Abstract
Brassinosteroids (BRs) induce plant tolerance to a wide spectrum of stresses. To study how BR induces stress tolerance, we manipulated the BR levels in cucumber (Cucumis sativus) through a chemical genetics approach and found that BR levels were positively correlated with the tolerance to photo-oxidative and cold stresses and resistance to Cucumber mosaic virus. We also showed that BR treatment enhanced NADPH oxidase activity and elevated H2O2 levels in apoplast. H2O2 levels were elevated as early as 3 h and returned to basal levels 3 d after BR treatment. BR-induced H2O2 accumulation was accompanied by increased tolerance to oxidative stress. Inhibition of NADPH oxidase and chemical scavenging of H2O2 reduced BR-induced oxidative and cold tolerance and defense gene expression. BR treatment induced expression of both regulatory genes, such as RBOH, MAPK1, and MAPK3, and genes involved in defense and antioxidant responses. These results strongly suggest that elevated H2O2 levels resulting from enhanced NADPH oxidase activity are involved in the BR-induced stress tolerance.
Plants are constantly exposed to a variety of biotic (i.e. pathogen infection and insect herbivory) and abiotic stresses (i.e. extreme temperature, drought, and salinity). To survive such stresses, plants have evolved intricate mechanisms to perceive external signals and activate optimal responses to environmental conditions. At the molecular level, the perception of extracellular stimuli and subsequent activation of appropriate responses require a complex interplay of signaling cascades. It has been shown that phytohormones, such as salicylic acid (SA), jasmonic acid, and abscisic acid (ABA), regulate the protective responses of plants to both biotic and abiotic stresses independently and through synergistic and antagonistic cross talk (Bostock, 2005; Lorenzo and Solano, 2005; Mauch-Mani and Mauch, 2005). Moreover, plant responses to different types of stresses are associated with generation of reactive oxygen species (ROS), suggesting that ROS may function as a common signal in signaling pathways of plant stress responses (Apel and Hirt, 2004; Torres and Dangl, 2005). More recent studies indicate extensive cross talk of plant signaling pathways for defense against pathogens with those for responses to abiotic stresses (Fujita et al., 2006).
For a long time, ROS was believed as a harmful byproduct in aerobic organisms. Extensive studies have shown that while high levels of ROS cause cell death, low levels of ROS have regulatory roles in plant stress responses. Application of ABA and SA as well as exposure to low temperature all resulted in a transient elevation of H2O2, leading to an increased tolerance to salt, high light, heat, and oxidative stress (Prasad et al., 1994; Dat et al., 1998; Zhang et al., 2001). It has been proposed that ROS plays a critical role in induced tolerance by activating or inducing stress response-related factors, such as mitogen-activated protein kinases (MAPKs), transcription factors, antioxidant enzymes, dehydrins, and low-temperature-induced, heat shock, and pathogenesis-related proteins (Gechev et al., 2006).
Brassinosteroids (BRs) are a group of naturally occurring plant steroids and are important for a broad spectrum of cellular and physiological processes, including stem elongation, pollen tube growth, leaf bending and epinasty, root inhibition, fruit development, ethylene biosynthesis, proton pump activity, xylem differentiation, photosynthesis, and gene expression (Li et al., 1996; Sasse, 1997; Clouse and Sasse, 1998; Dhaubhadel et al., 1999; Hu et al., 2000; Arteca and Arteca, 2001; Müssig et al., 2002; Yu et al., 2004; Fu et al., 2008). Important progress has been made in elucidating the BR signal transduction pathway. The discovery of BR-insensitive mutants in Arabidopsis (Arabidopsis thaliana), pea (Pisum sativum), tomato (Solanum lycopersicum), and rice (Oryza sativa) led to the isolation of the BRI1 gene and its homologs (Li and Chory, 1997; Yamamuro et al., 2000; Montoya et al., 2002; Nomura et al., 2003). BRI1 is a BR-binding Leu-rich repeat receptor located in the plasma membrane and functions in vitro as a Ser/Thr kinase (Wang et al., 2001). Recent studies have identified several other components in the BR signaling pathway (Li and Nam, 2002; Nam and Li, 2002; Mora-García et al., 2004), including BAK1 (for BRI1-associated receptor kinase 1), BIN2 (a GSK3/SHAGGY-like kinase), and BSU1 (for BRI1 suppressor 1, a phosphatase). BR binds to the extracellular domain of BRI1 and activates its intracellular kinase activity (Kinoshita et al., 2004). Activated BRI1 interacts with and activates its coreceptor BAK1 (Li et al., 2002; Nam and Li, 2002). The BIN2 kinase and BSU1 phosphatase function downstream of the receptor kinases and regulate the phosphorylation status of BZR1 and BZR2/BES1 transcription factors (Belkhadir and Chory, 2006; Vert and Chory, 2006). Dephosphorylated BZR1 and BZR2/BES1 recognize the promoters of BR target genes and regulate their expression (He et al., 2002; Yin et al., 2002).
In addition to its critical roles in growth regulation and photomorphogenesis, BRs can induce plant tolerance to a variety of abiotic stresses, such as high and low temperature stress, drought, and salinity injury (Krishna, 2003; Kagale et al., 2007). However, the underlying mechanisms for BR-mediated stress responses are not understood. It has been found that BR-induced increase in the basic thermotolerance is associated with increased heat shock protein synthesis and accumulation as well as increased expression of some components of translational machinery (Dhaubhadel et al., 1999, 2002). The mechanism by which BR induces protein synthesis during heat stress is unclear. BR may also play a role in plant responses to pathogens. BR induces resistance of tobacco (Nicotiana tabacum) and rice to bacterial and fungal pathogens (Nakashita et al., 2003). BR-induced disease resistance was not correlated with enhanced SA accumulation or increased expression of genes associated with SA-regulated systemic acquired resistance (SAR). Additionally, simultaneous treatment of plants with BR and SAR inducers resulted in additive protection against pathogen attack (Nakashita et al., 2003). Thus, BR-induced disease resistance is mediated by a novel signaling pathway distinct from the SA-regulated SAR pathway.
We have previously reported that BR enhances photosynthesis and chill tolerance (Yu et al., 2002, 2004). Subsequently, we have observed unexpectedly that BR also triggers a periodic increase in H2O2 level in cucumber (Cucumis sativus) leaves. H2O2 can function as a signaling molecule in response to various stimuli both in plant and animal cells (Neill et al., 2002). To study the role of elevated H2O2 level in BR-induced stress tolerance, we analyzed the effects of exogenous BR, inhibitors of BR biosynthesis and ROS production, and ROS scavengers on stress tolerance and associated gene expression in cucumber. These studies demonstrated that BR induced tolerance to both biotic and abiotic stresses in cucumber plants. In addition, we provide strong evidence that H2O2 plays a role in the BR-induced plant stress tolerance.
RESULTS
BR Induces Plant Stress Tolerance
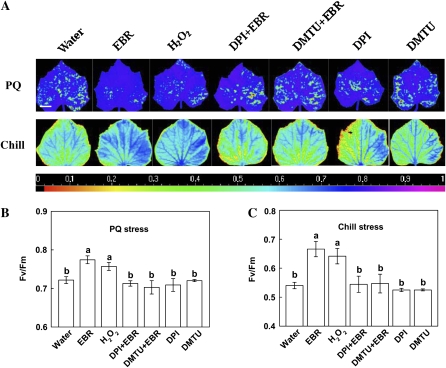
To determine whether BR induces stress tolerance in cucumber plants, we obtained four types of plants with different BR levels by applications of 24-epibrassinolide (EBR), one of the bioactive BRs, and brassinazole (Brz), a specific inhibitor of BR biosynthesis (Supplemental Fig.S1). We first compared the effects of EBR and Brz on plant sensitivity to paraquat (PQ), which causes photo-oxidative stress. When grown under continuous light, necrotic lesions appeared 1 d after PQ treatment on leaves of water-, Brz-, and Brz+EBR-treated plants but not on those of EBR-treated plants (Fig. 1A). To analyze the effects on photosynthetic efficiency, we compared the maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm; Maxwell and Johnson, 2000). Fluorescence images of Fv/Fm showed that PQ treatment resulted in a significant decrease in Fv/Fm in water-treated plants (Fig. 1). PQ-induced reduction in Fv/Fm was less in EBR-treated plants but greater in Brz-treated plants (Fig. 1B). Furthermore, EBR treatment restored Fv/Fm in Brz-treated plants close to that of water-treated plants.
Figure 1.
Effects of BR levels on resistance to PQ, chill, or CMV. A, Symptoms (up) and images of the maximum PSII quantum yield (Fv/Fm, down). The false color code depicted at the bottom of the image ranged from 0 (black) to 1.0 (purple). Plants treated with water, 0.1 μm EBR, 4 μm Brz, and 4 μm Brz + 0.1 μm EBR were challenged with 10 μm PQ at 600 μmol m−2 s−1 light intensity and 25°C for 1 d. Five plants were used for each treatment and the picture of one representative leaf is shown. Bars = 2.5 cm. B, Average Fv/Fm values. Fv/Fm was determined with the whole leaf as area of interest. Fv/Fm for control plants was 0.83. Data are the means of five replicates (±sd). Means denoted by the same letter did not significantly differ at P < 0.05 according to Tukey's test. C, ETRs determined after 1-d exposure to chill stress (8°C/200 μmol m−2 s−1) for plants treated with water, 0.1 μm EBR, 4 μm Brz, and 4 μm Brz + 0.1 μm EBR. Measurements were conducted at 25°C. Data are the means of five replicates (±sd). D, CMV incidence and MDA content determined at 14 dpi for plants treated with water, 0.1 μm EBR, 4 μm Brz, and 4 μm Brz + 0.1 μm EBR. Disease index is the mean (n = 9 leaves) of disease severities (0, light, to 1, severe). Data for MDA are the means of five replicates (±sd). Means denoted by the same letter did not significantly differ at P < 0.05 according to Tukey's test. FW, Fresh weight.
Electron transport rates (ETRs) were determined at 25°C after the chilling stress (8°C/200 μmol m−2 s−1) to assess the effects of EBR and Brz on chilling tolerance of cucumber seedlings. Chilling stress caused significant reduction in ETR. EBR treatment alleviated chilling stress and enhanced the ETR, whereas Brz treatment reduced ETR compared to water-treated plants. In addition, EBR treatment significantly restored ETR of Brz-treated plants (Fig. 1C).
We also examined the role of BR in plant responses to Cucumber mosaic virus (CMV) by comparing disease symptom development and CMV-induced lipid peroxidation based on the malondialdehyde (MDA) content after EBR or Brz treatment. Water-treated plants developed typical CMV symptoms by 10 d postinoculation (dpi). When CMV disease severity was rated at 14 dpi, Brz-treated plants had higher disease index and MDA content than water-treated plants (Fig. 1D), suggesting that BR biosynthesis was important for plant response to CMV. By contrast, CMV disease severity and MDA content in EBR-treated plants were lower than those in water-treated plants. In addition, application of EBR to Brz-treated plants restored resistance to CMV (Fig. 1D). These results indicate that BR enhances plant tolerance or resistance to both abiotic and biotic stresses.
Changes in Gene Expression in Response to BR Levels
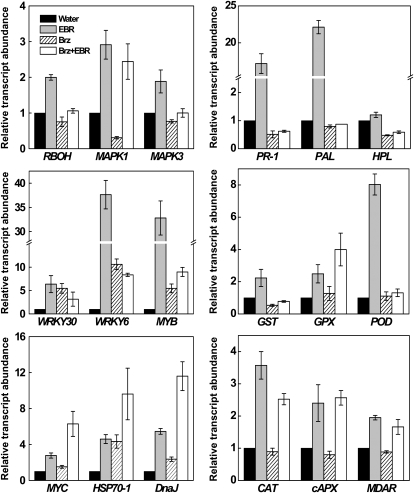
To analyze the underlying molecular mechanisms for BR-induced stress tolerance, we examined the effects of BR levels on expression of 18 stress-responsive genes. As shown in Figure 2, three regulatory genes, RBOH, MAPK1, and MAPK3, were up-regulated upon treatment with EBR but down-regulated after Brz treatment. Among the three genes, MAPK1 was most affected by Brz, whose expression was reduced by approximately 70%. Reductions of RBOH and MAPK3 expression by Brz were more moderate but also substantial. Again, application of EBR to Brz-treated plants rescued the repressed expression of the regulatory genes.
Figure 2.
Expression of stress-responsive genes in response to BR levels in cucumber seedlings. qRT-PCR analysis was performed to examine steady-state levels of mRNAs for 18 genes in plants treated with water, 0.1 μm EBR, 4 μm Brz, and 4 μm Brz + 0.1 μm EBR. Data are the means of three replicates (±sd).
Interestingly, expression of WRKY6 and MYB was induced >30-fold by EBR application (Fig. 2). EBR also induced expression of two other genes encoding transcription factors, WRKY30 and MYC. Unexpectedly, expression of the four transcription factor genes was also up-regulated after Brz treatment. EBR also induced expressions of genes encoding proteins involved in heat shock response (HSP and DnaJ), defense (PR-1, PAL, and HPL), detoxification (GST, GPX, and POD), and antioxidant (CAT, cAPX, and MDAR; Fig. 2). Brz treatment also induced expression of HSP and DnaJ but has little effect on expression of GPX, POD, CAT, PAL, cAPX, and MDAR. On the other hand, expression of PR-1, HPL, and GST were substantially reduced in Brz-treated plants. EBR and Brz had additive effect on the induction of HSP and DnaJ.
Changes in H2O2 by BR Levels
ROS act as second messengers in stress and hormone responses (Apel and Hirt, 2004; Kwak et al., 2006). To determine a possible role of ROS in BR-induced stress tolerance, we attempted to detect in situ accumulation of O2·− and H2O2 using nitroblue tetrazolium (NBT) and 3,3′-diaminobenzidine (DAB) staining procedures, respectively. Both procedures detected increased staining in EBR-treated leaves but decreased staining in Brz-treated leaves relative to that in water-treated leaves (Fig. 3A). However, EBR application to Brz-treated leaves partially restored O2·− and H2O2 levels. Interestingly, we observed enhanced staining on the edges of the leaf discs, probably due to wounding because it was not affected by EBR or Brz treatment (Fig. 3A). Similar effects of EBR and Brz on leaf H2O2 accumulation were observed when an independent spectrophotometric method was used (Fig. 3D).
Figure 3.
The roles of BR in regulation of ROS accumulation. A, In situ detection of leaf O2·− and H2O2. NBT and DAB stains were used to detect the presence of O2·− and H2O2 in leaves treated with water, 0.1 μm EBR, 4 μm Brz, and 4 μm Brz + 0.1 μm EBR. Leaf discs (1.5 cm in diameter) were harvested at 6 h after treatment and stained immediately. B, Cytochemical localization of H2O2 accumulation in mesophyll cells of cucumber leaves with CeCl3 staining and transmission electron microscopy. The plants were treated and harvested as described in (A). Arrows, CeCl3 precipitates; C, chloroplast; CW, cell wall; V, vacuole; IS, intercellular space. C, Blockage of EBR-induced H2O2 accumulation by DPI and DMTU. Plants were pretreated with 100 μm DPI or 5 mm DMTU for 8 h and then treated with 0.1 μm EBR. After 6 h, the DAB staining of leaf discs was performed. D, Quantitative measurements of H2O2 level and NADPH oxidase activity in cucumber leaves with different BR levels. Values are means ± sd (n = 6). Means denoted by the same letter did not significantly differ at P < 0.05 according to Tukey's test. FW, Fresh weight.
Using the CeCl3-based procedures, we showed that EBR-induced H2O2 was predominantly accumulated on the cell walls of mesophyll cells facing intercellular spaces but was undetectable in the cytosol or intracellular organelles, such as chloroplasts, mitochondria, nuclei, or vacuoles (Fig. 3B). In addition, EBR-induced H2O2 accumulation was sensitive to diphenyleneiodonium (DPI), a potent inhibitor of NADPH oxidase (Fig. 3C). Dimethylthiourea (DMTU), an H2O2 scavenger, also abolished EBR-induced H2O2 accumulation. These results suggest that BR-induced H2O2 accumulation is caused by increased activity of NADPH oxidase. To confirm this, we measured the activity of plasma membrane NADPH oxidase in extracts of leaf tissues. NADPH oxidase activities increased significantly in EBR-treated plants but decreased significantly in Brz-treated plants compared with that of water-treated plants (Fig. 3D). Again, EBR was effective in rescuing the repressed NADPH oxidase activity in Brz-treated plants.
Involvement of H2O2 in BR-Induced Stress Tolerance
To determine whether H2O2 accumulation contributes to BR-induced stress tolerance, we analyzed the effects of DPI and DMTU on EBR-induced tolerances to oxidative stress inflicted upon either PQ treatment or chilling stress (8°C) under 1,000 μmol m−2 s−1. Exposure to either stress caused necrotic lesions in water-treated plants (Supplemental Fig. S2). In EBR- or H2O2-treated plants, on the other hand, necrotic lesions were greatly reduced after PQ and chill treatment. Importantly, pretreatment with DPI or DMTU completely abolished the protective effects of EBR and H2O2 on plant tolerance to PQ and the chill (Fig. 4A). EBR and H2O2 treatment also alleviated significantly the decline of Fv/Fm after PQ treatment and the chill, and these protective effects were again almost completely blocked by DPI and DMTU (Fig. 4, B and C). These results strongly suggest that H2O2 is involved in the BR-induced stress tolerance.
Figure 4.
Requirement of H2O2 for EBR-induced resistance to PQ and chill. A, Images of the maximum PSII quantum yield (Fv/Fm) of PQ-challenged and chilled leaves (bar = 2.5 cm). The false color code depicted at the bottom of the image ranged from 0 (black) to 1.0 (purple). Plants were pretreated with 100 μm DPI or 5 mm DMTU for 8 h, and then plants were treated with 0.1 μm EBR or 10 mm H2O2. After 1 d, plants were challenged with 10 μm PQ or exposed to chill at high light intensity (8°C/1,000 μmol m−2 s−1). Single treatment of DPI or DMTU was included as negative control. B and C, Average Fv/Fm values of PQ-challenged (B) or chilled (C) leaves. Fv/Fm was determined with the whole leaf as area of interest. Fv/Fm for control plants was 0.83. Values are means ± sd (n = 5). Means denoted by the same letter did not significantly differ at P < 0.05 according to Tukey's test.
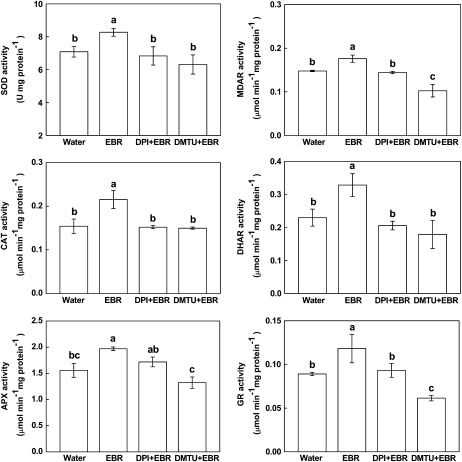
The expression of genes implicated in signal transduction (MAPK1), transcription (WRKY6), and stress tolerance (PR-1, PAL, CAT, and cAPX) was induced by EBR and H2O2 (Fig. 5). Again, DPI and DMTU pretreatment abolished or substantially reduced EBR-induced expression of these genes (Fig. 5). Likewise, both EBR and H2O2 induced expression of antioxidant genes CAT and cAPX, and EBR treatment increased activities of antioxidant enzymes (superoxide dismutase [SOD], catalase [CAT], ascorbate peroxidase [APX], monodehydroascorbate reductase [MDAR], dehydroascorbate reductase [DHAR], and glutathione reductase [GR]; Figs. 5 and 6). The increase in antioxidant gene expression and activities of antioxidant enzymes upon EBR treatment were also blocked by DPI and DMTU pretreatment (Figs. 5 and 6). These results support the involvement of H2O2 in BR-induced gene expressions.
Figure 5.
Involvement of H2O2 in EBR-induced up-regulation of stress-responsive genes. Plants were pretreated with 100 μm DPI or 5 mm DMTU for 8 h and then treated with 0.1 μm EBR or 10 mm H2O2. After 6 h, the steady-state transcript levels were assayed by qRT-PCR. Data are the means of three replicates (±sd).
Figure 6.
Involvement of H2O2 in EBR-induced up-regulation of antioxidant enzyme activity. Plants were pretreated with 100 μm DPI or 5 mm DMTU for 8 h and then treated with 0.1 μm EBR. After 6 h, the activities of antioxidant enzymes were determined. Values are means ± sd (n = 6). Means denoted by the same letter did not significantly differ at P < 0.05 according to Tukey's test.
Time Course of BR-Induced H2O2 Accumulation, Tolerance, Transcript Levels, and Activities of Antioxidant Enzymes
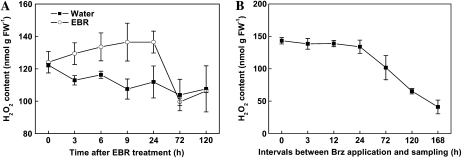
H2O2 levels were increased as early as at 3 h and remained elevated up to 24 h after EBR treatment (Fig. 7A). H2O2 levels were not significantly altered during the first 24 h after Brz treatment but were substantially reduced at 72 h after Brz treatment and continued to decline during the remaining period of the experiments (Fig. 7B). To further investigate the involvement of H2O2 in EBR-induced stress tolerance, we examined the effect of PQ-mediated oxidative stress applied at different intervals after EBR, H2O2, or Brz treatment. Plants were first sprayed with EBR, H2O2, or Brz and at various intervals after the treatment plants were subjected to oxidative stress treatment by applying PQ. The stress tolerance was then determined by measurement of Fv/Fm 1 d after PQ treatment. Enhanced tolerance of cucumber seedlings to PQ-induced oxidative stress was observed at 3 h after treatment with H2O2 and at 6 h after EBR treatment (Fig. 8; Supplemental Fig. S3A). Thus, H2O2 induced stress tolerance more rapidly than EBR (Fig. 8A). The maximum level of stress tolerance was observed at 12 h after treatment with H2O2 and at 24 h after EBR treatment (Fig. 8A). No significant level of stress tolerance was observed at 72 h after treatment with H2O2 and at 120 h after EBR treatment (Fig. 8A). The time course for Brz-induced decline in stress tolerance was highly correlated to that of Brz-induced decrease in H2O2 levels (r = 0.96, P < 0.01). Thus, like changes in H2O2 levels, stress tolerance was not significantly altered during the first 24 h but substantially reduced at 72 h after Brz treatment and continued to decline during the remaining period of the experiments (Fig. 8B; Supplemental Fig. S3B).
Figure 7.
Kinetics of changes in H2O2 content in EBR- or Brz-treated plants. A, Plants were treated with distilled water or 0.1 μm EBR. Leaf samples were harvested at indicated times (h) after EBR treatment. Values are means ± sd (n = 6). B, H2O2 content in leaves after different duration of Brz treatment. Brz (4 μm) treatment started at indicated times (h) before sampling. Brz treatment was repeated on alternative days until sampling. Time zero points were without Brz treatment. Values are means ± sd (n = 6). FW, Fresh weight.
Figure 8.
Levels of tolerance to PQ-induced oxidative stress at different times after EBR, H2O2, and Brz treatment. A, Oxidative stress tolerance induction curves of EBR or H2O2. PQ (10 μm) was applied at indicated times (h) after water, 0.1 μm EBR, or 10 mm H2O2 treatment. Time zero points indicate PQ treatment only. Fv/Fm was determined with the whole leaf as area of interest after 1 d at 600 μmol m−2 s−1 and 25°C. Fv/Fm for PQ-untreated leaves was 0.83. Values are means ± sd (n = 5). B, Oxidative stress tolerance of plants after different duration of Brz treatment. Brz (4 μm) treatment started at indicated times (h) before 10 μm PQ challenge. Brz treatment was repeated on alternative days until PQ challenge. Time zero points indicate PQ treatment only. Fv/Fm was determined using the whole leaf as area of interest after 1 d at 600 μmol m−2 s−1 and 25°C. Fv/Fm for PQ-untreated leaves was 0.83. Values are means ± sd (n = 5).
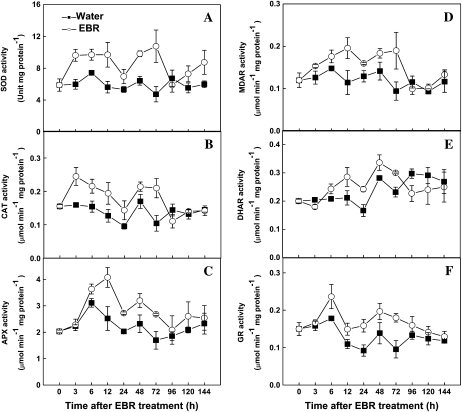
To characterize further the relationship of BR-induced H2O2 and enhanced stress tolerance, we analyzed the temporal changes in transcript levels of several stress-responsive genes and activities of antioxidant enzymes. The expression of RBOH and CAT started to increase at 3 h after EBR treatment, peaked at 12 h, and then declined. The cAPX expression increased at 3 h after EBR treatment and remained elevated for the following 9 h before declining to control level. PR-1 and PAL transcript levels also increased at 3 h, peaked at 12 h, and became undetectable at 72 h after EBR treatment (Fig. 9). Significant increases in activities of SOD, CAT, and MDAR were also detected at 3 h after EBR treatment (Fig. 10). For other antioxidant enzymes (APX, DHAR, and GR), significant increases occurred at 6 h. The activities of all these antioxidant enzymes declined to basal levels at 96 h after EBR treatment (Fig. 10). The decline of the antioxidant enzyme activities at 96 h after EBR treatment was accompanied by disappearance of EBR-induced stress tolerance at 120 h after EBR treatment (Fig. 8A).
Figure 9.
Time-course analysis of steady-state transcript levels of RBOH, CAT, cAPX, PR-1, and PAL in response to EBR. Leaf samples were harvested at indicated times (h) after 0.1 μm EBR treatment, and the steady-state transcript levels were assayed by qRT-PCR. Data are the means of three replicates (±sd).
Figure 10.
Time-course analysis of antioxidant enzymes activities in response to EBR. Leaf samples were harvested at indicated times (h) after 0.1 μm EBR treatment, and the activities of antioxidant enzymes were analyzed. Values are means ± sd (n = 6).
DISCUSSION
BR Induces Stress Tolerance in Cucumber
Several studies have shown that BR enhances plant tolerance to a variety of environmental stresses (Khripach et al., 2000; Dhaubhadel et al., 2002; Nakashita et al., 2003; Krishna, 2003; Kagale et al., 2007). However, it is difficult to analyze genetically the role and action mechanisms of BR in plant stress tolerance because of the strong and pleiotropic phenotypes of BR biosynthesis and signaling mutants, including extreme dwarfism, dark green and epinastic leaves, and delayed development (Khripach et al., 2000; Bishop and Koncz 2002; Müssig et al., 2002; Cao et al., 2005). Moreover, current techniques allow for measurement of stress-induced changes in the levels of only intermediates in the BR biosynthesis pathway but not of the bioactive brassinolide (Nakashita et al., 2003; Jager et al., 2008). As a result, application of Brz, a specific and potent inhibitor of BR biosynthesis (Asami et al., 2000, 2001), has been used to study the role of BR in plant stress responses. Consistent with the feedback control of BR biosynthesis (Bancos et al., 2002), Brz up-regulated BR biosynthetic genes and caused growth aberrations of cucumber seedlings (Supplemental Figs. S1 and S4). These observations support that endogenous BR contents in cucumber seedlings were altered by Brz application.
Our results demonstrated that EBR enhanced and Brz reduced tolerance to oxidative, cold, and CMV stresses in cucumber (Fig. 1). However, in comparison to the relatively fast induction (approximately 3 h) of tolerance by EBR application, the negative effect of Brz on stress tolerance was relatively slow (Fig. 8). Brz is a specific inhibitor for DWF4, a cytochrome P450 monooxygenase of the BR biosynthetic pathway (Asami et al., 2001), and it could not inhibit the downstream enzymes in the BR biosynthesis pathway. Accordingly, it needs a period of time to reduce the bioactive BR level. These results strongly suggest that BR-induced stress tolerance is quantitative in nature and is correlated with the BR levels. In other words, normal synthesis of BRs under nonstress conditions is expected to confer a certain level of stress tolerance, but an increase in BR accumulation under certain types of stress conditions will lead to a corresponding increase in stress tolerance. Conversely, reducing BR accumulation below its normal levels (e.g. after Brz application) leads to a corresponding decrease in stress tolerance. These studies support the involvement of BR in plant responses to various environmental stresses (Krishna, 2003; Nakashita et al., 2003; Kagale et al., 2007). There were significant increases in several intermetabolite levels in the BR biosynthesis pathway after Tobacco mosaic virus inoculation in tobacco leaves (Nakashita et al., 2003), whereas there were no consistent changes in the intermetabolite levels in leaves of pea in response to water stress (Jager et al., 2008). Accordingly, BR biosynthesis may be induced in some but not all types of stress conditions. Furthermore, we recently found that as in Brz-treated cucumber plants, the tomato BR biosynthesis dim mutant exhibited enhanced sensitivity to PQ-induced oxidative stress, and this phenotype can be rescued by exogenously applied BR. Most recently, we also found that EBR application to wild-type and BR-deficient mutant all increased their resistance to Cladosporium fulvum in tomato (data not shown). There was, however, no significant difference in the tolerance of wild-type and BR-deficient mutant to water stress in pea (Jager et al., 2008). Thus, BR may be involved in plant responses to some but not all types of plant stress conditions. Alternatively, the severe morphological or physiological phenotypes, such as the dwarf shoots with thick leaves and decreased stomatal conductance in BR-deficient pea plants, might complicate the water stress analysis because these phenotypes could lead to reduced transpiration and increased drought resistance.
Microarray analysis revealed that BR induces the expression of heat shock protein (HSP83, HSP70, Hsc70-3, and Hsc70-G7), heat shock factor (HSF3), and oxidative stress-related genes (GST, ATPA2, and ATP24a) in Arabidopsis (Goda et al., 2002; Müssig et al., 2002). Our quantitative reverse transcription (qRT)-PCR analysis revealed similar induction by EBR of HSP70, DnaJ, GST, and POD in cucumber (Fig. 2). BR induced defense and antioxidant genes in the absence of stresses. In contrast, expressions of cold and pathogenesis-related genes are reduced in Brz-treated cucumber seedlings or BR-deficient Arabidopsis mutants (Szekeres et al., 1996; Müssig et al., 2002). These results suggest that BR enhances plant stress tolerance by activating genes involved in plant defense and stress responses.
H2O2 Is Involved in BR-Induced Stress Tolerance in Cucumber
In this study, we have provided several lines of evidences that H2O2 is involved in BR-induced stress tolerance. First, EBR-induced stress tolerance was preceded by increased NADPH oxidase activity and elevated H2O2 levels (Fig. 3). Second, scavenging of H2O2 by DMTU or inhibiting H2O2 generation by DPI abolished EBR-induced stress tolerance (Fig. 4). Third, EBR-and Brz-induced changes in H2O2 levels were closely related to the changes in the tolerance to PQ-mediated oxidative stress (Figs.7 and 8; Supplemental Fig. S3), and exogenously applied H2O2 also induced the tolerance (Fig. 8A; Supplemental Fig.S3A). In addition, the temporal changes in H2O2 accumulation, transcript levels of antioxidant genes, and activities of antioxidant enzymes are consistent with the role of H2O2 in BR-induced stress tolerance (Figs. 7, 9, and 10). Of particular relevance is the observation that the interval between EBR treatment and stress challenge is critical for the magnitude of EBR-induced stress tolerance (Figs. 7 and 8). Accordingly, the variation of the efficiency of BR in enhancing plant stress tolerance in different studies (Khripach et al., 2000) is likely to be related to time interval between BR application and stress challenge.
H2O2 is considered as a central signaling molecule in plant responses to biotic and abiotic stresses (Foyer et al., 1997; Neill et al., 2002). It activates protective mechanisms for tolerance to chill in maize (Zea mays; Prasad et al., 1994), high temperature in mustard (Sinapis alba) seedlings (Dat et al., 1998), and light stress in Arabidopsis leaves (Karpinski et al., 1999). Perturbed H2O2 homeostasis or increased production of H2O2 enhanced the expression of antioxidant enzymes and acidic PR proteins in the absence of pathogen challenge (Chamnongpol et al., 1996; Takahashi et al., 1997; Wu et al., 1997). Likewise, we found that EBR treatment induced increase in both H2O2 and PR-1 mRNA levels (Figs. 2 and 3) without any significant effect on SA accumulation (Supplemental Fig. S5). However, BR did not induce changes in expression of PR genes in tobacco (Nakashita et al., 2003). This discrepancy might be caused by different interval between BR application and sampling time or the low sensitivity of northern blots to detect minor changes in expression of PR genes. In support of this, strong induction of PR-1 was transient and observed only within a short period of time after EBR treatment (Fig. 9). Verberne et al. (2003) have reported that ethylene is also required for the production or transmission of the mobile SAR signal in Tobacco mosaic virus-infected leaves. BR induced expression of 1-aminocyclopropane-1-carboxylic acid oxidase in cucumber (data not shown), and the increase in stress tolerance in BR-treated potato (Solanum tuberosum) tubers is associated with increased levels of ABA and ethylene and accumulation of phenolic and terpenoid compounds (Krishna, 2003). Moreover, microarray data indicate that there is cross talk between BR and jasmonic acid signaling pathways (Goda et al., 2002; Müssig et al., 2002). It is likely that BR-induced PR gene expression and stress tolerance are mediated by a complex set of signal transcription pathways with H2O2 as a common signal molecule in the activation of stress responses. It may also not be completely unexpected that signal transduction pathways mediating plant growth may cross talk with those mediating plant defense and stress responses.
In higher plants, ROS can be generated by several different pathways, including plasma membrane-localized NADPH oxidase, cell wall-localized peroxidases, and amine oxidases (Neill et al., 2002). We have presented evidence that NADPH oxidase is the potential source of BR-induced H2O2 generation. First, H2O2 accumulated mainly in the apoplast of mesophyll cells. Second, treatment with EBR significantly increased the activity of NADPH oxidase. Third, DPI, a potential inhibitor of NADPH oxidase, blocked EBR-induced production of H2O2. However, because DPI may also inhibit other oxidases (Bolwell et al., 1998), we cannot completely rule out the possibility that other oxidases may contribute to BR-induced ROS generation.
It has been reported that BRI1-associated coreceptor BAK1 and BKK1 (for BAK1-like 1) function not only in BR-dependent signaling in plant growth and development but also in the regulation of plant cell death. BAK1-deficient plants exhibited spreading necrosis accompanied by enhanced accumulation of ROS after infection by pathogens (Kemmerling et al., 2007). The bak1 bkk1 double mutants also accumulated enhanced ROS and exhibited seedling lethality (He et al., 2007). However, the role of BAK1 and BKK1 in plant cell death control is independent of BR (He et al., 2007; Kemmerling et al., 2007). It remains to be determined whether BRI1, BAK1, and BKK1 are required for BR-induced ROS production and stress tolerance.
The Signaling Pathways for BR-Induced Stress Tolerance
EBR induced genes encoding MAPK and transcription factors (Fig. 2). In plants, the MAPK cascade plays a crucial role in various biotic and abiotic stress responses and in hormone signaling, which often involves ROS (Nakagami et al., 2005). H2O2 can activate ANP1, an Arabidopsis MAPK kinase kinase to regulate the activities of MPK3 and MPK6 (Kovtun et al., 2000). A recently identified Ser/Thr protein kinase (OXI1) has also been shown to play a central role in ROS sensing and the activation of MPK3 and MPK6, which control the activation of different defense mechanisms (Rentel et al., 2004). Thus, MAPK cascades can mediate H2O2 signaling and may also play an important role in BR-induced stress tolerance.
In addition to the MAPK genes, BR-induced stress tolerance is associated with expression of a number of genes encoding WRKY, MYB, and MYC transcription factors. WRKY transcription factors have been implicated in the regulation of transcriptional reprogramming associated with plant immune responses (Eulgem and Somssich, 2007), and MYB and MYC transcription factors have been implicated as critical regulators of ABA-inducible gene expression under drought stress (Abe et al., 2003; Agarwal et al., 2006). Similarly, genome-wide expression analyses have shown that BR can regulate expression of genes encoding MYB and ERF transcription factors (Goda et al., 2002; Müssig et al., 2002). More recently, Kagale et al. (2007) showed that BR enhances expression of transcription factors of the CBF/DREB family in both unstressed and stress plants. The concerted induction of genes encoding these transcription factors suggests that BR-induced stress tolerance is mediated by transcriptional activation of genes involved in plant stress responses. Intriguingly, treatment of Brz also resulted in enhanced expression of transcription factors and heat shock proteins without concomitant induction of other defense genes expression. It is likely that BR deficiency may cause certain stress response and, as a result, induce expression of some but not all the transcription factors required for enhanced stress tolerance as observed in BR-treated plants (Szekeres et al., 1996; Kagale et al., 2007).
In conclusion, we have presented strong evidence that H2O2 mediates the transcriptional induction of defense or antioxidant genes by BR. Following perception of BR signal, NADPH oxidase may be activated to produce ROS, which initiates a protein phosphorylation cascade. Transcription factors may be activated via a phosphorylation cascade by MAPKs. Finally, the products of targets genes participate directly in cellular protection (Fig. 11). Further studies are needed to provide genetic evidence of the involvement of NADPH oxidase in BR-induced ROS generation, to identify the critical signaling components between BR perception and stress responses, and to elucidate the molecular mechanisms of cross talk between BR and other hormone signaling.
Figure 11.
A model for the induction of stress tolerance by BR in cucumber plants. Perception of BR by receptors results in the activation of plasma membrane-bound NADPH oxidase (RBOH). Activated NADPH oxidase results in elevated levels of H2O2, which functions as a signal molecule to activate stress response pathways.
MATERIALS AND METHODS
Plant Growth
Cucumber (Cucumis sativus ‘Jinyan No. 4’) seeds were sown in a growth chamber. Seven days after sowing, groups of eight seedlings were transplanted into a container (40 × 25 × 15 cm) filled with Hoagland nutrient solution. The growth conditions were as follows: a 12-h photoperiod, temperature of 25°C/17°C (day/night), and light intensity of 600 μmol m−2 s−1.
Experimental Design
To manipulate BR levels, we first treated cucumber seedlings with the BR biosynthesis inhibitor Brz by spraying a 4 μm solution (Brz dissolved in DMSO) to the tip and whole plants every 2 d from the cotyledon stage to the four-leaf stage. Both water- and Brz-treated plants were then divided equally into two groups for water or EBR treatments. Previous tests showed that a relatively moderate concentration of EBR at 0.1 μm is most effective, which was used in this experiment (Yu et al., 2004). This combination of treatments resulted in four types of plants: water (BR level unchanged), Brz (BR level reduced), EBR (BR level increased), and Brz + EBR (BR level reduced and then recovered) treatments. At the four-leaf stage, these four types of seedlings were then exposed to various forms of biotic or abiotic stresses. The third leaf from the bottom was used for analysis. For cold stress, seedlings were transferred to 8°C/200 μmol m−2 s−1 for 24 h and then returned to normal growth conditions for a 2-h recovery. Oxidative stress was induced by spraying with 10 μm PQ at 600 μmol m−2 s−1 and 25°C for 1 d. CMV was prepared from virus-infected leaf tissues by grounding in an inoculation buffer containing 0.1 m sodium phosphate (pH 7.5), 2% (w/v) polyvinylpyrrolidone (PVP), and 0.2% (w/v) Na2SO3 at ratio of 1:100. The extract was used for inoculation of the cucumber leaves. The injuries or disease index was evaluated after CMV infection. For time-course analysis of EBR-, Brz-, and H2O2-induced changes in the tolerance to oxidative stress, cucumber seedlings were first treated with EBR or H2O2 and challenged with 10 μm PQ at different time points after treatment, whereas plants with different duration of Brz treatment were challenged simultaneously with 10 μm PQ. To investigate the role of ROS in the resistance, leaves were pretreated with 100 μm DPI (an NADPH oxidase inhibitor) or 5 mm DMTU (an H2O2 and OH· scavenger) for 8 h, and then plants were treated with 0.1 μm BR or 10 mm H2O2. After 1 d, plants were sprayed with 10 μm PQ under the same conditions as described above or exposed to cold at 8°C and 1,000 μmol m−2 s−1 for 1.5 h. Stress tolerance was measured based on changes in the maximal quantum yield of PSII (Fv/Fm).
Analysis of Chlorophyll Fluorescence
Chlorophyll fluorescence was determined with an imaging pulse amplitude modulated fluorometer (IMAG-MAXI; Heinz Walz). For measurement of maximal quantum yield of PSII (Fv/Fm), plants were dark-adapted for 30 min. Minimal fluorescence (Fo) was measured during the weak measuring pulses and maximal fluorescence (Fm) was measured by a 0.8-s pulse light at about 4,000 μmol m−2 s−1. Fv/Fm was determined with the whole leaf as area of interest. The ETRs at a given actinic irradiance are determined according to White and Critchley (1999) and calculated as (Fm′ − Fs)/Fm′ × PAR × 0.5 × α, where (Fm′ − Fs)/Fm′ is the quantum yield of PSII (ΦPSII) in the light, PAR is the actinic irradiance, 0.5 is the assumed proportion of absorbed quanta used by PSII reaction centers, and α is the leaf absorbance for cucumber leaves, respectively.
Histochemical Staining of O2·− and H2O2
The histochemical staining of O2·− and H2O2 was performed as previously described (Jabs et al., 1996; Thordal-Christensen et al., 1997) with minor modifications. In the case of O2·−, leaf discs (1.5 cm in diameter) were vacuum infiltrated directly with 0.1 mg mL−1 NBT in 25 mm K-HEPES buffer (pH 7.8) and incubated at 25°C in the dark for 2 h. In the case of H2O2, leaf discs were vacuum infiltrated with 1 mg mL−1 DAB in 50 mm Tris-acetate (pH 3.8) and incubated at 25°C in dark for 24 h. In both cases, leaf discs were rinsed in 80% (v/v) ethanol for 10 min at 70°C, mounted in lactic acid/phenol/water (1:1:1; v/v), and photographed.
Cytochemical Detection of H2O2
H2O2 was visualized at the subcellular level using CeCl3 for localization (Bestwick et al., 1997). Electron-dense CeCl3 deposits are formed in the presence of H2O2 and are visible by transmission electron microscopy. Tissue pieces (1–2 mm2) were excised from the leaves and incubated in freshly prepared 5 mm CeCl3 in 50 mm MOPS at pH 7.2 for 1 h. The leaf sections were then fixed in 1.25% (v/v) glutaraldehyde and 1.25% (v/v) paraformaldehyde in 50 mm sodium cacodylate buffer, pH 7.2, for 1 h. After fixation, tissues were washed twice for 10 min in the same buffer and postfixed for 45 min in 1% (v/v) osmium tetroxide and then dehydrated in a graded ethanol series (30–100%; v/v) and embedded in Eponaraldite (Agar Aids). After 12 h in pure resin, followed by a change of fresh resin for 4 h, the samples were polymerized at 60°C for 48 h. Blocks were sectioned (70–90 nm) on a Reichert-Ultracut E microtome and mounted on uncoated copper grids (300 mesh). Sections were examined using a transmission electron microscope at an accelerating voltage of 75 kV.
Determination of H2O2 and MDA in Leaf Extracts
The concentration of H2O2 in leaves was measured by monitoring the absorbance of the titanium-peroxide complex at 415 nm using the method of Brennan and Frenkel (1977). The absorbance was quantified using a standard curve generated from known concentrations of H2O2. Oxidative damage to lipids was estimated by measuring the content of MDA in leaf homogenates, prepared with 10% TCA only. Samples were mixed with 10% TCA containing 0.65% 2-thiobarbituric acid (TBA) and heated at 95°C for 25 min, as by Hodges et al. (1999). MDA content was calculated by correcting for compounds, other than MDA, that absorb at 532 nm, by subtracting the absorbance at 532 nm of a solution containing plant extract incubated without TBA from an identical solution containing TBA.
Isolation of Plasma Membrane and the Determination of NADPH Oxidase Activity
Leaf plasma membranes were isolated using the two-phase aqueous polymer partition system (Larsson et al., 1987). Samples were homogenized in four volumes of the extraction buffer (50 mm Tris-HCl, pH 7.5, 0.25 m Suc, 1 mm ascorbic acid (AsA), 1 mm EDTA, 0.6% PVP, and 1 mm PMSF). The homogenate was filtered through four layers of cheesecloth, and the resulting filtrate was centrifuged at 10,000g for 15 min. Microsomal membranes were pelleted from the supernatant by centrifugation at 50,000g for 30 min. The pellet was suspended in 0.33 m Suc, 3 mm KCl, and 5 mm potassium phosphate, pH 7.8. The plasma membrane fraction was isolated by adding the microsomal suspension to an aqueous two-phase polymer system to give a final composition of 6.2% (w/w) Dextran T500, 6.2% (w/w) polyethylene glycol 3350, 0.33 m Suc, 3 mm KCl, and 5 mm potassium phosphate, pH 7.8. Three successive rounds of partitioning yielded the final upper phase. The upper phase produced was diluted 5-fold in Tris-HCl dilution buffer (10 mm, pH 7.4) containing 0.25 m Suc, 1 mm EDTA, 1 mm DTT, 1 mm AsA, and 1 mm PMSF. The fractions were centrifuged at 120,000g for 30 min. The pellets were then resuspended in Tris-HCl dilution buffer and used immediately for further analysis. All procedures were carried out at 4°C. Protein content of plasma membranes was determined according to the method of Bradford (1976) with BSA as standard.
The NADPH-dependent O2·−-generating activity in isolated plasma membrane vesicles was examined using SOD-inhibitable ferricytochrome c reduction. An aliquot of the isolated PM vesicles was added to a reaction mixture consisting of 50 mm HEPES-KOH (pH 7.8), 100 μm EDTA, 50 μm ferricytochrome c, and 100 μm NADPH in the presence or absence of SOD (200 units/mL, SOD from bovine erythrocytes; Sigma-Aldrich) and incubated at room temperature for 30 s. The activity was based on difference between A550 with or without SOD and the absorbance coefficient of 21.0 mm−1 cm−1.
Antioxidant Enzyme Extraction and Activity Assay
For the enzyme assays, 0.3 g of leaf were ground with 3 mL ice-cold 25 mm HEPES buffer (pH 7.8) containing 0.2 mm EDTA, 2 mm AsA, and 2% PVP. The homogenates were centrifuged at 4°C for 20 min at 12,000g, and the resulting supernatants were used for the determination of enzymatic activity. SOD activity was assayed by measuring the ability to inhibit the photochemical reduction of NBT following the method of Stewart and Bewley (1980). CAT activity was measured as a decline in A240 using the method of Patra et al. (1978). APX and DHAR activities were measured by a decrease in A290 and an increase in A265 according to Nakano and Asada (1981). MDAR activity was measured by using 1 unit ascorbate oxidase, and the oxidation rate of NADH was followed at 340 nm (Hossain et al., 1984). GR activity was measured according to Foyer and Halliwell (1976), which depends on the rate of decrease in the absorbance of NADPH at 340 nm. All spectrophotometric analyses were conducted on a SHIMADZU UV-2410PC spectrophotometer.
Total RNA Extraction and Gene Expression Analysis
Total RNA was extracted from cucumber leaves using Trizol according to the supplier's recommendation. Residual DNA was removed with purifying column. One microgram of total RNA was reverse transcribed using 0.5 μg of oligo(dT)12-18 (Invitrogen) and 200 units of Superscript II (Invitrogen) following the supplier's recommendation. On the basis of EST sequences, the gene-specific primers are shown in Supplemental Table I and used for amplification.
Quantitative real-time PCR was performed using the iCycler iQ real-time PCR detection system (Bio-Rad). PCRs were performed using the SYBR Green PCR Master Mix (Applied Biosystems). The PCR conditions consisted of denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s. A dissociation curve was generated at the end of each PCR cycle to verify that a single product was amplified using software provided with the iCycler iQ real-time PCR detection system. The identity of the PCR products was verified by single-strand sequencing using MegaBACE 1000 DNA analysis system (Amersham Biosciences). To minimize sample variations, mRNA expression of the target gene was normalized relative to the expression of the housekeeping gene actin. All experiments were repeated three times for cDNA prepared for two samples of cucumber leaves. The quantification of mRNA levels is based on the method of Livak and Schmittgen (2001). The threshold cycle (Ct) value of actin was subtracted from that of the gene of interest to obtain a ΔCt value. The Ct value of untreated control sample was subtracted from the ΔCt value to obtain a ΔΔCt value. The fold changes in expression level relative to the control were expressed as 2−ΔΔCt.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: RBOH (FJ036897), MAPK1 (FJ036898), MAPK3 (FJ0368902), WRKY30 (FJ036895), WRKY6 (FJ036899), MYB (FJ0368901), MYC (FJ036894), HSP70 (AJ249329), DnaJ (X67695), PR-1 (DQ641122), PAL (AF475285), HPL (AF229811), GST (FJ0368900), GPX (FJ036896), POD (M91373), CAT (AY274258), cAPX (D88649), MDAR (D26392), DWARF (EW968286), and actin (AAZ74666).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phenotypes of four types of plants with different BR levels.
Supplemental Figure S2. Oxidative symptoms of the PQ-challenged leaves after different treatments.
Supplemental Figure S3. Images of the maximum PSII quantum yield (Fv/Fm) of PQ-challenged leaves after different time of EBR treatment and different duration of Brz treatment.
Supplemental Figure S4. DWRF gene expression in four types of plants with different BR levels.
Supplemental Figure S5. SA accumulation after EBR or Brz treatment.
Supplemental Table S1. Primers used for real-time RT-PCR assays.
Supplementary Material
This work was supported by the National Basic Research Program of China (grant no. 2009CB119000), the National Natural Science Foundation of China (grant nos. 3050344 and 30671428), and the Program for Promotion of Basic Research Activities for Innovative Bioscience (PROBRAIN).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jing-Quan Yu (jqyu@zju.edu.cn).
The online version of this article contains Web-only data.
References
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281 37636–37645 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Arteca JM, Arteca RN (2001) Brassinosteroid-induced exaggerated growth in hydroponically grown Arabidopsis plants. Physiol Plant 112 104–112 [DOI] [PubMed] [Google Scholar]
- Asami T, Min YK, Nagata N, Yamagishi K, Takatsuto S, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S (2000) Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol 123 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Mizutani M, Fujioka S, Goda H, Min YK, Shimada Y, Nakano T, Takatsuto S, Matsuyama T, Nagata N, et al (2001) Selective interaction of triazole derivatives with DWF4, a cytochrome P450 monooxygenase of the brassinosteroid biosynthesis pathway, correlates with brassinosteroid deficiency in planta. J Biol Chem 276 25687–25691 [DOI] [PubMed] [Google Scholar]
- Bancos S, Nomura T, Sato T, Molnar G, Bishop GJ, Koncz C, Yokota T, Nagy F, Szekeres M (2002) Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiol 130 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y, Chory J (2006) Brassinosteroid signaling: a paradigm for steroid hormone signaling from the cell surface. Science 314 1410–1411 [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Koncz C (2002) Brassinosteroids and plant steroid hormone signaling. Plant Cell (Suppl) 14 S97–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM (1998) Comparative histochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol 116 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43 545–580 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle protein–dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Brennan T, Frenkel C (1977) Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SQ, Xu QT, Cao YJ, Qian K, An K, Zhu Y, Hu BZ, Zhao HF, Kuai BK (2005) Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant 123 57–66 [Google Scholar]
- Chamnongpol S, Willekens H, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1996) Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J 10 491–503 [Google Scholar]
- Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49 427–451 [DOI] [PubMed] [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaubhadel S, Browning KS, Gallie DR, Krishna P (2002) Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J 29 681–691 [DOI] [PubMed] [Google Scholar]
- Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40 333–342 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10 366–371 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133 21–25 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100 241–254 [Google Scholar]
- Fu FQ, Mao WH, Shi K, Zhou YH, Asami T, Yu JQ (2008) A role of brassinosteroids in early fruit development in cucumber. J Exp Bot 59 2299–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9 436–442 [DOI] [PubMed] [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28 1091–1101 [DOI] [PubMed] [Google Scholar]
- Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S (2002) Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 130 1319–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Yang YL, Li JM, Wang ZY (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci USA 99 10185–10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17 1109–1115 [DOI] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange PK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207 604–611 [DOI] [PubMed] [Google Scholar]
- Hossain MA, Nakano Y, Asada K (1984) Monodeydroascorbate reductase in spinach chloroplast and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25 385–395 [Google Scholar]
- Hu Y, Bao F, Li J (2000) Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J 24 693–701 [DOI] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 27 1853–1856 [DOI] [PubMed] [Google Scholar]
- Jager CE, Symons GM, Ross JJ, Reid JB (2008) Do brassinosteroids mediate the water stress response? Physiol Plant 133 417–425 [DOI] [PubMed] [Google Scholar]
- Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P (2007) Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225 353–364 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux PM (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284 654–657 [DOI] [PubMed] [Google Scholar]
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Abu Qamar S, Mengiste T, Betsuyaku S, Parker JE, Müssig C, et al (2007) The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17 1116–1122 [DOI] [PubMed] [Google Scholar]
- Khripach V, Zhabinskii V, De Groot A (2000) Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Ann Bot (Lond) 86 441–447 [Google Scholar]
- Kinoshita T, Caño-Delgado A, Seto H, Hiranuma S, Fujioka S, Yoshida S, Chory J (2004) Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433 167–171 [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22 289–297 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Nguyen V, Schroeder JI (2006) The role of reactive oxygen species in hormonal responses. Plant Physiol 141 323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C, Widell S, Kjellbom P (1987) Preparation of high purity plasma membranes. Methods Enzymol 148 558–568 [Google Scholar]
- Li JM, Chory J (1997) A putative leucine rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90 929–38 [DOI] [PubMed] [Google Scholar]
- Li JM, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272 398–401 [DOI] [PubMed] [Google Scholar]
- Li JM, Nam KH (2002) Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295 1299–1301 [DOI] [PubMed] [Google Scholar]
- Li JM, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110 213–222 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signaling network. Curr Opin Plant Biol 8 532–540 [DOI] [PubMed] [Google Scholar]
- Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8 409–414 [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51 659–668 [DOI] [PubMed] [Google Scholar]
- Montoya T, Nomura T, Farrar K, Kaneta T, Yokota T, Bishop GJ (2002) Cloning the tomato curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell 14 3163–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-García S, Vert G, Yin Y, Caño-Delgado A, Cheong H, Chory J (2004) Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev 18 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müssig C, Fischer S, Altmann T (2002) Brassinosteroid-regulated gene expression. Plant Physiol 129 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Pitzschke A, Hirt H (2005) Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 10 339–346 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22 867–880 [Google Scholar]
- Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S (2003) Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33 887–898 [DOI] [PubMed] [Google Scholar]
- Nam KH, Li JM (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110 203–212 [DOI] [PubMed] [Google Scholar]
- Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5 388–395 [DOI] [PubMed] [Google Scholar]
- Nomura T, Bishop G, Kaneta T, Reid JB, Chory J, Yokota T (2003) The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J 36 291–300 [DOI] [PubMed] [Google Scholar]
- Patra HK, Kar M, Mishra D (1978) Catalase activity in leaves and cotyledons during plant development and senescence. Biochem Physiol Pflanz 172 385–390 [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427 858–861 [DOI] [PubMed] [Google Scholar]
- Sasse JM (1997) Recent progress in brassinosteroid research. Physiol Plant 100 696–701 [Google Scholar]
- Stewart RRC, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65 245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85 171–182 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Chen Z, Du H, Liu Y, Klessig DF (1997) Development of necrosis and activation of disease resistance in transgenic tobacco plants with severely reduced catalase levels. Plant J 11 993–1005 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11 1187–1194 [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8 397–403 [DOI] [PubMed] [Google Scholar]
- Verberne MC, Hoekstra J, Bol JF, Linthorst HJM (2003) Signaling of systemic acquired resistance in tobacco depends on ethylene perception. Plant J 35 27–32 [DOI] [PubMed] [Google Scholar]
- Vert G, Chory J (2006) Downstream nuclear events in brassinosteroid signaling. Nature 441 96–100 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J (2001) BRI1 is a critical component of a plasmamembrane receptor for plant steroids. Nature 410 380–383 [DOI] [PubMed] [Google Scholar]
- White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59 63–72 [Google Scholar]
- Wu G, Shortt BJ, Lawrence EB, León J, Fitzsimmons KC, Levine EB, Raskin I, Shah DM (1997) Activation of host defense mechanisms by elevated production of H2O2 in transgenic plants. Plant Physiol 115 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12 1591–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wang ZY, Mora-Garcia S, Li JM, Yoshida S, Asami T, Chory J (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109 181–191 [DOI] [PubMed] [Google Scholar]
- Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55 1135–1143 [DOI] [PubMed] [Google Scholar]
- Yu JQ, Zhou YH, Ye SF, Huang LF (2002) 24-Epibrassinolide and abscisic acid protect cucumber seedlings from chilling injury. J Hortic Sci Biotechnol 77 470–473 [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.