence analysis
The human novel TSHβ sequence was subcloned into the pCR2.1 TOPO plasmid using the TOPO TA Cloning kit (Invitrogen; Carlsbad, CA). Briefly, the human novel TSHβ sequence was Taq-amplified from thyroid tissue using the following touchdown PCR program: 4 minutes at 95°C; 10 cycles of 95°C for 30 seconds, 65°C and -1/cycle for 60 seconds, and 72°C for 90 seconds; 20 cycles of 95°C for 30 seconds, 50°C for 60 seconds, and 72°C for 90 seconds; and a final 7 minute elongation step at 72°C. 2 μl of the resulting PCR product was included in the TOPO reaction and incubated at room temperature for 30 minutes prior to transformation of the TOP10 bacteria. Ampicillin-resistant clones were selected and plasmid DNA was isolated using the QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA). Positive clones were identified by restriction digest with EcoRI. Sequences were obtained from SeqWright (Houston, TX) using the M13R primer. Sequence analysis was performed using FinchTV v1.3.1s software and an NCBI BLAST search.
Acknowledgments
This work was supported in part by NIH grants DK035566 and DE015355. The authors are grateful to Dina Montufar-Solis for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Hayashizaki Y, Hiraoka Y, Endo Y, Miyai K, Matsubara K. Thyroid-stimulating hormone (TSH) deficiency caused by a single base substitution in the CAGYC region of the beta-subunit. Embo J. 1989;8:2291–2296. doi: 10.1002/j.1460-2075.1989.tb08355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain Behav Immun. 2007;21:384–392. doi: 10.1016/j.bbi.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JR. The immune system as a regulator of thyroid hormone activity. Exp Biol Med. 2006;231:229–236. doi: 10.1177/153537020623100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JR, Wang HC. Characterization of a novel set of resident intrathyroidal bone marrow-derived hematopoietic cells: potential for immune-endocrine interactions in thyroid homeostasis. J Exp Biol. 2004;207:55–65. doi: 10.1242/jeb.00710. [DOI] [PubMed] [Google Scholar]
- Kruger TE, Blalock JE. Cellular requirements for thyrotropin enhancement of in vitro antibody production. J Immunol. 1986;137:197–200. [PubMed] [Google Scholar]
- Kruger TE, Smith LR, Harbour DV, Blalock JE. Thyrotropin: an endogenous regulator of the in vitro immune response. J Immunol. 1989;142:744–747. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−Ct Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Smith EM, Phan M, Kruger TE, Coppenhaver DH, Blalock JE. Human lymphocyte production of immunoreactive thyrotropin. Proc Natl Acad Sci U S A. 1983;80:6010–6013. doi: 10.1073/pnas.80.19.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82:473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- Vincent BH, Montufar-Solis D, Teng BB, Amendt BA, Schaefer J, Klein JR. Bone marrow cells produce a novel TSH splice variant that is upregulated in the thyroid following systemic virus infection. Genes Immun. 2009;10:18–26. doi: 10.1038/gene.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Klein JR. Thymus-neuroendocrine interactions in extrathymic T cell development. Science. 1994;265:1860–1862. doi: 10.1126/science.8091211. [DOI] [PubMed] [Google Scholar]
- Wang J, Whetsell M, Klein JR. Local hormone networks and intestinal T cell homeostasis. Science. 1997;275:1937–1939. doi: 10.1126/science.275.5308.1937. [DOI] [PubMed] [Google Scholar]
- Wang SH, Koenig RJ. A locally secreted thyrotropin variant may regulate thyroid function in thyroid inflammatory disorders. Thyroid. 2009;19:5–6. doi: 10.1089/thy.2008.1564. [DOI] [PubMed] [Google Scholar]