Abstract
Vascular calcification plays a role in the pathogenesis of atherosclerosis, diabetes, and chronic kidney disease. Human aortic smooth muscle cells (HSMCs) undergo mineralization in response to elevated levels of inorganic phosphate (Pi) in an active and well-regulated process. This process involves increased activity of alkaline phosphatase and increased expression of core binding factor α-1, a bone-specific transcription factor, with the subsequent induction of osteocalcin. Mounting evidence suggests an essential role for the heme oxygenase 1 (HO-1)/ferritin system to maintain homeostasis of vascular function. We examined whether induction of HO-1 and ferritin alters mineralization of HSMCs provoked by high Pi. Upregulation of the HO-1/ferritin system inhibited HSMC calcification and osteoblastic differentiation. Of the products of the system, only ferritin and, to a lesser extent, biliverdin were responsible for the inhibition. Ferritin heavy chain and ceruloplasmin, which both possess ferroxidase activity, inhibited calcification; a site-directed mutant of ferritin heavy chain, which lacked ferroxidase activity, failed to inhibit calcification. In addition, osteoblastic transformation of HSMCs provoked by elevated Pi (assessed by upregulation of core binding factor α-1, osteocalcin, and alkaline phosphatase activity) was diminished by ferritin/ferroxidase activity. We conclude that induction of the HO-1/ferritin system prevents Pi-mediated calcification and osteoblastic differentiation of human smooth muscle cells mainly via the ferroxidase activity of ferritin.
Vascular calcification occurs in many pathologic conditions and can lead to devastating clinical consequences. For example, it has been related to increased risk for cardiovascular morbidities and complications such as atherosclerotic plaque burden,1–3 myocardial infarction,4,5 coronary artery disease,6,7 postangioplasty dissection,8 and increased ischemic episodes in peripheral vascular disease.9 Studies also have indicated that coronary calcification may be predictive of or associated with sudden cardiac death.10,11 Indeed, coronary calcification score measured by electron beam computed tomography has been shown to have a prognostic value for cardiovascular events comparable to that of the Framingham risk index.11 Vascular calcification follows two distinct patterns: (1) Intimal calcification that occurs with atherosclerotic plaques and (2) medial calcification, which is characterized by diffuse calcification of the media, particularly at the level of the internal elastic lamina, that does not necessarily accompany atherosclerosis. This pattern is commonly seen in patients with chronic kidney disease (CKD), who commonly exhibit hyperphosphatemia. The mechanism of vascular calcification is not completely understood, although abnormalities in mineral metabolism are considered important risk factors.
Many studies have demonstrated the role of high extracellular inorganic phosphate (Pi) to induce calcification of vascular cells in vitro12–16 in a process mediated by a sodium-dependent phosphate co-transporter that facilitates entry of Pi into vascular cells.17 This induces transition of human aortic smooth muscle cells (HSMCs) into osteoblast-like cells through a process that is accompanied by increased expression of core binding factor α-1 (Cbfa-1), which is an osteoblast-specific transcription factor required for osteoblast differentiation, bone matrix gene expression, and, consequently, bone mineralization.18 There is also an upregulation of alkaline phosphatase (ALP), an important enzyme in early osteogenesis and osteocalcin, a major noncollagenous protein found in bone matrix that is believed to regulate mineralization.19
Heme is a ubiquitous iron-containing molecule that is an absolute necessity for aerobic life. Current evidence suggests that heme can be pro-oxidant and potentially toxic.20–22 Heme induces the synthesis of heme oxygenase 1 (HO-1), the rate-limiting enzyme in the catabolism of heme.23 HO cleaves the porphyrin ring at the α-methene bridge to form biliverdin and carbon monoxide and releases free redox active iron. Biliverdin is then converted to bilirubin by biliverdin reductase.
Ferritin is another molecule strongly inducible by heme and iron. This is an iron storage protein that exhibits antioxidant properties, and it was shown to protect the endothelium against the damaging effects of heme and oxidants.24 Ferritin is a large (450 kD), spherical shell that can store up to 4500 Fe atoms in a safe, nontoxic form. It is made of 24 subunits of two types (heavy [H] and light [L] chain) whose proportion depends on the iron status of the cell, the tissue, and the organ.25 The H-chain has ferroxidase activity that is important not only for iron incorporation but also in controlling the potentially toxic Fe (II) ions, thereby reducing oxidative damage.26
In our investigations, we tested the role that heme may play in the process of extracellular calcification, and we observed that heme decreases extracellular matrix calcification in a dosage-responsive manner. These observations prompted us to hypothesize that one or more products of heme catabolism may inhibit HSMC mineralization.
RESULTS
Heme Decreases HSMC Calcification in a Dosage-Responsive Manner
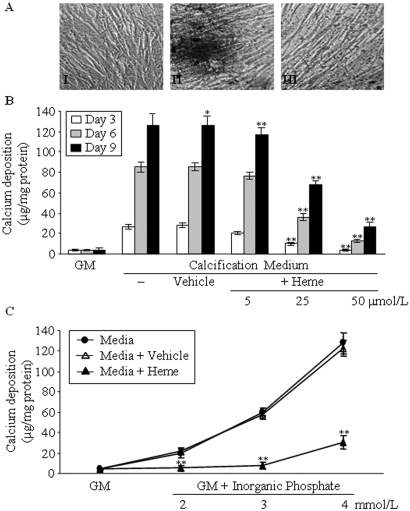
To develop an in vitro model, we cultured HSMCs in calcification medium. Granular deposits developed in HSMCs grown in calcification medium for 9 d (Figure 1AII) but not in the control culture grown in normal growth medium (GM; Figure 1AI). Intriguing, we found that addition of heme (50 μmol/L, 9 d) to the calcification medium inhibited calcium deposition as shown by von Kossa staining (Figure 1AIII). Extracellular calcium measurements showed that the inhibitory effect of heme on extracellular calcification is dosage dependent, with a highly significant (P < 0.01) suppression at a dosage of 25 μmol/L (Figure 1B).
Figure 1.
Heme inhibits HSMC calcification induced by elevated Pi in a dosage-dependent manner. (A) HSMCs were cultured in GM (I) or in calcification medium in the absence (II) or presence of heme (50 μmol/L; III) for 9 d. Von Kossa staining of cells was performed as described in the Concise Methods section. Representative picture of three separate experiments. (B) HSMCs were cultured in GM or in calcification medium alone or supplemented with NaOH (Vehicle, 1 mmol/L) or 5, 25, and 50 μmol/L heme (dissolved in NaOH at a final concentration of 1 mmol/L in each group). Calcium contents of cells were measured after 3 (□), 6 (  ), and 9 d (▪) of culture as described in the Concise Methods section and were normalized by protein content. Data are means ± SD of three independent experiments performed in duplicate. (C) HSMCs were cultured in GM alone or supplemented with 2, 3, or 4 mmol/L Pi (•). The media containing different amounts of Pi was supplemented with heme (50 μmol/L; ▴) or with NaOH (vehicle, 1 mmol/L; ▵). Calcium deposition was measured at day 9, and results were normalized by protein content of the cells. Data show the average of three separate experiments performed in duplicate. *P < 0.05; **P < 0.01. Magnification, ×100.
), and 9 d (▪) of culture as described in the Concise Methods section and were normalized by protein content. Data are means ± SD of three independent experiments performed in duplicate. (C) HSMCs were cultured in GM alone or supplemented with 2, 3, or 4 mmol/L Pi (•). The media containing different amounts of Pi was supplemented with heme (50 μmol/L; ▴) or with NaOH (vehicle, 1 mmol/L; ▵). Calcium deposition was measured at day 9, and results were normalized by protein content of the cells. Data show the average of three separate experiments performed in duplicate. *P < 0.05; **P < 0.01. Magnification, ×100.
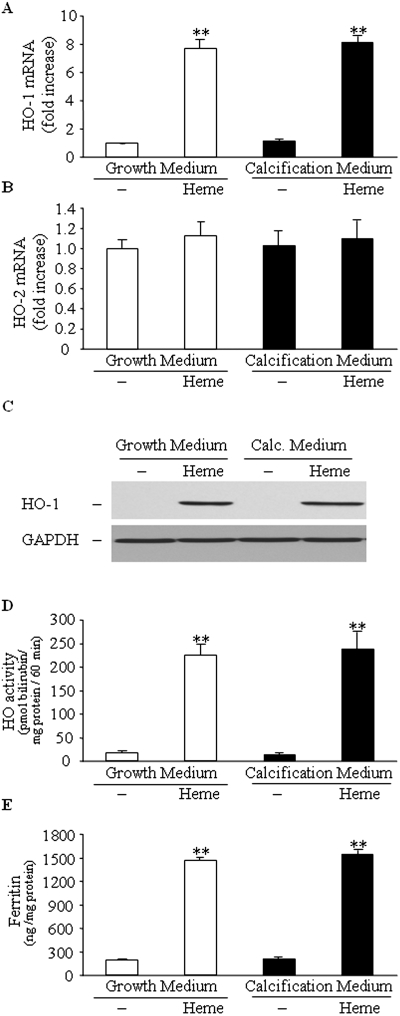
Heme is a strong inducer of HO-1, and, as expected, we found that HO-1 mRNA, protein, and HO activity were elevated in the cells cultured in heme-containing medium. Pi level of the medium did not affect this heme-mediated induction of HO-1 (Figure 2, A, C, and D). In addition, we found that heme did not significantly alter HO-2 expression (Figure 2B), and it induced expression of ferritin regardless of Pi level of the medium (Figure 2E).
Figure 2.
Heme induces HO-1 and ferritin in HSMCs. HSMCs were cultured in GM or in calcification medium in the absence or presence of heme (50 μmol/L) for 24 h. (A through E) HO-1 mRNA levels (A), HO-2 mRNA levels (B), HO-1 protein expression (C), HO activity (D), and ferritin expression (E) were measured as described in the Concise Methods section. Western blot was stripped and probed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and shown as a representative of three experiments. Data are means ± SD of three to five independent experiments each performed in duplicate. **P < 0.01.
Ferritin and Ferroxidase Activity Attenuate HSMC Calcification
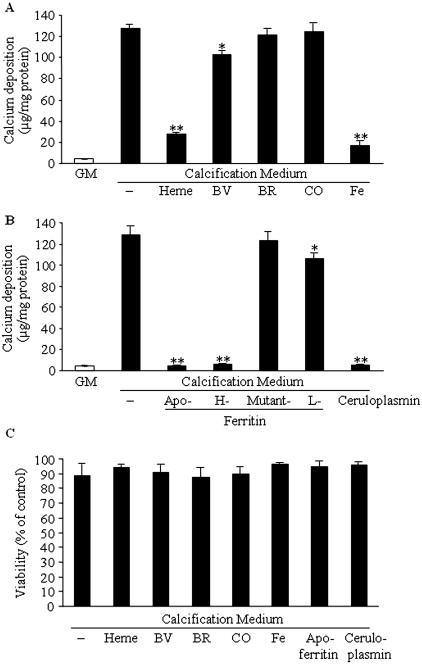
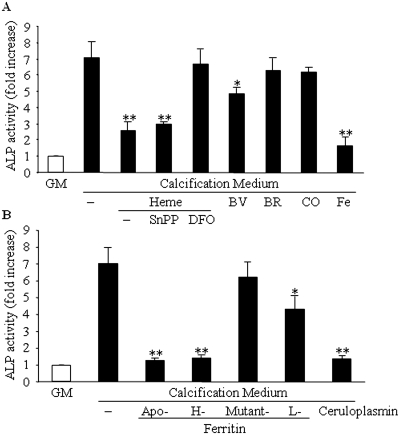
Heme induces HO-1 and ferritin; therefore, it was of interest to analyze which of the two had a major effect on calcification. We also analyzed the role of the end products of HO catalyzed heme degradation by adding them exogenously to the calcification medium. We found that iron, regardless of its ferric or ferrous state (50 μmol/L), completely inhibits calcification (the ferrous state, data not shown). Biliverdin at the concentration of 50 μmol/L provided a little but significant (P < 0.05) decrease in calcification (Figure 3A). Addition of CO (1%) or bilirubin (50 μmol/L) did not influence calcification (Figure 3A). Conversely, addition of apoferritin (2 mg/ml) or recombinant H-chain ferritin to the calcification medium abolished calcification (Figure 3B). These two ferritin types have ferroxidase activity; therefore, we tested another protein with ferroxidase activity, ceruloplasmin. Ceruloplasmin was found to mimic the effect of ferritins at a concentration of 4 mg/ml. The protective effect of L-ferritin was minor compared with that of H-ferritin and ceruplasmin. This may have the following explanation. The L-ferritin chains taken up by the cells may coassemble with the endogenous ferritin and thus expand the pool of active ferritins. The H-mutant 222 ferritin, which lacks both ferroxidase activity and iron-storing capability, was not protective at all against mineralization of HSMCs.
Figure 3.
Ferritin/ferroxidase activity is responsible for the inhibition of phosphate-induced HSMC calcification. (A) HSMCs were cultured in GM or in calcification medium alone or in the presence of heme (50 μmol/L), biliverdin (BV; 50 μmol/L), bilirubin (BR; 50 μmol/L), CO (1%), or iron (50 μmol/L) for 9 d. Calcium content of cells was measured and normalized by cellular protein content. (B) HSMCs were cultured in calcification medium alone or supplemented with apoferritin (2 mg/ml), H-ferritin (2 mg/ml), mutant 222 ferritin (2 mg/ml), ceruloplasmin (4 mg/ml), or L-ferritin (2 mg/ml). After 9 d, calcium deposition was measured as described in the Concise Methods section. Graphs show means ± SD of three separate experiments. *P < 0.05; **P < 0.01. (C) For investigation of whether any of the compounds cause significant toxicity, an MTT [3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-tetrazolium-bromide] assay was performed after 9 d of incubation. Data are means ± SD of five separate experiments. **P < 0.01.
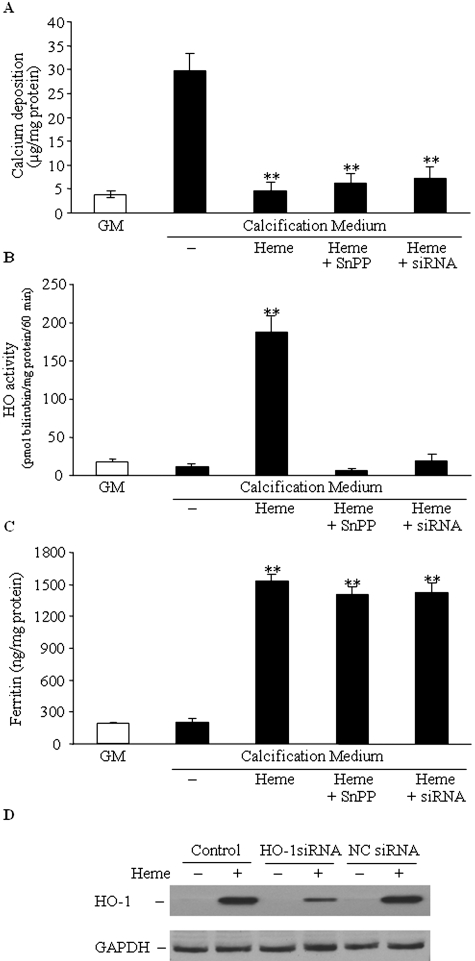
To confirm the protective role of ferritin, we inhibited HO using tin protoporphyrin (SnPP), a widely known inhibitor of HO activity, and also transfected the cells with small interfering RNA (siRNA) specific for HO-1. We confirmed the efficiency of siRNA and observed an approximately 70% decrease of HO-1 protein expression for up to 4 d after transfection (Figure 4D). In fact, cells treated with heme in the presence of SnPP or siRNA showed very low HO enzyme activity (Figure 4B). Treatment with SnPP or siRNA did not affect the heme-mediated ferritin induction (Figure 4C) and, more important, did not influence heme-mediated inhibition of calcification (Figure 4A), indicating the paramount role of ferritin in this protection.
Figure 4.
Ferritin induced by heme mediates the inhibition of phosphate-provoked HSMC calcification. When applied, cells were transfected with siRNA for HO-1 or negative control siRNA (NC) 24 h before the experiment. HSMCs were cultured in calcification medium in the presence of heme (50 μmol/L) or heme and SnPP (50 μmol/L each) for 4 d. (A through C) Calcium deposition (A), HO enzyme activity (B), and ferritin expression (C) were measured. (D) A typical Western blot shows efficacy of HO-1 knockdown by siRNA. Four days after transfection, cells were treated with heme (50 μmol/L) for 24 h, and level of HO-1 protein was determined. Data are means ± SD of three independent experiments each performed in duplicate. **P < 0.01.
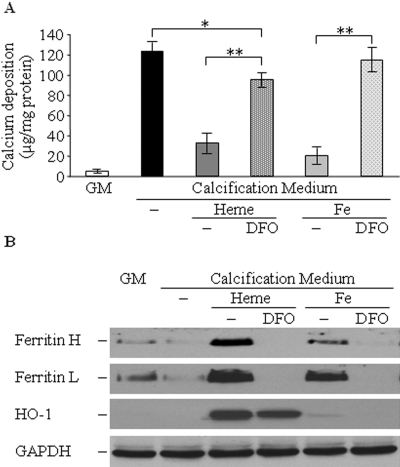
To confirm further the function of ferritin in the heme- or iron-induced inhibition of calcification, we selectively downregulated heme- or iron-induced ferritin synthesis by the iron chelator deferoxamine (DFO). Treatment of the cells with DFO together with equimolar amount of heme or Fe resulted in a complete block of heme- or Fe-induced ferritin synthesis of both H- and L-chains as shown by Western blot (Figure 5B). Downregulation of ferritin synthesis by DFO led to complete loss of inhibition of calcification by iron (Figure 5A). Moreover, co-treatment with heme and DFO resulted in downregulation of both chains of ferritin but not of HO-1 (Figure 5B), which was accompanied by substantial decrease in inhibition of calcification (Figure 5A). Mild but significant (P < 0.05) inhibition of calcification that may be attributed to biliverdin derived from HO-mediated heme degradation was noted.
Figure 5.
Downregulation of ferritin synthesis by DFO decreased heme- or iron-mediated inhibition of calcium deposition. (A) HSMCs were cultured in GM or in calcification medium alone or in the presence of heme (50 μmol/L) or iron (50 μmol/L), with or without equimolar amount of DFO for 9 d. Calcium content of cells was measured and normalized by cellular protein content. (B) Representative Western blots show expression of ferritin H- and L-chains, HO-1, and GAPDH of cells treated as described in A. Data are means ± SD of three separate experiments. *P < 0.05; **P < 0.01.
Ferritin Inhibits Osteoblastic Differentiation of HSMCs
It has been shown that vascular calcification in vivo shares similarities with bone mineralization; therefore, we asked whether ferritin and its ferroxidase activity solely inhibit mineralization or suppress the phenotype transition of HSMCs into osteoblast-like cells. We examined the activity of alkaline phosphatase (Figure 6). HSMCs maintained in calcification medium for 9 d showed an approximately seven-fold increase in ALP activity compared with control. Supplementation with heme provided a decrease in ALP activity. Similarly, exposures of cells to iron (50 μmol/L) abolished high Pi-induced ALP activity. Biliverdin (50 μmol/L) caused some inhibition (P < 0.05), whereas other end products of HO-mediated heme degradation—bilirubin (50 μmol/L) and CO (1%)—failed to decrease ALP activity. Co-treatment of the cells with heme and SnPP demonstrated similar changes in ALP activity as heme alone; conversely, co-treatment with heme and DFO did not affect the increased ALP activity. Importantly, apoferritin, H-ferritin, and ceruloplasmin also decreased the activity of ALP to the level seen in controls, but the H-mutant 222 ferritin was totally ineffective.
Figure 6.
Ferritin attenuates ALP activity induced by elevated Pi. (A) HSMCs were cultured in GM or in calcification medium alone or in the presence of heme (50 μmol/L), heme + SnPP (50 μmol/L each), heme + DFO (50 μmol/L each), biliverdin (BV; 50 μmol/L), bilirubin (BR; 50 μmol/L), CO (1%), or iron (50 μmol/L) for 9 d. (B) HSMCs were cultured in calcification medium alone or supplemented with apoferritin (2 mg/ml), H-ferritin (2 mg/ml), mutant 222 ferritin (2 mg/ml), ceruloplasmin (4 mg/ml), or L-ferritin (2 mg/ml) for 9 d. ALP activity of cells was measured as described in the Concise Methods section. Data are means ± SD of five independent experiments each performed in duplicate. *P < 0.05; **P < 0.01.
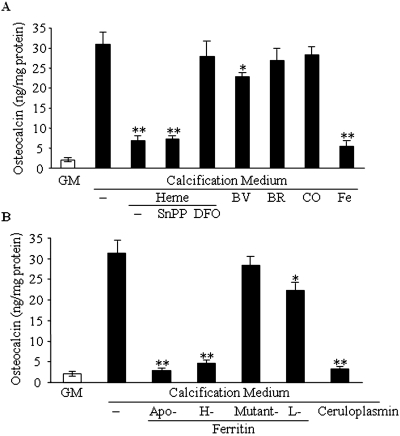
Next we investigated the presence of another bone-specific protein, osteocalcin, in the extracellular matrix. Maintaining of HSMCs in calcification medium for 9 d resulted in a >10-fold increase in osteocalcin content compared with control (Figure 7A). Heme decreased upregulation of osteocalcin, and SnPP did not alter this effect. In contrast, co-treatment of the cells with heme and DFO led to the loss of osteocalcin downregulation by heme. Iron inhibited upregulation of osteocalcin similarly to heme. In addition, biliverdin had a mild but significant effect (P < 0.05), whereas other products of HO reaction—bilirubin and CO—failed to downregulate high Pi-induced osteocalcin expression (Figure 7A). Apoferritin, H-ferritin, and ceruloplasmin abolished expression of osteocalcin, whereas H-mutant 222 had no effect at all (Figure 7B).
Figure 7.
Ferritin attenuates the upregulation of osteocalcin induced by elevated Pi. (A and B) HSMCs were treated as described at Figure 6, and osteocalcin levels were determined as described in the Concise Methods section. Data are means ± SD of three independent experiments each performed in duplicate. *P < 0.05; **P < 0.01.
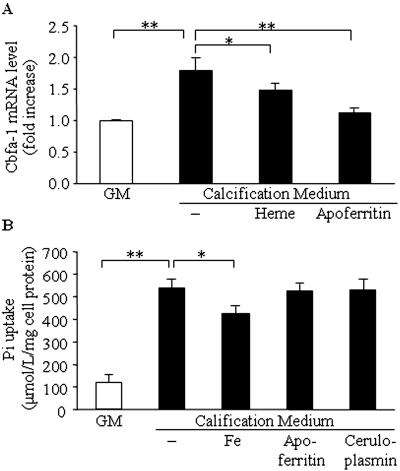
Finally, to explore the mechanism underlying the inhibition of mineralization, we examined the level of Cbfa-1, the “master gene” of osteoblast differentiation, in our in vitro model. Culturing HSMCs in calcification medium for 48 h resulted in a 1.8-fold increase in Cbfa-1 mRNA level compared with cells maintained in normal GM. Heme inhibited induction of Cbfa-1 mRNA (P < 0.05). Accordingly, apoferritin also significantly suppressed this Cbfa-1 induction (Figure 8A).
Figure 8.
(A) Both heme and apoferritin inhibit elevated Pi-induced increase in Cbfa-1 mRNA level. HSMCs were cultured in GM or in calcification medium alone or in the presence of heme (50 μmol/L) or apoferritin (2 mg/ml) for 48 h. Cbfa-1 mRNA levels were determined by quantitative reverse transcription–PCR as described in the Concise Methods section. Data are means ± SEM of five independent experiments performed in triplicate. *P < 0.05; **P < 0.01. (B) Intracellular Pi concentrations are not affected by apoferritin or ceruloplasmin. HSMCs were cultured in GM and calcification medium supplemented by iron (50 μmol/L), apoferritin (2 mg/ml), or ceruloplasmin (4 mg/ml) for 24 h. Cell lysates were used to measure Pi levels. Data are means ± SEM of three independent experiments performed in duplicate. *P < 0.05; **P < 0.01.
We also tested the intracellular levels of Pi (Figure 8B), and our results indicate neither apoferritin nor ceruloplasmin alters intracellular Pi levels after 24 h. Iron causes an approximately 25% decrease in the level of intracellular Pi that must be attributed to its phosphate-binding capacity; therefore, exposure of cells to iron inhibits osteoblastic differentiation via two mechanisms: (1) Increasing intracellular H-ferritin and (2) decreasing extracellular phosphate. Furthermore, we also examined the role of aluminum, which is both a trivalent cation and a strong phosphate binder. Although there was some inhibition of calcification, the extent was one third of that observed with heme or iron (data not shown).
DISCUSSION
CKD often translates to deranged metabolism of both phosphate and iron. Accumulation of phosphate starts relatively early in kidney disease, but overt hyperphosphatemia does not develop until the later stages of CKD. Evidence from clinical, animal, and in vitro studies indicate that such elevated phosphate level is an important inducer of vascular calcification. Analyzing data from hemodialysis patients reveals that the extent of elevated serum phosphate is positively correlated with mortality,27–29 and cardiovascular events are the major cause of mortality in this group of patients. In particular, development of calciphylaxis, which is a syndrome of vascular calcification and skin necrosis, is almost exclusively seen in patients with stage 5 CKD and correlates with extremely high fatal rates. Moreover, in patients with CKD, there is an accumulation of iron in reticuloendothelial cells that is accompanied by higher levels of plasma ferritin; however, this increase largely results because most of such iron is sequestered by reticuloendothelial cells and its availability to other cells is significantly reduced. This translates to depletion of intracellular ferritin and subsequent anemia of chronic disease. In inflammatory diseases such as CKD, cytokines released by activated leukocytes and other cells exert multiple effects.30 These contribute to the reduction in hemoglobin levels and increased hepatic synthesis of hepcidin that in turn binds to ferroportin, the transporter that allows egress of iron from reticuloendothelial macrophages and from intestinal epithelial cells. Binding of hepcidin leads to internalization and degradation of ferroportin. The corresponding sequestration of iron within the macrophages limits iron availability to all cells. On the basis of our observations, we suggest that such derangements in iron metabolism may facilitate Pi-induced vascular calcification; therefore, parenteral iron administration may be considered not only to replete iron and correct anemia but also to prevent vascular calcification via increasing intracellular ferritin expression and decreasing extracellular Pi level, especially when the inflammation is well controlled.
Previous studies indicated that elevated phosphate could induce SMC calcification as well as an osteochondrogenic phenotypic change. Evidence suggests a highly regulated cellular process whereby many different inducers and inhibitors of osteoblast differentiation have been recognized.31
Growing evidence indicates the importance of the HO-ferritin system in vascular homeostasis. Upregulation of HO-1 and ferritin occurs in the early phase of progression of atherosclerotic lesions,32–34 possibly reflecting cellular response to heme and/or heme-iron–generated lipid peroxidation products. There is growing evidence that induction of the HO/ferritin system is protective against atherosclerosis.34 Upregulation of HO-1 and ferritin inhibits cytotoxicity induced by oxidized LDL in endothelial cells35 and atherosclerotic lesion formation in LDL receptor knockout mice, whereas inhibition of HO enzyme activity by SnPP leads to accelerated atherosclerosis in these mice.36
In this study, we confirm that growing HSMC in Pi-containing calcification medium causes mineralization in a time-dependent manner, and, in agreement with other findings, we observed significant upregulation of specific osteoblast cell markers during the culture period, supporting that this transition is an active cell-regulated process. Observation of the inhibitory effect of heme prompted us to hypothesize that one or more products of heme catabolism might regulate HSMC mineralization.
To identify the mediator for inhibition of HSMC calcification and osteoblastic transformation, we first tested the products of heme degradation by HO. Iron almost completely attenuated extracellular calcification and upregulation of osteocalcin and ALP. Biliverdin was less effective, whereas bilirubin and CO failed to alter mineralization. Then we analyzed the possible role of ferritin that is also strongly upregulated by heme. We examined whether exogenous ferritin affected mineralization, because the uptake of exogenous apoferritin in a dosage-responsive manner has already been demonstrated.24 We found that apoferritin caused a dosage-responsive suppression of HSMC mineralization. Also exogenous H-ferritin and ceruloplasmin—two largely different proteins that share only ferroxidase activity—showed the same suppression of osteoblastic differentiation. The importance of ferroxidase activity in the process was confirmed by the finding that a structurally analogous molecule to H-ferritin, namely the recombinant H-ferritin mutant 222, which lacks ferroxidase activity and iron storage capability, was ineffectual. These results strongly support the notion that inhibition of mineralization may be attributed to ferritin and its ferroxidase activity.
Upregulation of H- and L-chains of ferritin in cells exposed to heme is driven at the translational level via labile iron provided from heme catalysis by HO.37 In addition, heme itself enhances ferritin expression by increasing its translational rate.38 This explains why induction of ferritin is not affected by inhibition of HO activity in cells exposed to heme, as observed in previous studies.24 Accordingly, in this study, cells treated with heme in the presence of SnPP or siRNA for HO-1 exhibited very low HO activity but high ferritin level. Treatment of cells with SnPP or siRNA for HO-1 did not affect heme-mediated ferritin induction and did not influence heme-mediated inhibition of mineralization. These results indicate that ferritin alone is capable of preventing HSMC calcification and differentiation after cells are exposed to heme. To confirm further the role of ferritin in the heme-induced inhibition of HSMC mineralization, we selectively downregulated heme-induced ferritin synthesis by the iron chelator DFO, which led to substantial loss of inhibition of calcification. The remaining mild inhibition of calcification may be attributed to biliverdin derived from enhanced HO-mediated heme degradation.
We observed an approximately 25% decrease in the level of intracellular Pi after exposure of cells to iron. Considering that ferric iron can bind up to five phosphates per mole, the decrease of intracellular Pi level resulted from the decrease of extracellular Pi level after iron treatment; therefore, inhibition of osteoblastic differentiation by iron occurs via two mechanisms: (1) Increasing intracellular H-ferritin and (2) decreasing extracellular Pi level.
The cellular mechanisms of vascular calcification still remain to be elucidated. Increased expression of Cbfa-1 is implicated in the transition of SMCs into osteoblast-like cells.12,16 That heme or apoferritin significantly suppressed Cbfa-1 induction by high Pi indicates that inhibition of mineralization by ferritin might occur via transcription factor Cbfa-1. H-ferritin has been found to localize also in the nucleus, where it may participate in the regulation of gene expression, for example in the suppression of β globin expression.39 This raises the possibility that it might be involved in the regulation of genes for HSMC differentiation into osteoblast-like cells, namely Cbfa-1. A relationship between calcification and iron metabolism has never been explored, although it should be noted that most patients who have CKD and are on dialysis have vascular calcification27–29 and deranged iron homeostasis.40
In conclusion, we report for the first time a novel role for ferritin in the context of HSMC mineralization. These results provide new insights into the mechanisms of vascular calcification and uncover the HO/ferritin pathway as a target for new strategies to prevent vascular calcification.
CONCISE METHODS
Cell Culture and Reagents
HSMCs were obtained from Cambrex Bioscience (Wokingham, United Kingdom), FBS from Life Technologies (Vienna, Austria), biliverdin from MP Biomedicals (Solon, OH), SnPP from Frontier Scientific (Logan, UT), 1% CO gas from Linde Gas (Repcelak, Hungary), and the gas chamber from Billups-Rothenburg (DelMar, CA). Unless otherwise mentioned, all other reagents were obtained from Sigma (St. Louis, MO). Cell cultures were maintained in GM DMEM (high glucose) containing 15% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and neomycin, and 1 mM sodium pyruvate. Cells were grown to confluence and used from passages 4 through 8. Iron was introduced as ammonium ferric citrate or ferric sulfate as well as ferrous form. To keep the ferrous state, the media were supplemented with 200 μmol/L ascorbic acid. Iron was dissolved in deionized water. siRNA specific to HO-1 and negative control siRNA were obtained from Ambion (Austin, TX) and were transfected with Oligofectamine Reagent (Invitrogen, Carlsbad, CA) 24 h before the experiment. Heme, biliverdin, and bilirubin were dissolved in NaOH. Final concentration of NaOH was kept below 2 mmol/L in all experiments. This amount of NaOH caused a little change in the pH of the medium (7.40 versus 7.46), which did not influence calcification and underlying gene expression of HSMCs.
Induction of Calcification
At confluence, cells were switched to calcification medium, which was prepared by addition of 4 mmol/L Pi to the GM. Both GM and calcification medium were changed every 2 d. For time-course experiments, the first day of culture in calcification medium was defined as day 0.
Quantification of Calcium Deposition
Cells grown on 48-well plates were washed twice with PBS and decalcified with 0.6 mol/L HCl for 24 h at 37°C. Calcium content of the supernatants was determined by the QuantiChrome Calcium Assay Kit (Gentaur, Brussels, Belgium). After decalcification, cells were solubilized with a solution of NaOH 0.1 mol/L and SDS 0.1%, and protein content of samples was measured with BCA protein assay kit (Pierce, Rockford, IL). Calcium content of the cells was normalized to protein content and expressed as μg/mg protein. Mineralization was determined by von Kossa staining.
ALP Activity Assay
Cells grown on six-well plates were washed with PBS twice, solubilized with 1% Triton X-100 in 0.9% NaCl, and assayed for ALP activity. Briefly, 130 μl of Alkaline Phosphatase Yellow Liquid Substrate (Sigma) was combined with 50 μg of protein samples and incubated at 37°C for 30 min, and then the kinetics of p-nitrophenol formation was followed for 30 min at 405 nm. Maximum slope of the kinetic curves was used for calculation.
HO Enzyme Activity Assay
Cells grown on P100 dishes were washed twice with HBSS, scraped, and centrifuged at 2000 × g for 15 min at 4°C. Cells were resuspended in 300 μl of potassium phosphate (100 mmol/L [pH 7.4]) buffer containing 2 mmol/L MgCl2, frozen and thawed three times, sonicated, and centrifuged at 18,000 × g for 10 min at 4°C. The supernatant containing cell microsomes was used to measure HO activity as described previously.24 HO activity is expressed as pmol bilirubin formed/mg cell protein per 60 min.
Western Blot to Detect HO-1 and Ferritin H- and L-Chains
For evaluation of HO-1 protein expression, cell lysate was electrophoresed in 12.5% SDS-PAGE. For ferritin H- and L-chain detection, cell lysate was subjected to 8% nondenaturing PAGE. Western blotting was performed with a polyclonal anti–HO-1 antibody at 1:2500 dilution (Calbiochem, San Diego, CA) or with mouse anti-human ferritin H- or L-chain antibodies (from P. Arosio) at 1:1000 dilution followed by horseradish peroxidase–labeled anti mouse IgG antibody. Antigen-antibody complexes were visualized with the horseradish peroxidase chemiluminescence system (Amersham Biosciences, Little Chalfont, United Kingdom). After detection, membranes were stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase.
Quantification of Ferritin and Osteocalcin
Ferritin content of cell lysate was measured with the IMx ferritin enzyme immunoassay (Abbott Laboratories, Abbott Park, Illinois). For osteocalcin detection, extracellular matrix of cells grown on six-well plates was dissolved in 300 μl of EDTA (0.5 mol/L [pH 6.9]). Osteocalcin content of the EDTA-solubilized extracellular matrix samples was quantified by an ELISA (Bender MedSystems, Burlingame, CA).
Quantitative Reverse Transcription–PCR
Total RNA was isolated and reverse-transcribed, and HO-1 mRNA was determined as described previously.41 For measurement of mRNA levels, the 25-μl reaction mixture contained 5 μl of reverse-transcribed sample, 0.3 nmol/L of forward (5′-CAGGCAGGCACAGTCTTC-3′) and reverse primers (5′-CAGAGGTGGCAGTGTCATC-3′) for Cbfa-1, forward (5′-GGTGATAGAAGAGGCCAAGACTG-3′) and reverse (5′GGTGTCATGGGTCAGCAGCT-3′) primers for HO-1, forward (5′-GCAATGTCAGCGGAAGTGGAA-3′) and reverse (5′-AAGTCACCTGAGGTGGTAGTT-3′) primers for HO-2, and 12.5 μl of iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). PCRs were carried out using the iCycler iQ Real-Time PCR System (Bio-Rad). Results were normalized by glyceraldehyde-3-phosphate dehydrogenase mRNA levels.
Ferritins and Ceruloplasmin
Apoferritin and ceruloplasmin were from Sigma. Human recombinant wild-type H- and L-chain ferritins and the H-chain mutant 222 deleted ferroxidase activity were expressed in Escherichia coli and purified as described previously.42 Final concentrations of ferritins were 2 mg/ml, which correspond to 4.5 μmol/L for apoferritin, 3.95 μmol/L for H-ferritin, and 4.19 μmol/L for L-ferritin. Final concentration of ceruloplasmin was 4 mg/ml, which corresponds to 32.7 μmol/L.
Phosphate Measurement
Pi content of the cell lysate was determined by the QuantiChrome phosphate Assay Kit (Gentaur). After 24 h of incubation, cells were washed twice with PBS and solubilized with 1% Triton, and the cell lysates were assayed for Pi. Phosphate content of the cells was normalized to protein content and expressed as μm/L per mg of cell protein.
CO Exposure
CO at a concentration of 1% (10,000 parts per million) in compressed air was mixed with compressed air containing 5% CO2 before being delivered into the culture incubator, yielding a final concentration of 400 parts per million CO. The incubator was humidified and maintained at 37°C. A CO analyzer was used to determine CO levels in the chamber. After the chamber had stabilized, no oscillations were measured in the CO concentration.
Statistical Analysis
Data are shown as means ± SD. Statistical analysis was performed by ANOVA test followed by post hoc, Newmann-Keuls test for multiple comparisons. P < 0.05 was considered significant, and P < 0.01 was considered highly significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by Hungarian government grants OTKA-K61546, ETT-337/2006, RET-06/2004, and MTA-DE-11003, and P.A. is supported by MIUR-PRiN-06.
We thank Erika Barna, Erzsébet Zavaczki, Zsolt Karányi, and Zsigmond Benkő for technical assistance.
Published online ahead of print. Publication date available at www.jasn.org.
A.Z. and V.J. contributed equally to this work.
G.B. and J.B. contributed equally to this work.
REFERENCES
- 1.Block GA, Port FK: Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: Recommendations for a change in management. Am J Kidney Dis 35: 1226–1237, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS: Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area: A histopathologic correlative study. Circulation 92: 2157–2162, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS: Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 31: 126–133, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Beadenkopf WG, Daoud AS, Love BM: Calcification in the coronary arteries and its relationship to arteriosclerosis and myocardial infarction. AJR Am J Roentgenol 92: 865–871, 1964 [PubMed] [Google Scholar]
- 5.Loecker TH, Schwartz RS, Cotta CW, Hickman JR: Fluoroscopic coronary artery calcification and associated coronary disease in asymptomatic young men. J Am Coll Cardiol 19: 1167–1172, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M: Medial artery calcification: A neglected harbinger of cardiovascular complications in non–insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 16: 978–983, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Olson JC, Edmundowicz D, Becker DJ, Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ: Coronary calcium in adults with type 1 diabetes: A stronger correlate of clinical coronary artery disease in men than in women. Diabetes 49: 1571–1578, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald PJ, Ports TA, Yock PG: Contribution of localized calcium deposits to dissection after angioplasty: An observational study using intravascular ultrasound. Circulation 86: 322–324, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Niskanen LK, Suhonen M, Siitonen O, Lehtinen JM, Uusitupa MI: Aortic and lower limb artery calcification in type 2 (noninsulin-dependent) diabetic patients and non-diabetic control subjects: A five year follow-up study. Atherosclerosis 84: 61–71, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Burke AP, Taylor A, Farb A, Malcom GT, Virmani R: Coronary calcification: Insights from sudden coronary death victims. Z Kardiol 89: 49–53, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Taylor AJ, Burke AP, O’Malley PG, Farb A, Malcom GT, Smialek J, Virmani R: A comparison of the Framingham risk index, coronary artery calcification, and culprit plaque morphology in sudden cardiac death. Circulation 101: 1243–1248, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM: Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 89: 1147–1154, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H: Vascular calcification and inorganic phosphate. Am J Kidney Dis 38: 34–37, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Giachelli CM: Vascular calcification: In vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol 43: 300–304, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Shioi A, Nishizawa Y, Jono S, Koyama H, Hosoi M, Morii H: β-Glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 15: 2003–2009, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: 10–17, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Li X, Yang HY, Giachelli CM: Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res 98: 905–912, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ: Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89: 765–771, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Giachelli CM, Steitz S, Jono S: Potential roles of bone matrix proteins in vascular calcification. Clin Calcium 9: 20–27, 1999 [Google Scholar]
- 20.Balla G, Vercellotti GM, Muller-Eberhard U, Eaton JW, Jacob HS: Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest 64: 648–655, 1991 [PubMed] [Google Scholar]
- 21.Balla J, Jacob HS, Balla G, Nath KA, Eaton JW, Vercellotti GM: Endothelial cell heme uptake from heme proteins: Sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A 90: 9285–9289, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nath KA, Grande JP, Croatt AJ, Likely S, Hebbel RP, Enright H: Intracellular targets in heme protein-induced renal injury. Kidney Int 53: 100–111, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Maines MD: Heme oxygenase system: Update 2005. Antioxid Redox Signal 7: 1761–1766, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM: Ferritin: A cytoprotective antioxidant strategem of endothelium. J Biol Chem 267: 18148–18153, 1992 [PubMed] [Google Scholar]
- 25.Theil EC: The ferritin family of iron storage proteins. Adv Enzymol Relat Areas Mol Biol 63: 421–449, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Arosio P, Levi S: Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med 33: 457–463, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Block GA: Prevalence and clinical consequences of elevated Ca x P product in hemodialysis patients. Clin Nephrol 54: 318–324, 2000 [PubMed] [Google Scholar]
- 28.Burke SK: Phosphate is a uremic toxin. J Ren Nutr 18: 27–32, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Zarychanski R, Houston DS: Anemia of chronic disease: A harmful disorder or an adaptive, beneficial response? CMAJ 179: 333–337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RC, Leopold JA, Loscalzo J: Vascular calcification: Pathobiological mechanisms and clinical implications. Circ Res 99: 1044–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY: Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol 152: 711–720, 1998 [PMC free article] [PubMed] [Google Scholar]
- 33.You SA, Archacki SR, Angheloiu G, Moravec CS, Rao S, Kinter M, Topol EJ, Wang Q: Proteomic approach to coronary atherosclerosis shows ferritin light chain as a significant marker: Evidence consistent with iron hypothesis in atherosclerosis. Physiol Genomics 13: 25–30, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Stocker R, Perrella MA: Heme oxygenase-1: A novel drug target for atherosclerotic diseases? Circulation 114: 2178–2189, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Juckett MB, Balla J, Balla G, Jessurun J, Jacob HS, Vercellotti GM: Ferritin protects endothelial cells from oxidized low density lipoprotein in vitro. Am J Pathol 147: 782–789, 1995 [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa K, Sugawara D, Wang XP, Suzuki K, Itabe H, Maruyama Y, Lusis AJ: Heme oxygenase-1 inhibits atherosclerotic lesion formation in LDL receptor knockout mice. Circ Res 88: 506–512, 2001 [DOI] [PubMed] [Google Scholar]
- 37.White K, Munro HN: Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem 263: 8938–8942, 1988 [PubMed] [Google Scholar]
- 38.Lin JJ, Daniels-McQueen S, Patino MM, Gaffield L, Walden WE, Thach RE: Derepression of ferritin messenger RNA translation by hemin in vitro. Science 247: 74–77, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Broyles RH, Belegu V, DeWitt CR, Shah SN, Stewart CA, Pye QN, Floyd RA: Specific repression of beta-globin promoter activity by nuclear ferritin. Proc Natl Acad Sci U S A 98: 9145–9150, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smrzova J, Balla J, Bárány P: Inflammation and resistance to erythropoiesis-stimulating agents: What do we know and what needs to be clarified? Nephrol Dial Transplant 8: 2–7, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Nagy E, Jeney V, Yachie A, Szabó RP, Wagner O, Vercellotti GM, Eaton JW, Balla G, Balla J: Oxidation of hemoglobin by lipid hydroperoxide associated with low-density lipoprotein (LDL) and increased cytotoxic effect by LDL oxidation in heme oxygenase-1 (HO-1) deficiency. Cell Mol Biol 30: 377–385, 2005 [PubMed] [Google Scholar]
- 42.Broxmeyer HE, Cooper S, Levi S, Arosio P: Mutated recombinant human heavy-chain ferritins and myelosuppression in vitro and in vivo: A link between ferritin ferroxidase activity and biological function. Proc Natl Acad Sci U S A 88: 770–774, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]