Abstract
Estrogen receptor-α (ERα) plays a critical role in male reproductive tract development and fertility. To determine whether estrogen-dependent and -independent ERα mechanisms are involved in male fertility, we examined male estrogen nonresponsive ERα knock-in mice. These animals have a point mutation (G525L) in the ligand-binding domain of ERα that significantly reduces interaction with, and response to, endogenous estrogens but does not affect growth factor activation of ligand-independent ERα pathways. Surprisingly, we found that ligand-independent ERα signaling is essential for concentrating epididymal sperm via regulation of efferent ductule fluid reabsorption. In contrast, estrogen-dependent ERα signaling is required for germ cell viability, most likely through support of Sertoli cell function. By treating estrogen nonresponsive ERα knock-in (ENERKI) mice with the ERα selective synthetic agonist propyl pyrazole triol, which is able to bind and activate G525L ERα in vivo, we discovered male fertility required neonatal estrogen-mediated ERα signaling. Thus, our work indicates both estrogen-dependent and -independent pathways play separable roles in male murine reproductive tract development and that the role of ERα in human infertility should be examined more closely.
Estrogen-dependent ERα signaling is required for germ cell viability, while estrogen-independent signaling is essential for concentrating epididymal sperm via regulation of efferent ductule fluid reabsorption.
Nuclear receptors (NRs) are transcription factors that are involved in a variety of biological processes including embryonic development, differentiation, reproduction, metabolism, and cell death (1,2). Estrogen receptors (ERs)-α and -β are closely related NRs with different tissue expression patterns and behaviors. Although ERα has traditionally been considered an important regulator of female development, its crucial role in the male reproductive tract was recently uncovered. Characterization of ERα knockout (αERKO) mice led to the surprising discovery that ERα is crucial for normal male mouse development and fertility (3). ERβ knockout males, on the other hand, are fertile with no apparent reproductive tract abnormalities (4). ERα is predominantly expressed in the efferent ductules and Leydig cells of the male reproductive tract (5,6). Efferent ductules reabsorb nearly 90% of the testicular fluid as sperm travel from the testis through the efferent ductules and into the epididymis (5,7). The benefits of concentrated epididymal sperm include improved gamete survival and maturation during storage, and increased genetic variation after ejaculation (8). αERKO efferent ductules are unable to reabsorb testicular fluid, due to decreased expression of sodium/hydrogen exchanger-3 (NHE3), carbonic anhydrase II (CAII), aquaporin-1 (AQP1), and the endocytotic apparatus (7,9,10). NHE3 is directly involved in reabsorption of 70% of the testicular fluid by actively pumping Na+ toward the basement membrane and H+ into the lumen (9,11). Water is absorbed as it moves passively toward the ion concentration gradient (9,11). CAII and AQP1 supply H+ for NHE3 and allow passive water movement via their pores, respectively. The unabsorbed testicular fluid in the αERKO mice causes atrophy of the seminiferous tubules due to fluid back pressure, dilution of sperm, and infertility (3,7). These studies have provided a great deal of information about the role of ERα in male mouse development. However, because the ERα gene is inactivated in αERKO mice, it has been difficult to define the roles of estrogen and growth factors, which activate two different ERα transcriptional activation domains.
In classical estrogen-mediated signaling, estrogen binds to the ERα C-terminal activation function 2 (AF)-2 domain (1). This induces conformational changes that allow ERα to dimerize and bind estrogen response elements upstream of target genes (12). ERα can also be activated in the absence of estrogen by growth factors like IGF-I or epidermal growth factor (12,13). During ligand-independent signaling, IGF-I or epidermal growth factor signaling stimulates kinases to phosphorylate the N-terminal AF-1 domain of ER (12,13). The transcriptional activity of target genes is dependent on coactivator and basal transcription factor recruitment (13,14). We previously generated a knock-in mouse model with a mutation in ERα [glycine 525 to leucine (G525L)] that permits exogenous regulation of its estrogen-mediated signaling pathways bur does not affect ligand-independent signaling (15). In these estrogen nonresponsive ERα knock-in (ENERKI) mice, the ligand-binding domain G525L mutation significantly reduces ERα interaction with and response to endogenous estrogens (15).
The goal of this study was to use ENERKI mice to distinguish between estrogen-dependent and -independent ERα mechanisms in the male reproductive tract and determine whether estrogen and/or growth factors contribute to infertility. In addition, because the ERα selective agonist propyl pyrazole triol (PPT) is able to bind and activate the mutant G525L ERα pathways in ENERKI mice (15), another objective was to investigate whether estrogen ERα signaling is crucial at a specific time during male reproductive tract development. This is the first report of in vivo modulation of estrogen-mediated ERα pathways in male mice.
Materials and Methods
Animals
The generation of ENERKI mice has been previously described (15). All procedures involving animals were conducted in accordance with the policies of the Institutional Animal Care and Use Committee at the University of Chicago. Animals were group housed in a barrier facility with 14-h light, 10-h dark cycles and provided food and water ad libitum. Mice were maintained on a regular or soy-free diet (2918 and 2919, respectively; Charles River Laboratories, Wilmington, MA). All results reported here are from animals on a regular diet, unless indicated otherwise.
Sample collection and histology
Male mice were euthanized by CO2 inhalation, followed by cervical dislocation, at 6, 12, 20, or 40 wk of age. The testis, efferent ducts, epididymis, and seminal vesicle were dissected out, weighed, and fixed overnight in 10% neutral buffered formalin. Tissues were embedded in paraffin and sectioned at the Human Tissue Research Center (The University of Chicago, Chicago, IL). Standard hematoxylin and eosin staining or immunohistochemistry (IHC) was then performed. Periodic acid-Schiff (PAS) staining was performed with a kit according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO).
IHC
IHC was performed as previously described (15). For NHE3 IHC, antigen retrieval was performed by incubating slides for 20 min in a steamer at 96 C. Slides were also incubated in 5% goat serum for 20 min at room temperature after peroxidase blocking. The rabbit polyclonal NHE3 antibody (Chemicon International, Temecula, CA) was applied at a 1:400 dilution for 1 h at room temperature. NHE3 efferent ductule staining intensity was quantified by an experienced pathologist (Maria Tretiakova, Human Tissue Resource Center, The University of Chicago, Chicago, IL) by scoring positively stained cells with the automated cellular imaging system (ACIS; ChromaVision, San Juan Capistrano, CA), as previously described (16,17). Briefly, stained sections were scanned and acquired using the ACIS imaging/microscopy system. Positive staining was measured by setting color-specific thresholds to determine brown (positively stained) and blue (negative) staining in the outlined areas of interest and calculate the ratio (percent) of positively stained efferent ductules to negatively stained efferent ductules. The integrated optical density per 10 μm2 of tissue is directly proportional to the concentration of the molecule recognized by the stain. For each section, at least 10 representative regions were analyzed.
Quantitative real-time PCR
Total RNA was prepared with the PicoPure RNA isolation kit according to the manufacturer’s directions (Molecular Devices, Sunnyvale, CA). One hundred nanograms of RNA were treated with deoxyribonuclease I (Invitrogen, Carlsbad, CA) before being reverse transcribed using the Superscript VILO cDNA synthesis kit (Invitrogen). Random hexamers was used to prime the cDNA synthesis reaction. 20 ng of the resultant cDNA products were used in a 20-μl quantitative real-time PCR (qRT-PCR) reaction using the QuantiTect SYBR Green PCR kit (QIAGEN, Valencia, CA). qRT-PCR was performed with QuantiTect primers for ribosomal protein L13A (RPL13A) and NHE3 (also called solute carrier family, member 3; QIAGEN). The reactions were carried out using the ABI StepOne real-time PCR system (Applied Biosystems, Foster City, CA) for 50 cycles (95 C for 15 sec, 55 C for 30 sec, 72 C for 40 sec) after an initial 15-min incubation at 95 C. RNA levels were determined for NHE3 and RPL13A with the ΔΔCT method using total mouse RNA as the reference. NHE3 expression was then normalized to the reference gene RPL13A and the relative expression was determined by normalizing to the wild-type control. The reported results represent the average ± sd of triplicate samples and are representative of two independent experiments with two to three mice per group.
Terminal deoxynucleotidyl transferase biotin-deoxyuridine 5-triphosphate nick end labeling (TUNEL) analysis
TUNEL staining was performed with the ApopTag peroxidase in situ apoptosis detection kit according to the manufacturer’s directions (Chemicon). The total number of TUNEL-positive cells in each testis section was determined by manual counting.
Serum gonadotropin and steroid hormone assays
Blood was collected by cardiac puncture from 12-wk-old euthanized animals. Coagulation was prevented by treating blood with heparin. Blood was centrifuged and plasma frozen at −80 C for later use. Serum RIAs were performed by Brigitte Mann (Northwestern University, Chicago, IL). Serum LH and FSH RIAs were performed using iodinated standards (rLH-RP3, rFSH-RP2) and antisera (anti-rLH-S11, anti-rFSH-S11) from the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD). Serum testosterone RIA was performed with the double-antibody kit (MP Biomedicals, Solon, OH) according to the manufacturer’s directions.
Continuous mating studies
Seven-, 12-, 20-, and 40-wk-old male mice were individually housed and two 6- to 8-wk-old CD-1 female mice (Charles River Laboratories) were placed in each cage. The first visibly pregnant female in each cage was removed and placed in a separate cage to give birth so the cages would not become overcrowded. The second female remained in the cage with the male for 2 months. The number of litters, pups per litter, and date of each birth were recorded. A male was considered fertile if he impregnated one female. At the end of the 2-month mating period, the remaining animals were euthanized.
Injections
Mice were maintained on a soy-free diet (2919; Charles River Laboratories) for all injection experiments. Compounds were prepared in a vehicle of 2% ethanol, 10% Cremophor EL (Sigma-Aldrich), and 88% 1× PBS at defined concentrations so that the treatment volume was 0.01 ml/g body weight. Male mice were sc injected with 0–100,000 μg/kg of PPT (a kind gift from Dr. John Katzenellenbogen, University of Illinois, Urbana, IL). Seven to 10 mice were used for each treatment group. In the first group, mice were injected once a week from 4 to 20 wk of age. In the second group, mice were injected every fourth day from 4 d to 4 wk of age and then once a week from 4 to 20 wk of age. Twelve-wk-old males were mated for 2 months as described above and euthanized at 20 wk of age.
Statistical analysis
All reported values represent the mean ± sd. Differences were considered significant at P < 0.05 using factoral ANOVA with appropriate post hoc tests or the Fisher exact test (SigmaStat 3.5; Systat Software, San Jose, CA).
Results
ENERKI efferent ductules exhibit normal histology
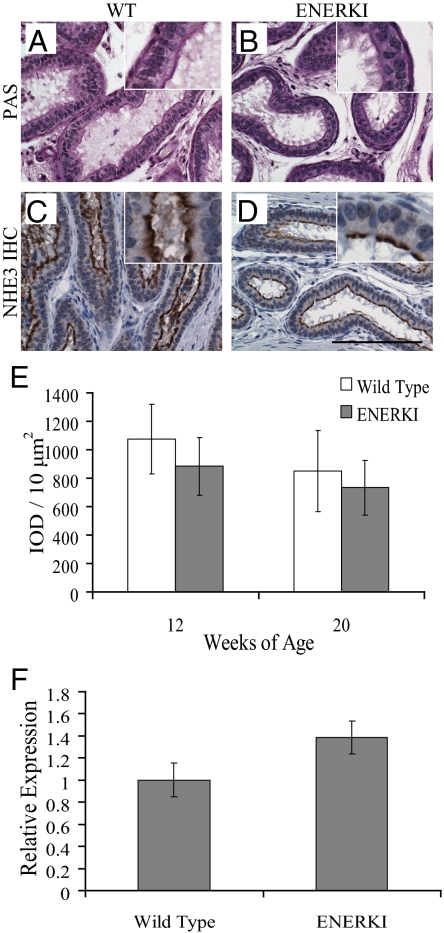
ENERKI efferent ductule histology was examined because αERKO males are infertile due to impaired efferent ductule fluid reabsorption (3,7). αERKO efferent ductules are dilated due to excess unabsorbed testicular fluid and have a decreased number of endocytotic vesicles and microvilli (7,10,18). In fact, αERKO efferent ductules are abnormal at birth; the epithelium lacks the microvilli that should start appearing by d 5, and NHE3 is never expressed (Hess, R., personal communication). Surprisingly, there were no remarkable histological differences between wild-type and ENERKI efferent ductules at 12 and 20 wk of age (Fig. 1, A and B). Efferent ductules of both genotypes were lined with a columnar epithelium consisting of ciliated and nonciliated cells. The nonciliated cells contained a well-developed brush border, endocytotic vesicles, and PAS+ lysosomes, which are indicative of functional endocytosis apparatus (Fig. 1, A and B). ENERKI efferent ductule histology was therefore normal, unlike the ducts of the αERKO mice.
Figure 1.
ENERKI efferent ductules exhibit normal histology. Representative efferent ductule tissue PAS staining and IHC for NHE3 from 12- or 20-wk-old wild-type (WT) (A and C) and ENERKI (B and D) mice. Efferent ductules of all genotypes were lined with a columnar epithelium consisting of ciliated and nonciliated cells (A and B). Similar levels of NHE3 were found along the brush border of nonciliated cells in both genotypes (C and D). Quantification of immunohistochemical staining confirmed wild-type and ENERKI NHE3 levels were not statistically different (E). The reported results represent the average integrated optical density (IOD) per 10 μm2 of tissue (which is directly proportional to the concentration of the molecule recognized by the stain) ± sd of three to seven samples per genotype. qRT-PCR results showing relative expression levels of wild-type and ENERKI efferent ductule NHE3 transcript levels (F). NHE3 expression levels of 20-wk-old mice were normalized to the wild-type control. The reported results represent the average ± sd of triplicate samples. ENERKI NHE3 transcript levels are not significantly different from wild-type levels. Bar, 100 μm.
To confirm that ENERKI efferent ductules function normally, several proteins involved in testicular fluid reabsorption were measured by immunohistochemistry; levels of all these proteins were decreased in the αERKO ducts (9). Because the NHE3 promoter region contains an estrogen response element site, ERα transcriptional regulation of NHE3 is expected (9). Growth factor signaling may activate efferent ductule NHE3 by several different mechanisms, including the phosphatidylinositol 3-kinase-AKT pathway, as shown in vitro (19). NHE3 immunohistochemical staining was abundant along the brush border of nonciliated cells in wild-type and ENERKI efferent ductules of 12- and 20-wk-old animals (Fig. 1, C and D). Quantification of immunohistochemical staining with an ACIS confirmed wild-type and ENERKI NHE3 levels were not statistically different (Fig. 1E). In addition, qRT-PCR showed NHE3 transcript levels from the efferent ductules of 20-wk-old wild-type and ENERKI animals were not significantly different (Fig. 1F). Notably, efferent ductule morphology and NHE3 expression levels were also the same between wild-type and ENERKI animals at 40 wk of age (data not shown). Immunohistochemical staining for AQP1 and CAII was present along the brush border of the efferent ductule epithelium in both genotypes, and CAII was also present in the cytoplasm (supplemental Fig. 1, A–D, published as supplemental data on The Endocrine Society’s Journals Online web site at http:// endo.endojournals.org). Because the decreased levels of CAII and AQP1 in αERKO efferent ductules are thought to be secondary effects due to microvilli loss (9), it is not surprising ENERKI CAII and AQP1 levels were normal. Clusterin (also called sulfated glycoprotein-2), a protein that dissociates from spermatozoa and is endocytosed by nonciliated efferent ductule cells (10), was also equally expressed among both genotypes by IHC, indicating ENERKI endocytotic apparatus was functional (supplemental Fig. 1, E and F). These results confirm that ENERKI efferent ductules function normally and that ligand-independent ERα signaling is crucial in regulating efferent ductule function.
Heterogeneous testicular seminiferous tubule degradation occurs in older ENERKI males
We hypothesized that ENERKI mice would have normal testes because they have functional efferent ductules. In αERKO males, unabsorbed testicular fluid builds up in the efferent ductules and accumulates in the testis, causing an increase in testicular weight at 11 wk of age (7). This extra fluid is hypothesized to cause atrophy of the seminiferous tubules and dilution of sperm by blocking blood flow, which results in a significant decrease in testis weight by 26 wk of age (3,7). In fact, young αERKO mice exhibit testis degeneration as early as 3–6 wk of age and complete atrophy by 21 wk of age (3).
Testes were collected from 6-, 12-, 20-, and 40-wk-old wild-type and ENERKI animals and analyzed for morphological and histological indication of function and development. Testis weights were similar between 6- and 12-wk-old wild-type and ENERKI animals (supplemental Table 1), providing further evidence that ENERKI efferent ductules function normally. There were no remarkable histological differences among the testes of 12-wk-old animals (supplemental Fig. 2, A and B). ENERKI testicular sperm counts were also equivalent to wild-type levels at 6 and 12 wk of age (supplemental Table 2).
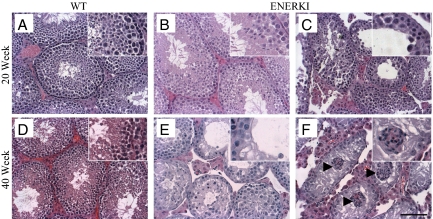
Surprisingly, even though ENERKI animals of all ages had normal efferent ductules and 6–12 wk mice had normal testes, older ENERKI males exhibited an altered testicular phenotype. Testes from 20- and 40-wk-old wild-type mice had healthy seminiferous tubules supporting normal spermatogenesis (Fig. 2, A and D). Testis weights from 20- and 40-wk-old ENERKI males were significantly smaller than those of wild-type animals (supplemental Table 1), and at 20 wk of age, 45% of testes from ENERKI males had a disorganized seminiferous epithelium with diffuse atrophy and loss of postmeiotic round and elongating spermatids in the seminiferous tubules (Fig. 2C). Sertoli cell vacuolization and retraction of the Sertoli cell cytoplasm caused an increased lumen diameter in these tubules (Fig. 2C). The phenotype was heterogeneous, with some seminiferous tubules appearing normal (Fig. 2B) and others being affected (Fig. 2C). By 40 wk of age, 75% of ENERKI males had a severe disruption of the seminiferous epithelium (Fig. 2E). Some tubules also showed unusual clusters of Sertoli cells in the lumen of the tubules (Fig. 2F). By 20 and 40 wk of age, ENERKI testicular sperm numbers were decreased by 45 and 87% from wild-type levels, respectively (supplemental Table 2). Positive immunohistochemical staining for the 70-kDa heat-shock protein, a chaperone protein required for germ cell meiosis and thus present in pachytene spermatocytes (20), showed spermatogenic cells were present only within some of the ENERKI disordered seminiferous tubules (supplemental Fig. 2, C and D). This heterogeneous and progressive testicular phenotype resembled that of young αERKO mice, which exhibited testis degeneration as early as 3–6 wk of age and complete atrophy by 21 wk of age (3), indicating that the loss of estrogen-dependent ERα signaling in ENERKI animals affected testicular function. Therefore, a separate defect, in addition to impaired efferent ductule fluid reabsorption in the αERKO animals, contributes to both the αERKO and ENERKI testicular phenotypes.
Figure 2.
Heterogeneous testicular abnormalities occur in the seminiferous epithelium of 20- and 40-wk-old ENERKI mice. Representative testicular hematoxylin-and-eosin staining from 20- or 40-wk-old wild-type (WT) (A and D) and ENERKI (B, C, E, and F) mice. Wild-type testicular tissues from 20- (A) and 40-wk-old (D) mice had healthy seminiferous tubules supporting normal spermatogenesis. Some 20-wk-old ENERKI seminiferous tubules were normal (B), whereas others had severe abnormalities that included disorganization of the seminiferous epithelium, loss of sperm, Sertoli cell vacuolization (asterisks), and retraction of the Sertoli cell cytoplasm leading to an increased lumen diameter (C). Some 40-wk-old ENERKI testes exhibited these same abnormalities (E), whereas in others the injury progressed to reveal a Sertoli cell-only phenotype, in which the seminiferous epithelium lacked the presence of any germ cells (F). Some tubules also showed unusual clusters of Sertoli cells in the lumen of the tubules (arrowheads) (F). Bar, 100 μm.
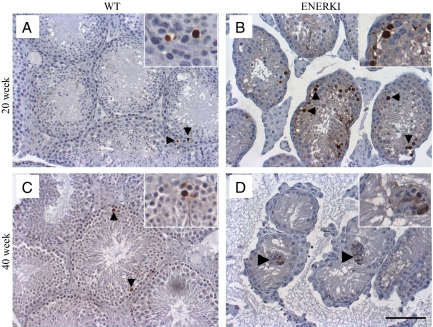
TUNEL staining was performed to determine the mechanism of germ cell loss in the ENERKI animals. During spermatogenesis, male germ cells undergo tightly regulated phases of development in the seminiferous tubules of the testis (21,22). Spermatogonial stem cells undergo mitotic division to differentiate into spermatocytes, which subsequently go through meiosis to differentiate into haploid round spermatids. Spermatids then elongate and are transported to the epididymis. A low incidence of spontaneous apoptotic spermatogonia and spermatocytes was observed in the testis of 20- and 40-wk-old wild-type animals (Fig. 3, A and C). Significantly higher numbers of TUNEL-positive cells occurred in the seminiferous tubules of 20-wk-old ENERKI testes compared with wild-type tissues (Fig. 3, A and B). The morphology of the ENERKI TUNEL-positive cells indicated they were predominantly apoptotic meiotic spermatocytes (Fig. 3B). These results demonstrated that estrogen-dependent ERα signaling is important for the progression and/or viability of these germ cells. There were no differences between TUNEL staining in 40-wk-old wild-type and ENERKI animals because most ENERKI seminiferous tubules were devoid of germ cells at this point (Fig. 3, C and D). However, a few ENERKI Sertoli cells were TUNEL positive within the clusters of the Sertoli cells found in the lumen (Fig. 3D).
Figure 3.
ENERKI mice exhibit increased testis TUNEL-positive germ cells compared with wild-type mice. Representative testicular TUNEL analysis from 20- and 40-wk-old wild-type (WT) (A and C) and ENERKI (B and D) mice. TUNEL analysis revealed there were significantly increased numbers apoptotic germ cells (black arrowheads) in 20-wk-old ENERKI testis sections (B) compared with wild-type sections (A) (P < 0.04). TUNEL-positive ENERKI germ cells were predominantly meiotic spermatocytes. Forty-week-old TUNEL germ cell staining was not significantly different between wild-type (C) and ENERKI (D) animals because most ENERKI tubules did not contain germ cells at this point. A few ENERKI Sertoli cells were TUNEL positive within the clusters of the Sertoli cells found in the lumen (large arrowhead) but not among the Sertoli cells that still remained adhered to the basement membrane (D). Bar, 100 μm.
ENERKI epididymal tissues have decreased sperm and proteinaceous debris
Epididymal tissues from wild-type and ENERKI animals were also examined. Histological analysis revealed that the caput (head) and cauda (tail) of the epididymis from 12-wk-old mice of both genotypes were similar (supplemental Fig. 3, A and B). ENERKI epididymal sperm counts were also equivalent to wild-type levels at 6 and 12 wk of age (supplemental Table 2). However, 55 and 71% of caput epididymides and 55 and 50% of cauda epididymides, from 20- and 40-wk-old ENERKI mice, respectively, had a reduction in the number of visible sperm (supplemental Fig. 3, C–F). Many ENERKI epididymal lumens were completely devoid of sperm. Thirty-six and 50% of 20- and 40-wk-old ENERKI cauda epididymides, respectively, also contained viscous proteinaceous debris (supplemental Fig. 3F), which we hypothesize arise from sperm undergoing degradation. By 20 and 40 wk of age, ENERKI sperm numbers were significantly decreased in the caput epididymis and cauda epididymis, respectively (supplemental Table 2). The epididymal phenotype is most likely due to the testicular loss of sperm. In comparison, αERKO cauda epididymal sperm counts were reduced by 83% at 15 wk of age (3). Surprisingly, ENERKI sperm motility was not affected (supplemental Table 2). Sperm motility of αERKO mice were significantly decreased by 15 wk of age (3). However, ENERKI cauda epididymal sperm tended to exhibit fewer fast and straightforward movements than sperm of wild-type males (data not shown). Therefore, both estrogen-dependent and -independent ERα signaling may play a role in sperm maturation in the epididymis.
Serum gonadotropin and steroid hormone assays
ENERKI serum hormone levels were measured to determine whether ENERKI males, like αERKO males (3), exhibit dysregulation of the hypothalamic-pituitary axis. In αERKO males, the lack of estrogen-dependent ERα signaling in the testis leads to a lack of estrogen-negative feedback in the brain, leading to slightly increased LH and significantly elevated testosterone levels. ENERKI male serum LH levels were slightly higher and testosterone levels were significantly greater than wild-type levels, respectively (Table 1). The 4-fold higher ENERKI testosterone levels over wild-type levels (Table 1) most likely caused the increased ENERKI seminal vesicle weights (supplemental Table 1) because the seminal vesicle is an androgen-sensitive organ. In support of this hypothesis, histological examination revealed the seminal vesicles were dilated with excess proteinaceous secretions (data not shown). FSH levels were not significantly different (Table 1).
Table 1.
Male serum hormone levels
| 12-wk mice | LH (ng/ml) | FSH (ng/ml) | T (ng/ml) |
|---|---|---|---|
| WT (n = 5–13) | 0.35 ± 0.07 | 13.45 ± 3.55 | 1.63 ± 0.74 |
| ENERKI (n = 10–24) | 0.67 ± 0.10 | 12.72 ± 2.57 | 6.42 ± 1.10a |
Data expressed as mean (±sem). T, Testosterone.
ENERKI value significantly different from WT value (P < 0.01).
ENERKI males are subfertile
Continuous mating studies were performed to determine whether ENERKI males are fertile. Nearly 100% of wild-type animals were fertile at all ages examined (Table 2). Wild-type and heterozygous animals exhibited similar fertility rates (data not shown), indicating one intact ERα allele is sufficient to induce normal reproductive tissue growth. ENERKI males were subfertile, with 0–25% siring litters at 7, 12, or 20 wk of age (Table 2). ENERKI males were never fertile at 40 wk of age (Table 2). There were no differences in the numbers of pups per litter (Table 2). However, the number of days until the birth of the first litter was significantly higher than wild-type levels in the 7-wk-old group of ENERKI males (Table 2). The one fertile 12-wk-old ENERKI male also took a longer time to sire a litter than wild-type animals, but this difference was not significant due to the small sample size (Table 2). ENERKI males were therefore subfertile, unlike the infertile αERKO animals (3), indicating that ligand-independent pathways were able to partially rescue the αERKO fertility defect by restoring normal efferent ductule function.
Table 2.
Continuous mating studies
| Mice | Fertile, % | Pups per litter | Days to first litter |
|---|---|---|---|
| 7 wk | |||
| WT (n = 12) | 100 ± 0 | 10.7 ± 0.6 | 24.8 ± 0.8 |
| ENERKI (n = 12) | 25 ± 13a | 12.2 ± 1.4 | 38.2 ± 5.9a |
| 12 wk | |||
| WT (n = 12) | 100 ± 0 | 11.1 ± 0.6 | 26.0 ± 1.3 |
| ENERKI (n = 11) | 9 ± 9a | 3.0 | 38.0 |
| 20 wk | |||
| WT (n = 12) | 100 ± 0 | 11.0 ± 0.8 | 26.6 ± 1.8 |
| ENERKI (n = 13) | 23 ± 12a | 11.6 ± 0.8 | 30.4 ± 2.7 |
| 40 wk | |||
| WT (n = 11) | 83 ± 12 | 12.6 ± 0.6 | 28.2 ± 2.0 |
| ENERKI (n = 11) | 0 ± 0a | N/A | N/A |
Data expressed as mean (±sem).
ENERKI value significantly different from WT value (P < 0.01). There is no significant difference between ENERKI values.
ENERKI fertility is restored through neonatal PPT supplementation
ENERKI males were treated with PPT in an attempt to restore normal reproductive tract development and fertility because previous studies demonstrated a uterotrophic response could be stimulated in ENERKI females with PPT treatments to activate G525L ERα signaling in vivo (15). PPT treatments were initiated at 4 d or at the beginning of puberty at 4 wk of age and continued to 20 wk of age. When 10,000 μg/kg PPT treatments were initiated in ENERKI mice at 4 wk of age, animals remained subfertile, just like vehicle controls (Table 3). However, when 10,000 μg/kg PPT treatments were begun at 4 days of age, fertility was restored, with 71% siring litters (Table 3). These data indicate neonatal PPT treatments were needed to induce fertility in ENERKI males. Interestingly, 75% of ENERKI males were fertile when they were injected with 10,000 μg/kg PPT every fourth day from 4 d to 4 wk of age and weekly thereafter until the conclusion of puberty, around 7 wk of age (Table 3). These surprising results demonstrate neonatal and pubertal estrogen-dependent ERα signaling alone may be sufficient to induce normal adult fertility rates. In addition, there were no remarkable histological differences between 20-wk-old wild-type and neonatal PPT-treated male reproductive tracts (data not shown).
Table 3.
PPT-treated male continuous mating studies
| 12-wk mice | Treatment | Fertile, % | Pups per litter, n | Days to first litter |
|---|---|---|---|---|
| ENERKI (n = 7) | Vehicle every fourth day from 0 to 4 wk, weekly from 4 to 20 wk | 14 ± 14 | 10.5 ± 1.5 | 25.0 ± 5.0 |
| ENERKI (n = 9) | 10,000 μg/kg PPT weekly from 4 to 20 wk | 33 ± 17 | 11.3 ± 0.7 | 28.7 ± 4.4 |
| ENERKI (n = 7) | 10,000 μg/kg PPT every fourth day from 0 to 4 wk, weekly from 4 to 20 wk | 71 ± 18 | 10.8 ± 0.9 | 26.4 ± 1.9 |
| ENERKI (n = 8) | 10,000 μg/kg PPT every fourth day from 0 to 4 wk, weekly from 4 to 7 wk | 75 ± 16 | 8.0 ± 1.3 | 21.9 ± 0.7 |
Data expressed as mean (±sem).
Discussion
In the current study, we examined the male reproductive tract of ENERKI mice, which have a G525L point mutation in the ligand-binding domain of ERα that interferes with estrogen binding. Notably, ligand-independent ERα signaling is important for concentrating epididymal sperm via regulation of efferent ductule fluid reabsorption, whereas estrogen-mediated ERα signaling is essential for germ cell progression and/or viability, most likely through support of Sertoli cell function, as described below. Ligand-independent ERα activation has been hypothesized to be important in tissues in which estrogen levels are low, such as the male reproductive tract or in nonreproductive tissues (23,24); our results confirm this hypothesis. Our finding that ligand-independent ERα signaling is crucial for male reproductive tract development is supported by the suggestion that ERα evolved from ancestors that were unable to bind ligands (25,26). Because the ERα selective agonist PPT is able to activate G525L ERα pathways in vivo (15), we also treated ENERKI animals with PPT during different times of development and found that neonatal estrogen-dependent ERα signaling is crucial for male fertility.
We hypothesize that the atrophic ENERKI seminiferous tubules result from disrupted Sertoli cell support. Cross talk between germ and Sertoli cells is important in spermatogenesis because Sertoli cells provide physical support, nutrients, and differentiation factors to the developing germ cells (27,28,29). The abundant Sertoli cell vacuoles found in 20- and 40-wk-old ENERKI seminiferous tubules are prototypical and sensitive indicators of Sertoli cell dysfunction (30). In addition, the presence of aggregates of Sertoli cells in the lumen of the seminiferous tubules, with no apparent connection to the basal lamina, in 40-wk-old ENERKI animals has been previously described to be a normal feature in the testis of sterile mice. It has been proposed that prolonged Sertoli cell inactivity may lead to this effect (31,32,33). Taken together, these observations indicate that altered Sertoli cell function and resultant disruption in the supportive capacity of the seminiferous epithelium is likely the primary mechanism leading to the age-dependent testicular phenotype and subfertility in the male ENERKI mice.
Importantly, the elevated LH and testosterone levels of the ENERKI mice most likely do not contribute to their seminiferous tubule atrophy. Previous studies have shown that mice treated with the 5α-reductase inhibitor finasteride for 83 wk developed high LH and testosterone serum levels and Leydig cell hyperplasia, but their seminiferous tubule structure and spermatogenesis were not affected (34). The LH and testosterone levels of finasteride-treated males were similar to those of the ENERKI animals (34). Thus, elevated LH and testosterone levels most likely do not cause disruption of spermatogenesis. Notably, 27 and 75% of 20- and 40-wk-old ENERKI males also developed Leydig cell hyperplasia, respectively (data not shown). Leydig cell hyperplasia and increased steroidogenic enzyme gene expression and activity have all been shown to contribute to elevated αERKO testosterone levels (35,36). Further investigation is needed to determine which of these mechanisms contribute to high ENERKI testosterone levels.
Although ENERKI males were subfertile from 7 to 20 wk of age, we expected more robust fertility in the younger males because the reproductive tract histology and sperm counts of 6- and 12-wk-old ENERKI males were indistinguishable from those of wild-type animals. We hypothesize that the reduced fertility of young ENERKI males may be due to impaired sexual behaviors, similar to αERKO mating activities, which are compromised (37). The fact that it took longer for 7- and 12-wk-old ENERKI males to sire litters, compared with wild-type males, supports this idea. Future studies will determine whether estrogen-mediated ERα signaling is crucial for normal male sexual behaviors.
Our work provides strong evidence that neonatal estrogen signaling is required in the development of normal adult fertility rates. Interestingly, environmental exposure to exogenous estrogenic compounds during fetal and early childhood development has long been postulated to cause impaired sperm production and decreased adult fertility rates (38). Our studies indicate ENERKI mice will be a valuable tool in helping to determine the amount and timing of estrogen-dependent ERα signaling required for normal adult male reproductive tract development and fertility rates.
More than 10% of couples are unable to conceive, and male infertility contributes to 30–50% of these cases (39). Our data suggest that the role of ERα in human infertility should be examined more closely. Because only one human male has been reported to have an inactivating ERα mutation (40), it is unlikely that mutations leading to ERα deletion account for a significant percentage of human male infertility cases. However, the role of ERα polymorphisms in male infertility remains to be established, and we hypothesize that polymorphisms that affect either estrogen- or growth factor-mediated ERα may be crucial for male fertility. Although several ERα polymorphic sites have been identified, there are conflicting data about the role of these sites in sperm number and fertility (41).
Our work also indicates that it will be important to examine the role of other NR ligand-independent pathways in vivo. This is the first report, to our knowledge, of a knock-in mouse model that was used to define the separable mechanistic contributions of the AF-1 and AF-2 domains of a NR during development. Examination of the role of ligand-independent NR signaling in vivo may lead to an improved understanding of the regulation of both normal biological processes and the many reproductive and metabolic diseases caused by impaired NR signaling including infertility, obesity, diabetes, and cancer (1). The AF-1 domain of many NRs, including the progesterone receptor, androgen receptor, glucocorticoid receptor, retinoic acid receptor, retinoid X receptor, and peroxisome proliferator-activated receptor, can be activated in a ligand-independent manner via phosphorylation by MAPKs (42). A better understanding of ligand-independent NR signaling may lead to new therapeutic approaches for diseases caused by aberrant NR signaling.
In summary, ENERKI male animals were used to successfully separate the contributions of different ERα mechanisms during development. The phenotype of ENERKI males reveals the importance of ligand-independent and estrogen-mediated ERα signaling in regulating efferent ductule function and developing germ cells, respectively. PPT treatments demonstrate that neonatal estrogen-mediated ERα activation may be crucial for normal male reproductive tract development and fertility in adult animals. ENERKI animals should prove to be a valuable model for studying the relative contributions of estrogen-dependent and -independent ERα pathways in vivo.
Supplementary Material
Acknowledgments
We are grateful to Kay Carnes (University of Illinois, Urbana-Champaign, Illinois), Rex Hess (University of Illinois), Carla Kim (Children’s Hospital Boston, Boston, Massachusetts), Jon Levine (Northwestern University, Chicago, Illinois), and Kay Macleod (The University of Chicago, Chicago, Illinois) for helpful discussions and advice. We appreciate the excellent technical skills of Brigitte Mann (Northwestern University) and Maria Tretiakova (The University of Chicago).
Footnotes
This work was supported in part by Department of Defense Breast Cancer Research Program predoctoral traineeship Grant W81XWH-04-1-0347 (to K.W.S.) and National Cancer Institute Grant CA89089 (to G.L.G.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 5, 2009
Abbreviations: ACIS, Automated cellular imaging system; AF, activation function; AQP1, aquaporin-1; CAII, carbonic anhydrase II; ENERKI, estrogen nonresponsive ERα knock-in; ER, estrogen receptor; αERKO, ERα knockout; G525L, glycine 525 to leucine; IHC, immunohistochemistry; NHE3, sodium/hydrogen exchanger-3; NR, nuclear receptor; PAS, periodic acid-Schiff; PPT, propyl pyrazole triol; qRT-PCR, quantitative real-time PCR; RPL13A, ribosomal protein L13A; TUNEL, terminal deoxynucleotidyl transferase biotin-deoxyuridine 5-triphosphate nick end labeling.
References
- Gronemeyer H, Gustafsson JA, Laudet V 2004 Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 3:950–964 [DOI] [PubMed] [Google Scholar]
- Lonard DM, O'Malley BW 2006 The expanding cosmos of nuclear receptor coactivators. Cell 125:411–414 [DOI] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS 1996 Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137:4796–4805 [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA 2003 Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol 1:52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA 2002 Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23:870–881 [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB 1997 A role for oestrogens in the male reproductive system. Nature 390:509–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B, Hermo L 1988 Efferent ducts, epididymis, and vas deferens structure, function, and their regulation. In: Knobil E, Neill J, eds. The physiology of reproduction. New York: Raven Press; 99–1080 [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA 2001 Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci USA 98:14132–14137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M, Bouma J, Nie R, Zhou Q, Carnes K, Lubahn DB, Hess RA 2001 Morphological analysis of endocytosis in efferent ductules of estrogen receptor-α knockout male mouse. Anat Rec 263:10–18 [DOI] [PubMed] [Google Scholar]
- Hansen LA, Clulow J, Jones RC 1999 The role of Na+-H+ exchange in fluid and solute transport in the rat efferent ducts. Exp Physiol 84:521–527 [PubMed] [Google Scholar]
- Hewitt SC, Kenneth KS 2002 Estrogen receptors: structure, mechanisms and function. Rev Endocr Metab Disord 3:193–200 [DOI] [PubMed] [Google Scholar]
- Coleman KM, Smith CL 2001 Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci 6:1379–1391 [DOI] [PubMed] [Google Scholar]
- Klinge CM 2000 Estrogen receptor interaction with co-activators and co-repressors. Steroids 65:227–251 [DOI] [PubMed] [Google Scholar]
- Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL 2008 An estrogen receptor α knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology 149:2970–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretiakova M, Turkyilmaz M, Grushko T, Kocherginsky M, Rubin C, Teh B, Yang XJ 2006 Topoisomerase IIα expression in Wilms tumors. J Clin Pathol 59:1272–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong H, Mathur PS, Greene GL 2008 Inhibition of mammary tumorigenesis in the C3(1)/SV40 mouse model by green tea. Breast Cancer Res Treat 107:359–369 [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J 2000 Morphologic changes in efferent ductules and epididymis in estrogen receptor-α knockout mice. J Androl 21:107–121 [PubMed] [Google Scholar]
- Lee-Kwon W, Johns DC, Cha B, Cavet M, Park J, Tsichlis P, Donowitz M 2001 Constitutively active phosphatidylinositol 3-kinase and AKT are sufficient to stimulate the epithelial Na+/H+ exchanger 3. J Biol Chem 276:31296–31304 [DOI] [PubMed] [Google Scholar]
- Eddy EM 1999 Role of heat shock protein HSP70–2 in spermatogenesis. Rev Reprod 4:23–30 [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N 1998 Spermatogenesis. Hum Reprod 13(Suppl 1):1–8 [DOI] [PubMed] [Google Scholar]
- Whitehead SA, Nussey SS 2001 Endocrinology: an integrated approach. London: Taylor, Francis [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, Lowik C, Maggi A 2003 In vivo imaging of transcriptionally active estrogen receptors. Nat Med 9:82–86 [DOI] [PubMed] [Google Scholar]
- Escriva H, Delaunay F, Laudet V 2000 Ligand binding and nuclear receptor evolution. Bioessays 22:717–727 [DOI] [PubMed] [Google Scholar]
- Paris M, Pettersson K, Schubert M, Bertrand S, Pongratz I, Escriva H, Laudet V 2008 An amphioxus orthologue of the estrogen receptor that does not bind estradiol: insights into estrogen receptor evolution. BMC Evol Biol 8:219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD 1995 Interactions between germ cells and Sertoli cells in the testis. Biol Reprod 52:211–216 [DOI] [PubMed] [Google Scholar]
- Petersen C, Soder O 2006 The Sertoli cell -a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res 66:153–161 [DOI] [PubMed] [Google Scholar]
- Jégou B 1993 The Sertoli-germ cell communication network in mammals. Int Rev Cytol 147:25–96 [PubMed] [Google Scholar]
- Boekelheide K, Johnson KJ, Richburg JH 2005 Sertoli cell toxicants. In: Skinner MK, Griswold MD eds. Sertoli cell biology. San Diego: Elsevier Science (USA); 345–382 [Google Scholar]
- Russell LD, Brinster RL 1996 Ultrastructural observations of spermatogenesis following transplantation of rat testis cells into mouse seminiferous tubules. J Androl 17:615–627 [PubMed] [Google Scholar]
- Russell LD, França LR, Brinster RL 1996 Ultrastructural observations of spermatogenesis in mice resulting from transplantation of mouse spermatogonia. J Androl 17:603–614 [PubMed] [Google Scholar]
- Parreira GG, Ogawa T, Avarbock MR, França LR, Brinster RL, Russell LD 1998 Development of germ cell transplants in mice. Biol Reprod 59:1360–1370 [DOI] [PubMed] [Google Scholar]
- Prahalada S, Majka JA, Soper KA, Nett TM, Bagdon WJ, Peter CP, Burek JD, MacDonald JS, van Zwieten MJ 1994 Leydig cell hyperplasia and adenomas in mice treated with finasteride, a 5α-reductase inhibitor: a possible mechanism. Fundam Appl Toxicol 22:211–219 [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP 2003 Estrogen receptor-α gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology 144:84–93 [DOI] [PubMed] [Google Scholar]
- Delbès G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R 2005 Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor α. Endocrinology 146:2454–2461 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW 1997 Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA 94:1476–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE 1993 Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341:1392–1395 [DOI] [PubMed] [Google Scholar]
- Maduro MR, Lamb DJ 2002 Understanding new genetics of male infertility. J Urol 168:2197–2205 [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubhan DB, Korach KS 1994 Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- Krausz C, Giachini C 2007 Genetic risk factors in male infertility. Arch Androl 53:125–133 [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C 2003 Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal 15:355–366 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.