Abstract
Leptin has profound effects on adipose tissue metabolism. However, it remains unclear whether direct leptin signaling in adipocytes is involved. We addressed this question by transplanting inguinal adipose tissue stromal vascular cells (SVCs) from 4- to 5-wk-old wild-type (WT) and leptin receptor-deficient [Leprdb/db (db)] mice to inguinal and sternal subcutaneous sites in Ncr nude mice. Both WT and db SVCs gave rise to mature adipocytes with normal morphologies 3 mo after the transplantation. The average adipocyte size (μm2/cell) was not significantly different between WT and db transplants at either the inguinal (1,630 ± 103 vs. 1,491 ± 74) or the sternal site (1,788 ± 107 vs. 1,596 ± 92). Expression levels of β3-adrenergic receptor, a major mediator of lipid mobilization, were indistinguishable between WT and db transplants and similar to those of the hosts. Additionally, adipocyte sizes of inguinal transplants and endogenous inguinal adipose tissues were closely correlated (β = 0.76, P < 0.001), suggesting that the metabolic milieu of host mice has significant effects on adipocyte size of the transplants. Contrary to the indifference to donor's Lepr genotype, adipocyte size of the transplants was significantly affected by the donor's sex in a leptin receptor-dependent manner. In WT transplants, female SVCs gave rise to smaller adipocytes than male SVCs (1,358 ± 127 vs. 2,133 ± 171, P < 0.05). However, this sex difference was not significant in db transplants (1,537 ± 121 vs. 1,655 ± 140, P = 0.22). These data suggest that: 1) long-form receptor-mediated direct leptin signaling has no significant cell-autonomous effect on adipocyte differentiation and metabolism in adult mice, 2) sex may affect adipocyte metabolism via genetic and/or epigenetic programming, and 3) leptin may potentiate sexual dimorphism in adipocyte metabolism.
Keywords: β3-adrenergic receptor, sex, genetic and epigenetic programming, adipocyte progenitor cells, db mutation
leptin, a hormone produced primarily by adipocytes, has profound effects on systemic energy balance and adipose tissue metabolism (48, 95). Leptin administration decreases food intake and increases energy expenditure, resulting in preferential reduction of fat mass in both wild-type (WT) and leptin-deficient (Lepob/ob) mice (12, 36, 61). The mechanism by which leptin exerts these effects has been a subject of intensive research. The central nervous system (CNS) is believed to be the primary site of action through which leptin exerts its effect on food intake and energy expenditure (2, 12, 18, 24, 71). Recent studies indicate that leptin also regulates lipid metabolism in adipocytes through neuronal and/or neuroendocrine mechanisms, increasing lipolysis via sympathetic activation and decreasing lipogenesis by activating phosphoinositol-3 kinase in the CNS (11, 62, 72). However, whether direct leptin signaling in adipocytes contributes to the overall antilipogenic effect of leptin under the physiological conditions remains unclear.
Among the known isoforms of the leptin receptor, the long-form receptor (or Rb) is responsible for mediating leptin signaling via the JAK-STAT pathway and is the primary mediator of the metabolic effects of leptin (15, 16, 47, 80). Expression of the Rb isoform has been detected in many tissues, including adipose tissues [both adipocytes and stromal vascular cells (SVCs)], although levels of Rb expression are much lower in adipose tissues compared with those in the hypothalamus (15, 47). Treatment of cultured adipocytes and adipose tissue explants with leptin acutely stimulates lipolysis and fatty acid oxidation, in part by activation of AMP-activated kinase (30, 56, 73, 87, 97). However, leptin is ineffective in eliciting these effects in adipose tissue explants from leptin receptor-deficient (Leprdb/db) mice, which lack the Rb isoform (15, 47). Exogenous leptin administration or overexpression of leptin by recombinant adenovirus in rats reduces triglyceride content in adipocytes of denervated fat pads (69, 88), suggesting that direct leptin action in adipocytes may play a role in mediating antilipogenic effects of leptin under these circumstances. However, we have shown that in the presence of an intact Lepr gene in the CNS, selective inactivation of the signaling function of the leptin receptor in peripheral tissues and organs that include adipose tissue, liver, intestine, and pancreas has no significant effects on systemic energy balance and adipose tissue metabolism in mice (34). No changes in adipose tissue mass, adipocyte size, or expression levels of several key adipokines and metabolic genes, including leptin, adiponectin, β3-adrenergic receptor (β3-AR), and fatty acid synthase, are detected in this knockout mouse model (34). These data suggest that direct leptin signaling in adipocytes may not be important in maintaining normal energy homeostasis or adipose tissue metabolism under the physiological conditions.
In this study, we employed preadipocyte transplantation experiments to further investigate whether a lack of the Rb receptor in preadipocytes/adipocytes affects adipose differentiation and metabolism when the cells are transplanted to normal host mice with an intact leptin signaling system. Preadipocytes, such as those found in the stromal-vascular fraction of adipose tissue (SVC) or immortalized 3T3-F442A cells, are capable of adipocyte differentiation when transplanted to immunodeficient or allogeneic mice (33, 37, 51, 98). We took the transplantation approach in this study for the following two reasons. First, transplanted preadipocytes are expected to achieve full adipocyte differentiation in vivo, whereas induced adipose differentiation of SVCs or 3T3-F442A cells is usually incomplete in vitro (33, 51). Cultured 3T3-F442A adipocytes are multilocular and express only < 1% of normal tissue levels of leptin, a marker of mature adipocytes (33, 51). In contrast, transplanted 3T3-F442A cells are able to differentiate into unilocular adipocytes that express leptin at levels comparable to those in normal tissues (51). Second, in contrast to in vitro experiments in which only acute effects of leptin can be examined, transplantation experiments allow long-term effects of direct leptin signaling in adipocytes to be examined under physiological circumstance. In this study, we used SVCs isolated from inguinal adipose tissues of Lepr+/+ and Leprdb/db mice (both male and female) as donors of SVCs, and male Ncr nude mice as hosts for the SVCs. Ncr nude mice, which are deficient in T cell-mediated immunity, have been widely used as hosts for tumor transplantation and 3T3-F442A preadipocyte transplantation (51, 96). Ncr nude mice display normal energy balance despite being hairless and athymic. Body weight, body fat mass, and plasma concentrations of insulin and leptin are similar between Ncr nude mice and WT C57BL/6J mice (See Supplemental Table 1 in the online version of this article.). Tissue morphology, adipocyte size, and expression level of β3-AR of the transplants were examined. While our results suggest that direct leptin signaling in adipocytes has no significant effect on adipocyte differentiation and metabolism in adult mice, leptin signaling may be required for the development of sex-related differences in adipocyte size.
MATERIALS AND METHODS
Animal husbandry.
Ncr-nude mice (Nu/Nu) were purchased from Taconic (Hudson, NY). C57BL/6J Leprdb/+ mice and C57BL/6-Tg (ACTbEGFP) 1Osb/J mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). The green fluorescent protein (GFP) transgene in 57BL/6-Tg (ACTbEGFP) 1Osb/J mouse is under the control of β-actin promoter and is expressed in many tissues of the donor mice, including mature adipocytes. Pups were weaned at the age of 3 wk, separated according to sex, and maintained in a barrier facility (12:12-h light-dark cycle) with ad libitum access to water and rodent breeder chow (rodent chow 5058; PicoLab). Mice carrying transplants were also maintained in the barrier facility with ad libitum access to rodent breeder chow and water. All animal experimentation was conducted in accordance with accepted standards of humane animal care, and all protocols were approved by the Columbia University Institutional Animal Care and Use Committee.
Generation of donor mice.
C57BL/6J Leprdb/+ mice were mated with C57BL/6-Tg (ACTbEGFP) 1Osb/J mice to generate C57BL/6J Leprdb/+ mice that carry one copy of the GFP transgene, which were then mated with C57BL/6J Leprdb/+ mice to generate GFP-transgenic and nontransgenic Lepr+/+ and Leprdb/db mice (WT and db). Homozygous db and WT pups were identified using a PCR-based genotyping method as previously described (45). GFP transgenic mice were identified by their green fluorescent feet under a hand-held UV lamp (model UVM-57, UVP, Upland, CA).
Isolation and expansion of adipose tissue SVCs.
SVCs were isolated from inguinal adipose tissues of 4- to 5-wk-old littermates by collagenase digestion as previously described (35). Adipose tissues from 8–10 mice were pooled for SVC isolation. Red blood cells were removed by resuspending SVCs in ice-cold erythrocyte lysis buffer for 10 min (0.154 M NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA). Washed SVCs were then cultured in DMEM supplemented with 10% fetal bovine serum and 100 IU/ml penicillin and streptomycin in the presence of 5% CO2 at 37°C for a total of 7–8 days (3 passages). The initial plating density was ∼0.04 million cells/cm2 or 1.2 million cells/60-mm dish. Cultured SVCs were trypsinized (passed) every 2–3 days when they reached confluence. The primarily purpose of the in vitro culture was to expand SVCs and to remove damaged and nonadherent cells. Such in vitro expansion has no significant effects on the ability of adipocyte progenitor cells to undergo adipocyte differentiation in vivo or in vitro (84). WT and db SVCs were processed in parallel by following the same isolation and expansion protocols, and the same numbers of cells were transplanted. Rates of in vitro adipocyte differentiation of cultured SVCs were assessed as previously described (93). Briefly, confluent cultures of SVCs were treated with rosiglitazone (1 μg/ml), a potent proliferator-activated receptor-γ agonist, in DMEM supplemented with insulin and fetal bovine serum for 3 days followed by an additional 9-day culture in the same supplemented DMEM without rosiglitazone. At the end of the 12-day culture, cells were fixed and stained for cellular lipids with oil-red-O. No significant difference in the rates of proliferation or in vitro adipose differentiation was observed between the two groups (WT vs. db or male vs. female) (see online Supplemental Fig. 1). These results are consistent with earlier observations that leptin has no direct effects on the proliferation and adipocyte differentiation of rat primary SVCs (85). At the end of the in vitro expansion, SVCs were harvested in the culture medium (50 million cells/ml) for transplantation.
Transplantation of adipose tissue SVCs in athymic nude mice.
Cultured adipose tissue SVCs were injected subcutaneously using 23-gauge needle syringes into either the inguinal (2.5 million SVC/site) or the sternal site (5–10 million SVCs/site) in 8-wk-old male Ncr nude mice under anesthesia. GFP-transgenic WT and db SVCs were transplanted pairwise to the contralateral inguinal sites of the same host mouse for comparison. Transplanted cells were distinguished from endogenous adipocytes by the expression of the donor-specific GFP transgene. Both GFP transgenic and nontransgenic SVCs were used for transplantations at the sternal site, where there was no endogenous adipose tissue. Each host mouse received one sternal transplant (sTx) . Both inguinal transplants (iTxs) and sTxs were allowed to grow for 3 mo before they were excised for characterization. All transplants were collected in the basal state (after a 5-h food deprivation during the light cycle) immediately after euthanasia. For histological analysis, transplants and endogenous adipose tissues were fixed in 4% paraformaldehyde-PBS for paraffin-embedding and tissue section.
Histological characterization of transplants and endogenous adipose tissues.
Consecutive tissue sections (12- to 15-μm thick) were used for hematoxylin and eosin staining, and immunostainings of GFP (anti-GFP antibody; Clontech Laboratories, Mountain View, CA) and β3-AR antibody (Santa Cruz Biotechnology, Santa Cruz, CA). GFP immunostaining was carried out by following the protocol recommended by the manufacturer. For β3-AR immunostaining, db and WT inguinal transplants from the same host or native inguinal adipose tissues of Lepr+/+ and Leprdb/db mice were paired and processed on the same slide. All samples were processed in a single batch, and the time for final color development was kept the same for all samples. The intensity of β3-AR immunostaining on the plasma membrane of adipocytes was quantified by densitometry in a manner similar to that described by Masliah et al. (53). Briefly, plasma membranes of similar widths were chosen and random areas of the plasma membrane were boxed. The optical densities of the boxed areas were then determined by densitometry (Quantity One; Bio-Rad, Hercules, CA). To avoid potential bias, the quantitative analysis was done in a blind fashion. The average adipocyte β3-AR immunoreactivity for each tissue specimen was derived from measurements of at least 50 adipocytes.
Determination of adipocyte size of transplants and endogenous adipose tissues.
Digital images of hematoxylin and eosin-stained tissue sections were used for adipocyte size analysis with Image Pro Plus Program (IPP; Media Cybernetics, Bethesda, MD). IPP is able to measure the cross-sectional area and roundness of each adipocyte and tallies the number of total adipocytes measured for each tissue section. We set the lower and upper limits of acceptable values for the cross-sectional area of an adipocyte to exclude extremely small or large circular areas that did not correspond to adipocytes. We also set the acceptable range of a roundness index between 0 and 3 to exclude adjacent adipocytes with damaged plasma membrane rim, which would be otherwise counted by IPP as one cell with the roundness index less than 3. By setting the acceptable ranges for the cross-sectional area and roundness, we were able to eliminate most, if not all, artificial counts that did not correspond to adipocytes. The remaining artificial counts were toggled off manually. The average adipocyte size [expressed as the average cross-sectional area per cell (μm2/cell)] of each transplant or tissue sample was calculated based on the values of at least 200 adipocytes. To convert IPP readouts to the corresponding metric values, we measured cell sizes of a group of isolated adipocytes using both micrometer ruler and IPP as described (4, 94), and related the two sets of data to obtain a conversion formula, area in μm2/cell = 0.565 × IPP readout (arbitrary units) + 449 (see online Supplementary Fig. 2). The correlation coefficient between the two sets of measurements was 0.999 (P < 0.001).
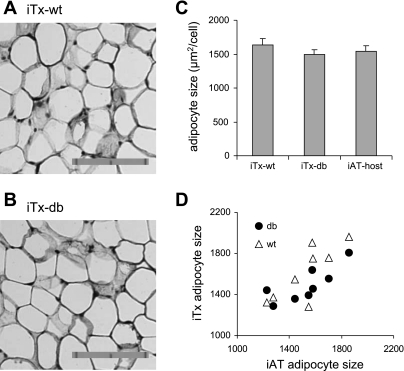
Fig. 2.
Quantitative analysis of adipocyte size in the WT and db inguinal transplants. GFP-immunostaining of representative WT (A) and leptin receptor-deficient [Leprdb/db(db); B] iTx. Donor SVCs were isolated from inguinal adipose tissues of 4- to 5-wk-old GFP-transgenic WT and Leprdb/db littermates. The bar represents 0.1 mm in two 0.05-mm units. C: quantitative measurements of adipocyte size (the average cross-sectional area of each adipocyte, μm2/cell) in the WT inguinal transplants (iTx-WT, n = 8) and db inguinal transplants (iTx-db, n = 8), and the endogenous iAT of the hosts (iAT-host) (means ± SE). D: correlation in adipocyte size between the iTx and the endogenous iAT tissue (r = 0.76, P < 0.001).
Statistical analysis.
Statistical analyses were performed using Statistica V6 (StatSoft, Tulsa, OK). All data are expressed as means ± SE. Paired t-tests were used to assess effects of Lepr-genotype of donor SVCs on adipocyte size and β3-AR expression levels in paired WT and db inguinal transplants and in native inguinal adipose tissues of the WT and db mice. Multiple regression analysis was used to assess effects of endogenous adipocyte size of hosts and Lepr-genotype of donor SVCs on adipocyte size of inguinal transplants. Factorial ANOVA was used to assess effects of the Lepr genotype, sex of donors, and the interactions of the two on adipocyte size in sTxs, which include transplants derived from donors of either sex. Post hoc Tukey tests were used to determine genotype effects within a specific sex group or sex effects within a specific genotype group.
RESULTS
Transplanted adipose tissue SVCs develop normally into mature adipose tissues at sternal and inguinal subcutaneous sites of host mice.
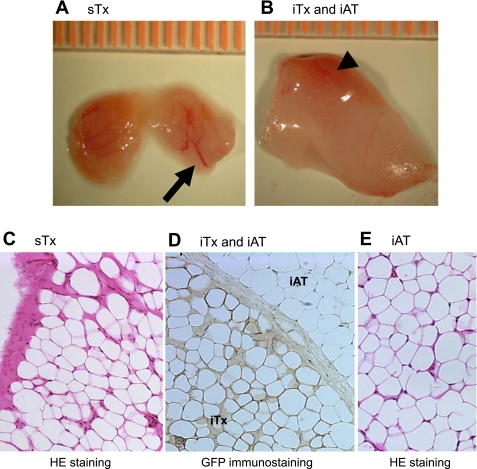
In mice carrying sTxs, the transplants became visible underneath the skin between 1–2 mo posttransplantation, and remained visible during a 6-mo follow-up (data not shown). The gross morphology of excised sTx was similar to that of endogenous inguinal adipose tissues (iAT), except that the transplants contained more visible blood vessels (Fig. 1A). iTxs were usually found embedded in the endogenous inguinal fat pads, and were slightly redder than the surrounding endogenous fat tissues because of more extensive vascularization (Fig. 1B). Mature adipocytes of sTx (Fig. 1C) and iTx (Fig. 1D) were histologically indistinguishable from those of endogenous iAT (Figs. 1, D and E). In the inguinal fat pads that carried iTx, GFP-positive adipocytes were found clustered together and embedded in the endogenous fat tissue sometimes bordered by a layer of thickened connective tissue (Fig. 1D). When GFP-positive SVCs were transplanted in the sternal site, all of the mature adipocytes in the resultant sTx were GFP-positive (data not shown). These results suggest that there was no recruitment of host adipocyte progenitor cells to transplantation sites.
Fig. 1.
Adipose tissue morphogenesis at the sternal (sTx) and inguinal (iTx) transplantation sites. Gross morphology of a sTx (A) and an iTx with the endogenous inguinal adipose tissue (iAT) (B). The transplants were derived from iAT SVCs of the wild-type (WT) male C57BL/6J donor mice. The arrow in A points to blood vessels in the sTx. The arrowhead in B points to the iTx, which is distinct from the paler endogenous iAT by virtue of its redder hue. C–E: tissue morphology of the transplants. Hematoxylin and eosin (HE) staining of a sTx (C), green fluorescent protein (GFP)-immunostaining of an iTx and the surrounding endogenous iAT (D), and HE staining of the endogenous iAT of a host mouse (E). Adipocytes of the iTx are identified by their GFP-staining, as opposed to the GFP-negative adipocytes of the host (D).
Deficiency of direct leptin signaling does not affect adipocyte size or β3-AR expression in transplants.
To evaluate the physiological role of direct leptin signaling in regulating adipocyte differentiation and metabolism, we generated and characterized paired inguinal transplants derived from SVCs isolated from GFP-transgenic WT C57BL/6J (WT) and Leprdb/db (db) littermates. The gross morphology of WT and db iTx was not significantly different (Figs. 2, A and B). To assess the overall effect of direct leptin signaling on triglyceride accumulation, we measured adipocyte size of iTx and the corresponding endogenous iAT using Image-Pro-Plus (IPP). Adipocyte size (μm2/cell) of db transplants (iTx-db; 1,491 ± 74) was not significantly different from that of WT transplants (iTx-WT; 1,630 ± 103) (P = 0.32 by paired t-test, n = 8 per group) (Fig. 2C). Adipocyte sizes of WT and db transplants were both similar to those of endogenous adipocytes of the host (iAT-host; 1,538 ± 85) (Fig. 2, C and D), which ranged from 1,226 to 1,860 (μm2/cell) among individual animals. By multiple regression analysis, adipocyte size of the endogenous inguinal fat pads was a major predictor of adipocyte size of the transplants, accounting for 61% variations of the latter (β = 0.762, P < 0.001). Lepr-genotype of donor SVCs was not a significant predictor of adipocyte size of transplants (β = 0.182, P = 0.30).
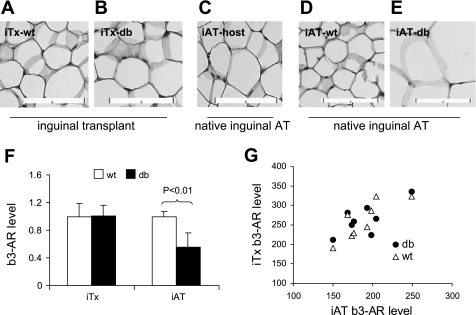
To further assess the lack of difference in adipocyte size between db and WT transplants, we examined gene expression of β3-AR in these transplants. β3-AR is the primary mediator of sympathetic regulation of lipid mobilization in both white and brown adipose tissues (5, 21, 50, 78). Decreased β3-AR expression and reduced lipolysis are characteristics of animals deficient in leptin signaling, such as Lepob/ob mice and Leprfa/fa rats (44, 91). To determine whether a lack of direct leptin signaling in adipocytes affects β3-AR expression, we quantified and compared β3-AR immunostaining on plasma membrane of mature adipocytes in the paired WT and db inguinal transplants (iTx-WT and iTx-db, respectively), and in the endogenous inguinal adipose tissues of host nude mice (iAT-host) (Fig. 3, A–C). β3-AR immunostaining levels were not significantly different between iTx-WT and iTx-db (Fig. 3F) and were significantly correlated between the transplants and the endogenous iAT of host mice [r = 0.73, P < 0.05 (iTx-db) and r = 0.82, P < 0.05 (iTx-WT), Fig. 3G]. In contrast, β3-AR density was 45% lower in the native iATs of Leprdb/db mice (iAT-db) compared with that of the WT mice (iAT-WT) (P < 0.01) (Figs. 3, D–F), suggesting that leptin signaling may affect adipocyte β3-AR expression indirectly. The apparent lack of direct leptin signaling effect on adipocyte β3-AR expression is consistent with the lack of direct leptin signaling effect on adipocyte size and metabolism.
Fig. 3.
Comparison of β3-adrenergic receptor (β3-AR) expression in iAT-WT and iAT-db. β3-AR immunostaining of the representative pairs of iTx-WT (A) and iTx-db (B), the endogenous iAT-host (C), the native iAT of male 8-wk-old WT mice (iAT-WT) (D), and Leprdb/db mice (iAT-db) (E). The bar represents 0.1 mm. F: quantification of average β3-AR expression levels on adipocyte plasma membrane of the iTx-WT and iTx-db (n = 8 per genotype), and of the native iAT of the WT and db mice (n = 5 per genotype). The densities of β3-AR immunostaining in the db transplants and Leprdb/db iAT are expressed relative to those of the WT transplants and the iAT-WT, respectively. G: correlation of β3-AR expression levels (in arbitrary units) between the inguinal transplants (iTx), and the hosts' iAT tissues (r = 0.82, P < 0.05 for WT; and r = 0.73, P < 0.05. for db).
Sex of SVC donors influences adipocyte size in WT transplants.
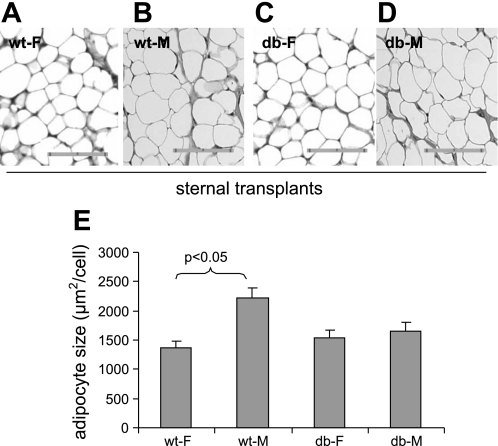
Sex and/or gonadal steroids have significant effects on adipose tissue metabolism (25, 39, 41, 58). Sexual dimorphism in the regulation of adipocyte size and metabolism in humans and rodent models has been well documented (3, 49, 60). Leptin signaling promotes sexual maturation (1, 52, 92), and lack of leptin signaling function delays sexual maturation in both humans and rodents (20, 28, 57). Therefore, we investigated potential effects of Lepr genotype, sex, and interactions between the two on adipocyte size of sTxs. SVCs from inguinal adipose tissues of male and female WT and Leprdb/db littermates were transplanted to the sternal site of male athymic nude mice and the transplants were excised 3 mo later for analysis. SVCs from both male and female donors all gave rise to fat tissues with similar gross tissue morphology. Figure 4, A–D shows a set of representative sTxs derived from SVCs of the WT female and male mice (WT-F and WT-M) and Leprdb/db female and male mice (db-F and db-M). Adipocyte size of the transplants was again determined using IPP (Fig. 4E). Multifactorial ANOVA was used to assess the effects of sex, Lepr genotype, and potential interactions between the two on adipocyte size of the sTxs in a data set that includes values of both sexes (n = 18). The sex of donor SVCs had significant effects on the size of adipocytes (μm2/cell) in the transplants [1,447 ± 88 (female) vs. 1,937 ± 110 (male) (F = 12.1, P < 0.01) (Fig. 4E)], and interactions between Lepr genotype and sex also had significant effects on adipocyte size of the transplants (F = 7.0, P < 0.05). By post hoc analysis, adipocyte size of WT-F transplants (1,358 ± 127) was significantly smaller than that of WT-M transplants (2,133 ± 171) (P < 0.05 by post hoc Tukey test); there was no significant difference in adipocyte size between db-F (1,537 ± 121) and db-M transplants (1,655 ± 140) (P = 0.82). These results suggest that sex difference in adipocyte size of transplants requires intact leptin signaling system in either the donor mice or the transplanted SVCs. Consistent with the results of inguinal transplants, Lepr genotype of SVCs had no significant effect on adipocyte size of sTxs (1,788 ± 107, WT vs. 1,596 ± 92, db; n = 8 and 10, respectively) (Fig. 4E). Adipocyte size of male sTxs were similar to those of inguinal transplants, which were all derived from male SVCs (Fig. 3G). Post hoc tests confirmed that adipocyte size was not significantly different between WT and db sTx of either sex.
Fig. 4.
Effects of sex and Lepr-genotype of donor SVCs on adipocyte size of sTxs. Hematoxylin and eosin-stained tissue sections of representative the WT female (WT-F) (A), WT male (WT-M) (B), db female (db-F) (C), and db male (db-M) (D) sTxs. The bar represents 0.1 mm in two 0.05-mm units. E: quantitative measurements of adipocyte size in sTxs grouped by sex and Lepr-genotype of SVC donors (n = 18). Data are expressed as unweighted means ± SE. A statistically significant difference (P < 0.05) between WT-M and WT-F transplants is indicated by post hoc Tukey tests.
DISCUSSION
We have described a preadipocyte (SVC) transplantation method that enables distinction of cell autonomous vs. noncell autonomous effects on adipose tissue biogenesis and metabolism. Using this technique, we find that absence of the Rb isoform of the leptin receptor in preadipocytes/adipocytes has no significant effect on either the differentiation or the size of mature adipocytes in vivo. Tissue morphology and adipocyte sizes of mature adipocytes derived from Leprdb/db SVCs (db) were indistinguishable from those derived from the WT SVCs when the SVCs were transplanted to either inguinal or sternal subcutaneous sites of host mice with intact leptin signaling. Moreover, contrary to the markedly decreased expression of β3-AR in the native adipose tissues of Leprdb/db mice, expression levels of β3-AR in db transplants were normal and not significantly different from those of WT transplants, further supporting the inference that Rb-mediated signaling in adipocytes per se does not play a significant role in regulating adipocyte metabolism.
The similarities in tissue morphology, adipocyte size, and β3-AR expression between inguinal transplants (either WT or db) and the endogenous inguinal adipose tissue of hosts suggest that environmental factors or the metabolic milieu of hosts dominates over the direct leptin signaling in determining adipocyte metabolism in the transplants. Our results are consistent with those of earlier studies. Meade et al. (55) have shown that when adipose tissues from the WT (lean) or Leprdb/db mice were transplanted to the sites underneath the kidney capsule of db/db and lean host mice, adipocytes in the transplants, regardless of their original sizes in donor mice, attained the sizes similar to those of endogenous adipocytes of the hosts mice within a month. Similarly, while β3-AR expression is markedly decreased in the adipocytes of leptin-deficient ob/ob mice (22), adipocyte β3-AR expression is not acutely regulated by leptin administration or denervation (70), suggesting that leptin signaling influences β3-AR expression indirectly (27, 38, 70), possibly through changes in adiposity and/or levels of other hormones. We have previously shown that deletion of the signaling domain of the leptin receptor in multiple peripheral tissues and organs, including adipose tissue, liver, and pancreas, has no significant effect on energy balance in mice; adipose tissue mass, adipocyte size, and β3-AR expression levels were not affected (34).
Contrary to the results of in vivo studies (18, 24, 34), leptin induces lipolysis and fatty acid oxidation in adipose tissue explants or cultured cells in vitro (30, 56, 73, 87, 97). The apparent discrepancy may, in part, result from differences in the concentration of leptin, or more relevantly, of free leptin, which is the biologically active form (90). Supraphysiological concentrations of leptin were used in some in vitro studies (73, 87, 97). Even in the studies in which physiological concentrations of leptin were used (30, 73), it is possible that concentrations of free leptin were much higher than those found in vivo, as the majority of circulating leptin exists as bound and biologically inactive forms under the normal physiological conditions in lean individuals (42, 59, 74). It is also possible that while effects of direct leptin signaling on adipocyte metabolism are discernable in well-controlled in vitro experiments, they are insignificant relative to the effects exerted by central leptin action and other regulators in vivo.
We also observed significant sex-related differences in adipocyte metabolism in the sTxs. SVCs from female WT donors gave rise to smaller adipocytes than those from male WT donors. The difference in adipocyte size between male and female WT transplants is reminiscent of the sex-related difference observed in the native inguinal adipose tissues of the donor mice (C57BL/6J) (see online Supplemental Fig. 3), underscoring the physiological relevance of the results. Although the present study appears to be the first to show a sex-related difference in adipocyte size of inguinal subcutaneous adipose tissues in C57BL/6J mice, several earlier studies have shown that in perigonadal fat pads, adipocyte size of virgin females is smaller than those of age-matching males in various rodent models (9, 40, 43, 75). In Wistar rats, sexual dimorphism and depot-related differences in adipocyte size were also observed during normal growth and in response to caloric restriction (63). During normal growth, increases in the size of inguinal subcutaneous fat pads were due primarily to adipocyte hypertrophy in male rats, but to adipocyte hyperplasia in female rats. However, when the rats were subjected to mild-to-moderate caloric restrictions, female rats defended fat cell number at the expense of fat cell size, whereas the converse was seen for male rats (63). Clearly, understanding sexual dimorphism in the regulation of adipocyte size and number is of importance with regard to the regulation of body fat distribution and comorbidities of obesity (8, 13, 26, 65, 81).
The sex-related difference in adipocyte size of transplants is apparently a result of cell-autonomous function of the donor SVCs. The difference appears to be programmed in adipocyte progenitor cells prior to the adipose differentiation of transplanted SVCs in host mice, suggesting that genetic and/or epigenetic programming in adipocyte progenitor cells plays a role in the development of sexual dimorphism in adipocyte metabolism. Sexual dimorphisms in the structural organization and function of the brain have also been observed, and are believed to be due to both cell-autonomous fulfillment of sex-specific genetic programs and epigenetic effects of prenatal and early postnatal hormonal exposure (7, 10). Thus, it is possible that sex-specific genetic and/or epigenetic programming, which may affect sensitivities to gonadal steroids or expression levels of key metabolic genes, such as lipoprotein lipase (3, 29, 54), occurs in preadipocytes and/or their precursors, and persists in transplanted SVCs.
It is equally interesting to note that the manifestations of sex-related differences in adipocyte size appear to require normal leptin signaling activities in donor animals and/or their SVCs as such sex-related differences were absent in db transplants. Leptin is critically important in regulating sexual maturation via the hypothalamus (1, 20, 57). It is possible that the deficiency of leptin signaling in Leprdb/db mice may prevent the sex-specific programming in adipocyte progenitor cells, which results in the lack of sexual dimorphism in adipocyte size in db transplants. However, it is also possible that the manifestation of the sex-related differences may require direct leptin signaling in SVCs prior and/or after transplantation. Cross-talk between the signaling pathways of leptin and estrogen, as demonstrated recently in the CNS (17, 31), may exist in adipocytes and their progenitor cells. Finally, although direct leptin signaling has no significant effect on the overall metabolism in adipocytes, the results of this study do not rule out potential roles of leptin signaling in regulating other cellular functions or actions of other hormones. Additionally, other endocrine abnormalities (e.g., hyperinsulinemia, hyperglycemia, and hypercortisolemia) in the Leprdb/db mice, which may be more exaggerated in one sex than the other, may also contribute to genotype and sex interactions in the transplants (19).
The source of mature adipocytes in the transplants was proven to be donors by the presence of donor-specific GFP transgene. However, only a small percentage (0.01–0.1%) of transplanted SVCs differentiated into mature adipocytes in vivo. Such a result is not surprising since SVCs are a mixed cell population and only a small faction of SVCs may be adipocyte precursors. Consistently, we observed only a small faction of SVCs undergoing adipose differentiation in vitro after the cells were treated with rosiglitazone (see online Supplemental Fig. 1). Rosiglitazone activates peroxisome proliferator activated-receptor-γ, which is a key regulator of adipocyte differentiation (77, 82). Recent studies have shown that adipocyte progenitor cells may reside in the vasculature of adipose tissue and comprise ∼0.08% of total stromal vascular fraction of adipose tissue (67, 79). The physiological condition of the host, such as the stress level and systemic energy balance, appears to have a major effect on the survival of transplanted SVCs. Size and number of mature adipocytes in the transplants recovered from different host mice varied significantly even when the same numbers of cells from the same SVC preparation were transplanted. On the other hand, no consistent difference in the number of mature adipocytes between the WT and db transplants or between the female and male transplants was observed. It is worth noting, however, that the variation in the number of mature adipocytes in the transplants does not appear to affect the tight correlation in adipocyte size and β3-AR level between the transplants and the endogenous adipose tissues of the hosts.
Our results also show that adult mice (8 wk or older) are fully capable of supporting adipogenesis of the transplanted SVCs without the aid of exogenous stimulatory drugs, consistent with the notion that adipogenesis in human adipose tissues continues throughout life under the normal physiological conditions (76). There was little mosaicism of GFP-positive and GFP-negative adipocytes in either inguinal or sTxs. GFP-positive adipocytes clustered at the transplantation sites in the inguinal fat pads, and adipocytes in the sTxs were uniformly GFP-positive when GFP-transgenic SVCs were transplanted, suggesting that there was no recruitment of adipocyte progenitor cells of the host to transplantation sites. Thus, adipocyte progenitor cells are likely produced or stored locally for the continuous turnover of adipose tissue in vivo (79), unlike adipose tissue macrophages, which originate mainly from bone marrow cells (89).
Adipose tissue transplants are biologically active and may affect adipose tissue cellularity and systemic energy metabolism of the host. Transplanting epididymal fat tissue of donor mice to subcutaneous sites of recipient mice, which leads to a 10% increases in adipose tissue mass in the hosts, decreases adipocyte size of the endogenous epididymal and retroperitoneal fat pads, but does not affect fat cell number in these fat pads (68). However, fat transplantation-induced compensatory decreases in endogenous fat mass and adipocyte size were not observed in Siberian hamster (46). Transplanting of the WT adipose tissue to Lepob/ob mice also corrects the metabolic abnormalities in these mice, primarily by virtue of the production of leptin by the transplanted adipocytes (32). We did not observe any significant effects of transplants on the size and morphology of the surrounding endogenous adipocytes or any gross changes in energy-metabolism of the hosts. A likely explanation for this observation is that the masses of adipose tissue derived from transplanted SVCs were very small (10–30 mg), accounting for less than ∼1% of the total fat mass of the host vs. a 10% increase in the study by Rooks et al. (68). Low transplant mass is, in fact, desirable in determining the potential metabolic effect of direct leptin signaling in the transplants.
Perspectives and Significance
Body fat distribution, independent of the overall adiposity, is an important determinant comorbidity of obesity; abdominal obesity is a component of metabolic syndrome and is associated with increased risk for diabetes and cardiovascular diseases (8, 26, 64, 66, 81). The adverse consequences of abdominal obesity may be due in part to the anatomic locations and metabolic characteristics of visceral fat, such as the proximity to the portal system and the higher lipolytic activity associated with greater sensitivity to β3-adrenergic stimulation and lower sensitivity to insulin (14, 50, 81, 83, 86). However, it is unclear what determines the metabolic characteristics of anatomically distinct adipose tissues and how body fat distribution is influenced by genetic and environmental factors, and by age and sex. The results of the present study suggest that genetic and/or epigenetic modifications in adipocyte progenitor cells may mediate some of the sexual dimorphism in adipocyte metabolism. It is conceivable that genetic and/or epigenetic modifications in adipocyte progenitor cells may also play a role in determining depot-related metabolic differences (6, 23). Our results also suggest that adipocyte progenitor cells are likely produced or stored locally. Thus, the turnover and renewal of adipocytes, which occur continuously throughout life (76), could be influenced or limited by the proliferative capacity or the size of the repository of progenitor cells in a depot-specific manner. Depletion of the progenitor cells in obese and aging individuals may affect adipose tissue renewal and storage capacity, which may, in turn, lead to elevated circulating levels of free fatty acids, insulin resistance, and ectopic fat deposition. The preadipocyte transplantation technique described here is a useful experimental paradigm for characterizing and quantifying the respective roles of cell-autonomous functions, both genetic and epigenetic, and environmental factors (both hormonal and neuronal) in regulating adipocyte biogenesis and metabolism.
GRANTS
This work was supported by an American Diabetes Association Career Development Award, and National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK-63034, P30-DK-26687, P30-DK-63068.
Supplementary Material
Acknowledgments
The authors thank Rudy Leibel for helpful discussions and review of the manuscript, Qiong Li, Quen Xu, and Xi Sun for assistance in tissue section and staining, and Foster Chen and Chen Zhou for assistance in genotyping and quantification of adipocyte size.
REFERENCES
- 1.Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest 99: 391–395, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Anderson LA, McTernan PG, Barnett AH, Kumar S. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab 86: 5045–5051, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Ashwell M, Priest P, Bondoux M, Sowter C, McPherson CK. Human fat cell sizing–a quick, simple method. J Lipid Res 17: 190–192, 1976. [PubMed] [Google Scholar]
- 5.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol Regul Integr Comp Physiol 275: R1399–R1411, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Bassett DR, Craig BW. Influence of early nutrition on growth and adipose tissue characteristics in male and female rats. J Appl Physiol 64: 1249–1256, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Becu-Villalobos D, Gonzalez Iglesias A, Diaz-Torga G, Hockl P, Libertun C. Brain sexual differentiation and gonadotropins secretion in the rat. Cell Mol Neurobiol 17: 699–715, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med 120: S3–S8, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann P, Militzer K, Schmidt P, Buttner D. Sex differences in age development of a mouse inbred strain: body composition, adipocyte size and organ weights of liver, heart and muscles. Lab Anim 29: 102–109, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Beyer C, Eusterschulte B, Pilgrim C, Reisert I. Sex steroids do not alter sex differences in tyrosine hydroxylase activity of dopaminergic neurons in vitro. Cell Tissue Res 270: 547–552, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14: 667–675, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269: 546–549, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Carr MC The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404–2411, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Carr MC, Brunzell JD. Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J Clin Endocrinol Metab 89: 2601–2607, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Chua SC Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271: 994–996, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55: 978–987, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108: 1113–1121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman DL Diabetes-obesity syndromes in mice. Diabetes 31: 1–6, 1982. [DOI] [PubMed] [Google Scholar]
- 20.Coleman DL Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14: 141–148, 1978. [DOI] [PubMed] [Google Scholar]
- 21.Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: β-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol 18: 2123–2131, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW. Impaired expression and functional activity of the β3- and β1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol Endocrinol 8: 518–527, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Cryer A, Jones HM. The development of white adipose tissue. Effect of litter size on the lipoprotein lipase activity of four adipose-tissue depots, serum immunoreactive insulin and tissue cellularity during the first year of life in male and female rats. Biochem J 186: 805–815, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC Jr. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115: 3484–3493, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280: 35983–35991, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 10: 497–511, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138: 839–842, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Fried SK, Kral JG. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int J Obes 11: 129–140, 1987. [PubMed] [Google Scholar]
- 30.Fruhbeck G, Aguado M, Martinez JA. In vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptin. Biochem Biophys Res Commun 240: 590–594, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294: E817–E826, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 105: 271–278, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5: 19–27, 1975. [DOI] [PubMed] [Google Scholar]
- 34.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, Loh D, Li C, Chua S Jr, Zhang Y. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology 148: 3987–3997, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Guo KY, Halo P, Leibel RL, Zhang Y. Effects of obesity on the relationship of leptin mRNA expression and adipocyte size in anatomically distinct fat depots in mice. Am J Physiol Regul Integr Comp Physiol 287: R112–R119, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269: 543–546, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Hausman GJ Responsiveness to adipogenic agents in stromal-vascular cultures derived from lean and preobese pig fetuses: an ontogeny study. J Anim Sci 70: 106–114, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson PR, Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res 13: 2–11, 1972. [PubMed] [Google Scholar]
- 41.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97: 12735–12740, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan J, Brabant G, Brinsuk M, Tank J, Horn R, Luft FC, Busjahn A. Heritability of free and receptor-bound leptin in normal twins. Am J Physiol Regul Integr Comp Physiol 288: R1411–R1416, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan ML, Trout JR, Leveille GA. Adipocyte size distribution in ob/ob mice during preobese and obese phases of development. Proc Soc Exp Biol Med 153: 476–482, 1976. [DOI] [PubMed] [Google Scholar]
- 44.Kortner G, Petrova O, Vogt S, Schmidt I. Sympathetically and nonsympathetically mediated onset of excess fat deposition in Zucker rats. Am J Physiol Endocrinol Metab 267: E947–E953, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Kowalski TJ, Liu SM, Leibel RL, Chua SC Jr. Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes 50: 425–435, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Lacy EL, Bartness TJ. Autologous fat transplants influence compensatory white adipose tissue mass increases after lipectomy. Am J Physiol Regul Integr Comp Physiol 286: R61–R70, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Leibel RL The role of leptin in the control of body weight. Nutr Rev 60: S15–S19, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Lemonnier D Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J Clin Invest 51: 2907–2915, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lonnqvist F, Thome A, Nilsell K, Hoffstedt J, Arner P. A pathogenic role of visceral fat β3-adrenoceptors in obesity. J Clin Invest 95: 1109–1116, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3–F442A preadipocytes. Proc Natl Acad Sci USA 94: 4300–4305, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab 82: 1066–1070, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Masliah E, Terry RD, Alford M, DeTeresa R, Hansen LA. Cortical and subcortical patterns of synaptophysin-like immunoreactivity in Alzheimer's disease. Am J Pathol 138: 235–246, 1991. [PMC free article] [PubMed] [Google Scholar]
- 54.McTernan PG, Anwar A, Eggo MC, Barnett AH, Stewart PM, Kumar S. Gender differences in the regulation of P450 aromatase expression and activity in human adipose tissue. Int J Obes Relat Metab Disord 24: 875–881, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Meade CJ, Ashwell M, Sowter C. Is genetically transmitted obesity due to an adipose tissue defect? Proc R Soc Lond B Biol Sci 205: 395–410, 1979. [DOI] [PubMed] [Google Scholar]
- 56.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, Leranth C, Vondracek-Klepper S, Lewis C, Chang A, Parducz A. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci 14: 101–116, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Ogier V, Ziegler O, Mejean L, Nicolas JP, Stricker-Krongrad A. Obesity is associated with decreasing levels of the circulating soluble leptin receptor in humans. Int J Obes Relat Metab Disord 26: 496–503, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Pedersen SB, Jonler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab 78: 1354–1359, 1994. [DOI] [PubMed] [Google Scholar]
- 61.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995. [DOI] [PubMed] [Google Scholar]
- 62.Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol 291: R1613–R1621, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Porter MH, Fine JB, Cutchins AG, Bai Y, DiGirolamo M. Sexual dimorphism in the response of adipose mass and cellularity to graded caloric restriction. Obes Res 12: 131–140, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Rader DJ Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med 120: S12–S18, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol 95: 136–147, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Reisin E, Alpert MA. Definition of the metabolic syndrome: current proposals and controversies. Am J Med Sci 330: 269–272, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 135: 240–249, 2008. [DOI] [PubMed] [Google Scholar]
- 68.Rooks C, Bennet T, Bartness TJ, Harris RB. Compensation for an increase in body fat caused by donor transplants into mice. Am J Physiol Regul Integr Comp Physiol 286: R1149–R1155, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Rooks CR, Penn DM, Kelso E, Bowers RR, Bartness TJ, Harris RB. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am J Physiol Regul Integr Comp Physiol 289: R92–R102, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Scarpace PJ, Matheny M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am J Physiol Endocrinol Metab 275: E259–E264, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett 416: 193–197, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, Cusin I, Rohner-Jeanrenaud F, Burger AG, Zapf J, Meier CA. Direct effects of leptin on brown and white adipose tissue. J Clin Invest 100: 2858–2864, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sinha MK, Opentanova I, Ohannesian JP, Kolaczynski JW, Heiman ML, Hale J, Becker GW, Bowsher RR, Stephens TW, Caro JF. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J Clin Invest 98: 1277–1282, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinnett-Smith PA, Waddington D. Size distribution of adipocytes and variation in adipocyte number in lines of mice selected for high or low body fat. Comp Biochem Physiol Comp Physiol 102: 573–578, 1992. [DOI] [PubMed] [Google Scholar]
- 76.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature 453: 783–787, 2008. [DOI] [PubMed] [Google Scholar]
- 77.Spiegelman BM Peroxisome proliferator-activated receptor-γ: a key regulator of adipogenesis and systemic insulin sensitivity. Eur J Med Res 2: 457–464, 1997. [PubMed] [Google Scholar]
- 78.Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB. Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem 270: 29483–29492, 1995. [DOI] [PubMed] [Google Scholar]
- 79.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science 322: 583–586, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Woolf EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263–1271, 1995. [DOI] [PubMed] [Google Scholar]
- 81.Tchernof A Visceral adipocytes and the metabolic syndrome. Nutr Rev 65: S24–S29, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR-γ2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol 15: 351–357, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van RL, Roncari DA. Complete differentiation in vivo of implanted cultured adipocyte precursors from adult rats. Cell Tissue Res 225: 557–566, 1982. [DOI] [PubMed] [Google Scholar]
- 85.Wagoner B, Hausman DB, Harris RB. Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am J Physiol Regul Integr Comp Physiol 290: R1557–R1564, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Wajchenberg BL Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697–738, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Wang MY, Lee Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem 274: 17541–17544, 1999. [DOI] [PubMed] [Google Scholar]
- 88.Wang ZW, Zhou YT, Lee Y, Higa M, Kalra SP, Unger RH. Hyperleptinemia depletes fat from denervated fat tissue. Biochem Biophys Res Commun 260: 653–657, 1999. [DOI] [PubMed] [Google Scholar]
- 89.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang G, Ge H, Boucher A, Yu X, Li C. Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol 18: 1354–1362, 2004. [DOI] [PubMed] [Google Scholar]
- 91.Young JB, Landsberg L. Diminished sympathetic nervous system activity in genetically obese (ob/ob) mouse. Am J Physiol Endocrinol Metab 245: E148–E154, 1983. [DOI] [PubMed] [Google Scholar]
- 92.Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fujii S, Nakao K. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest 105: 749–755, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Chen L, Chen N, Yu YH. Expression of monocyte chemoattractant protein-1 is modulated by lipids, but is independent of cell-size and cell-aging. Adipocytes 2: 37–46, 2006. [Google Scholar]
- 94.Zhang Y, Guo KY, Diaz PA, Heo M, Leibel RL. Determinants of leptin gene expression in fat depots of lean mice. Am J Physiol Regul Integr Comp Physiol 282: R226–R234, 2002. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]
- 96.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA 102: 755–760, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou YT, Shimabukuro M, Koyama K, Lee Y, Wang MY, Trieu F, Newgard CB, Unger RH. Induction by leptin of uncoupling protein-2 and enzymes of fatty acid oxidation. Proc Natl Acad Sci USA 94: 6386–6390, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211–228, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.