Abstract
The hematopoietic system of mice is established during the early to midgestational stage of development. However, the earliest lymphohematopoietic progenitors that appear during mouse development have been less well characterized compared with the hematopoietic stem cell compartment of fetal liver and bone marrow. We isolated the earliest lymphohematopoietic progenitors by using embryonic stem (ES) cell culture in vitro. Cells with the c-Kit+Lin− cell surface phenotype were present abundantly in ES cells cocultured with stromal cell lines. We further separated the cells into two distinct cell subsets based on AA4.1 expression. Although AA4.1+ and AA4.1− cells had equivalent potency to generate myeloid cell lineages, the lymphoid potential in ES-cell-derived cells was largely restricted to the cells expressing AA4.1. The same cell type was present abundantly in the early yolk sac and in fewer numbers (≈5% of that in the yolk sac) in the caudal half of the developing embryos. These data suggest that AA4.1 is a cell surface marker that can identify the earliest lymphohematopoietic progenitors in mouse development.
Keywords: embryonic stem cell, embryonic, hematopoiesis, yolk sac
Hematopoietic stem cells (HSCs) are a rare subset of cells that are capable of self-renewal and differentiation into all mature blood cell lineages (1). During mouse development, the first hematopoietic cells appear in yolk sac (YS) blood islands (2) and subsequently in the aorta-gonad-mesonephros (AGM) (3) and placental regions (4, 5). After the establishment of circulation, hematopoietic progenitors colonize the fetal liver (FL) and later the bone marrow (BM) and spleen (6). Although the site where HSCs initially originate in the mouse embryo is controversial (7–11), in late fetal life, the BM becomes the major site of hematopoiesis where HSCs reside, and it produces mature blood cells throughout life (12, 13). Antibody-mediated phenotyping of cell surface molecules and cell sorting techniques have been used to identify and isolate a rare HSC compartment from hematopoietic tissues, and the techniques are especially successful to isolate pure HSCs from FL and BM (13–16).
Embryonic stem (ES) cells, pluripotent cell lines derived from the inner cell mass of blastocysts (17, 18), or induced pluripotent stem cells generated from fibroblasts by the transduction of transcription factors (19) are an alternative source of hematopoietic cells. Pluripotent stem cell lines can be a theoretically unlimited source of HSCs because the cells expand indefinitely in an undifferentiated state in vitro (20–22). Extrapolated to humans, pluripotent stem cell lines can be a potential candidate source for treating hematopoietic disorders. ES cell differentiation in vitro recapitulates early embryonic development in vivo, thus providing a potent analytical tool of early development. The potency of ES cells to generate myeloid and lymphoid lineage cells in vitro has been reported (23–26). Despite these observations, ES-cell-derived hematopoietic cells in vitro are known to have only a limited ability to engraft adult mice and lack long-term multilineage repopulating activity in vivo (27), although ES cells could contribute to the hematopoietic system for at least 10 months when replanted into blastocysts giving rise to chimeric mice (28–30). The presence of lymphohematopoietic progenitors in ES cell cultures was suggested initially by a report that examined the hematopoietic potential of cells derived from overexpressed BCR/ABL in ES cells: the ES cells acquired improved ability to engraft mice, and hematopoietic progenitors with both myeloid and lymphoid potential were demonstrated by in vivo assay (31). In addition, HOXB4 with or without enforced expression of Cdx4 was recently reported to confer long-term multilineage repopulating capability on ES-cell-derived progenitors (32, 33). However, whether these genes modified the transplantability of preexisting HSCs or changed the self-renewal and differentiation potential of ES-cell-derived hematopoietic progenitors into HSC-like properties is not known, because these experiments depended on exogenously introduced genes.
We examined cell surface phenotypes and differentiation potentials of ES-cell-derived hematopoietic progenitors developed on stromal cell coculture to determine the earliest lymphohematopoietic progenitors in early embryos. We found that a cell population positive for both c-Kit and AA4.1 expression appearing on the early ES cell culture had both myeloid and lymphoid lineage potentials. In embryos developing in utero, AA4.1+ lymphohematopoietic progenitors were found mainly in the YS, and some in the caudal half (CH) of embryos. The cell population represents the earliest subset of cells with lymphohematopoietic properties during development.
Results
Expression of AA4.1 on ES-Cell-Derived Hematopoietic Progenitors.
To derive hematopoietic cells from undifferentiated mouse ES cells, we cocultured ES cells on stromal cell lines; ST2 (34) or macrophage colony-stimulating factor-deficient OP9 (35, 36) was used for this purpose (Fig. 1A). Stepwise culture on the ST2 stromal cell line was established based on the OP9 system (23). We could not find any differences in the timing of hematopoietic cell appearance, expression of cell surface molecules, and characteristics of generated hematopoietic cells (described below) between the OP9 and ST2 systems. In both culture systems, starting from singly dissociated cells, ES cells extensively divided and formed colonies consisting of differentiated cells on stromal cells (Fig. 1B Left). On day 5 or day 6 of differentiation, a single cell suspension was replated onto a fresh stromal cell layer. Small hematopoietic cell clusters were observable a couple of days later (Fig. 1B Center). These cells grew rapidly and formed large hematopoietic clusters by 2 weeks of differentiation (Fig. 1B Right).
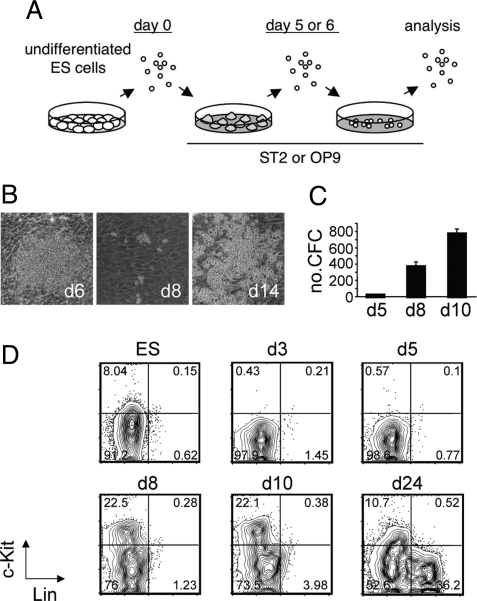
Fig. 1.
Differentiation of embryonic stem (ES) cells into hematopoietic cells. (A) A schematic overview of the culture system. (B) Representative pictures of hematopoietic differentiation of ES cells on ST2 stromal cells. (C) Hematopoietic colony-forming activity during ES cell differentiation. The numbers of colony-forming cells (CFCs) per well of the secondary plates plotted against the time course of culture are presented. These cultures were initiated with 105 cells on day 5. Values are the mean ± SD of triplicate cultures. A representative result from 2 independent experiments is shown. (D) Expression of c-Kit and lineage markers (Lin) during hematopoietic differentiation of ES cells. The plots shown are pregated on forward scatter/side scatter (FSC/SSC). The percentages of each fraction are indicated.
We examined the time point at which hematopoietic activity appears during in vitro differentiation of ES cells by checking colony-forming activity in methylcellulose in the presence of cytokines (stem cell factor, Flt3 ligand, IL-11, IL-3, granulocyte-macrophage colony-stimulating factor, thrombopoietin, and erythropoietin). As shown in Fig. 1C, we could detect very small numbers of colony-forming cells (CFCs) on day 5 of differentiation. A dramatic increase (>20-fold) of the CFC number was observed in the next 3 days of culture (by day 8). The number of CFCs increased by 2-fold in the following 2 days.
To find hematopoietic subsets during the differentiation of ES cells, we first examined the time course for expressions of c-kit (CD117) and mature blood cell lineage markers (Lin, Mac-1, Gr-1, Ter119, B220, CD3, CD4, and CD8) in ES cell cultures (Fig. 1D). The tyrosine kinase receptor c-Kit is known to be expressed on embryonic and adult hematopoietic stem and progenitor cells (37, 38) and is functionally important for these cells (37). Undifferentiated ES cells expressed low levels of c-Kit. We detected very few, if any, c-Kit+ cells from day 3 to day 5 of differentiation, indicating that c-Kit expression in undifferentiated ES cells is down-regulated upon differentiation (39). Consistent with the colony-forming activity in methylcellulose, c-Kit+ cells became abundant on day 8 of differentiation. Some Linlow cells became apparent after day 10 of differentiation. Abundant Lin+ cells were observed after 2 weeks of differentiation. Because of the selective induction of hematopoietic cell lineages in our culture system and the cell collection process that harvests cells only by pipetting, the contamination of c-Kit+ cell lineages other than hematopoietic cells (40) was unlikely in our analyses after passage on days 5–6.
Further characterization of ES-cell-derived c-Kit+ cells was carried out from day 8 to day 9 of differentiation before the presence of Lin+ cells became overt, because HSCs quickly lose their HSC properties in culture (41, 42). Among the stem cell markers examined, AA4.1 (CD93 or C1qRp) was expressed heterogeneously in ES-cell-derived c-Kit+Lin− cells (Fig. 2A). AA4.1 is expressed on candidate HSCs present in the developing embryo (43–45), but expression of the molecule is known to be from low to undetectable in BM HSCs (46). The c-Kit+AA4.1− and c-Kit+AA4.1+ cells were present in a ratio of ≈1:8 to 1:2, depending on the experiments, when analyzed on day 9 of differentiation. Notably, we obtained the same pattern of expression for these molecules using either the D3 or the R1 ES cell line.
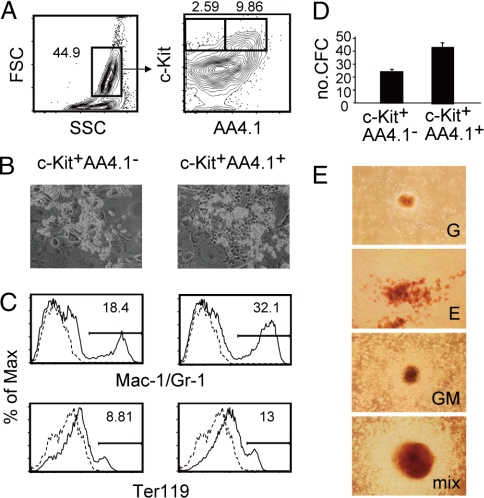
Fig. 2.
AA4.1 expression and myeloid lineage potential. (A) Expression of AA4.1 on cells present at day 9 of embryonic stem (ES) cell differentiation. The numbers indicate the percentages within the parent population. (B) Hematopoietic clusters developed from AA4.1+ and AA4.1− cells after 1 week of culture on OP9 stromal cells. (C) Expression of monocyte/macrophage and granulocyte (Mac-1/Gr-1) and erythroid (Ter119) lineage markers. AA4.1+ and AA4.1− cells cultured for 2 weeks on OP9 stromal cells were analyzed by flow cytometry. The percentages of positive cells are indicated. (D) Hematopoietic colony-forming activity of AA4.1+ and AA4.1− cells. The data are presented as the numbers of colony-forming cells (CFCs) per 500 cells. Values are the mean ± SD of triplicate cultures. A representative result from 2 independent experiments is shown. (E) Representative pictures of granulocyte (G), erythroid (E), granulocyte–macrophage (GM), and mixed-type (mix) colonies.
Myeloid Lineage Potential of c-Kit+AA44.1− and c-Kit+AA4.1+ Cells.
We next examined the differentiation potential of c-Kit+AA4.1− and c-Kit+AA4.1+ cells. The cells were sorted on day 9 of differentiation and cultured on an OP9 stromal cell layer (Fig. S1). Both cell populations proliferated well on stromal cells (Fig. 2B) and gave rise to monocyte/macrophage and granulocyte (Mac-1/Gr-1) and erythroid (Ter119) lineage cells (Fig. 2C). We also placed the sorted cells in methylcellulose culture with cytokines. As shown in Fig. 2D, c-Kit+AA4.1+ cells were approximately 2 times more enriched in CFC activity compared with c-Kit+AA4.1− cells. Both cell populations generated multilineage colonies in addition to single-lineage-containing colonies (Fig. 2E). These results indicated that both c-Kit+AA4.1+ and c-Kit+AA4.1− cell subsets possess multilineage myeloid potential in culture. The CFCs were present only in the c-Kit+ cell fractions on day 5, and the CFC activity of the c-Kit− cells was only ≈20-fold less than that of c-Kit+AA4.1+ cells on day 9, as determined by our assay.
c-Kit+AA4.1+ Cells Have Properties of Definitive Hematopoietic Progenitors.
We next examined either primitive or definitive hematopoiesis initiated by c-Kit+AA4.1+ and c-Kit+AA4.1− cell subsets. For this purpose, we cultured c-Kit+AA4.1+ and c-Kit+AA4.1− cells sorted on day 9 of differentiation for 1 week on OP9 stromal cells and then examined the expression of β-major and β-H1 globin genes as definitive and primitive erythrocyte markers, respectively, by RT-PCR. We detected β-major expression in both cell cultures initiated from c-Kit+AA4.1+ and c-Kit+AA4.1− cells; in contrast, we could not detect β-H1 expression in either cell culture, although the gene was detectable on embryonic day 12.5 (E12.5) FL (Fig. 3A). The result indicated that both c-Kit+AA4.1+ and c-Kit+AA4.1− compartments represent definitive-type hematopoietic progenitors. It should be noted that neither c-Kit+AA4.1+ nor c-Kit+AA4.1− cell subset themselves expressed these globin genes, further validating the immature properties of these progenitors. These results prompted us to examine endogenous HOXB4 expression in the ES-cell-derived progenitors because HOXB4 reportedly promotes definitive hematopoiesis from ES and YS cells (32). As shown in Fig. 3B, both c-Kit+AA4.1+ and c-Kit+AA4.1− cell subsets show expression of HOXB4, although the expression level appears to be low compared with E12.5 FL. The result suggested that the endogenous HOXB4 expression, although low, is sufficient to allow ES cells to enact at least part of the developmental program of definitive hematopoiesis.
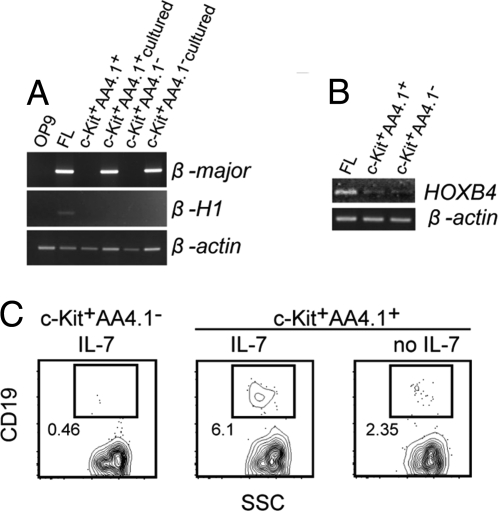
Fig. 3.
The β-globin expression and lymphoid potential of AA4.1+ and AA4.1− cells. (A) RT-PCR analysis of β-major and β-H1 expression in c-Kit+AA4.1+ and c-Kit+AA4.1− cells or c-Kit+AA4.1+ and c-Kit+AA4.1− cells cultured for a week on OP9 stromal cells. Lanes of OP9 and embryonic day 12.5 (E12.5) fetal liver (FL) are shown as controls. (B) RT-PCR analysis of HOXB4 expression on AA4.1+ and AA4.1− cells. Expression in E12.5 FL is shown as a control. (C) B lymphocyte potential of AA4.1+ and AA4.1− cells. Sorted cell subsets were cultured under B cell culture conditions for 4 weeks on OP9 stromal cells and then analyzed for CD19 expression. A representative result from 3 independent experiments is shown. The plots shown are pregated on FSC. The numbers indicate the percentages of the positive fraction.
We further tested whether these cell subsets possess lymphoid potential. For this purpose, the cells were placed onto OP9 stromal cells and cultured under B lymphocyte culture conditions in the presence of IL-7. Remarkably, the potential to generate B lymphocytes was clearly separated depending on AA4.1 expression. The c-Kit+AA4.1+ cells gave rise to B lymphocytes under the culture conditions, whereas c-Kit+AA4.1− cells generated very few, if any, B lymphocytes (Fig. 3C). Removal of IL-7 from the culture medium diminished the number of CD19+ cells (Fig. 3C), further validating the lymphoid nature of these cells (47). The residual B cells in the absence of IL-7 are presumably produced depending on endogenous IL-7 produced from OP9 stromal cells (48). This result indicated that the potential to generate lymphocytes is largely restricted to c-Kit+AA4.1+ cells, although both c-Kit+AA4.1+ and c-Kit+AA4.1− cells have myeloid potentials. Together, these results demonstrated that c-Kit+AA4.1+ cells derived from ES cells in vitro are definitive lymphohematopoietic progenitors.
To study the hierarchical relationship between c-Kit+AA4.1− and c-Kit+AA4.1+ cells, we sorted both cell populations on day 9 of differentiation, cultured them for 2 days on OP9, and then analyzed the cells to determine the expression of c-Kit and AA4.1. Both cell subsets readily became positive for the lineage markers. Although c-Kit+AA4.1+ cells generated a few c-Kit+AA4.1− cells, c-Kit+AA4.1− cells did not give rise to c-Kit+AA4.1+ cells (Fig. S2).
Transplantation into Mice.
Genetically unmodified ES-cell-derived progenitors are generally considered to have only a limited ability to engraft mice. We reevaluated their transplantability. First, we transplanted EGFP-expressing c-Kit+AA4.1+cells, c-Kit+AA4.1− cells, or unfractionated cells (H-2b) harvested on day 8 and day 9 of differentiation into lethally irradiated adult C57BL/Ka (H-2b) mice along with helper whole BM cells or into sublethally irradiated RAG2−/−γc−/− mice (H-2b), which completely lack T, B, and NK cell lineages (49). In these experiments, we could not detect any ES-cell-derived cells in peripheral blood, BM, and spleen when analyzed at 1, 4, and 12 weeks after transplantation. The results indicated that in vitro ES-cell-derived cells do not engraft adult mice and that immune surveillance through T, B, and NK cells cannot explain ES cell inability to settle in mice. Next, we transplanted c-Kit+AA4.1+ cells, c-Kit+AA4.1− cells, or unfractionated day-9 cells into sublethally irradiated newborn RAG2/γc-deficient mice via the facial vein (10). However, even in newborn recipients, we could not detect any lymphoid and myeloid lineage cells derived from ES cells (Fig. S3). We also tested an ES cell line overexpressing Bcl-2, an antiapoptotic molecule that we had previously established (22). The overexpression of Bcl-2 is known to increase the repopulation potential of HSCs in mice (50). However, overexpression of Bcl-2 did not improve the transplantability of ES-cell-derived cells in newborn RAG2/γc-deficient mice (Fig. S4), suggesting that protecting cells from apoptosis is not sufficient to allow ES-cell-derived stem cells to engraft mice.
As shown in Fig. S5, the expressions of CXCR4 (CD184), integrin α4 (CD49d), and integrin β1 (CD29), molecules involved in the homing and retention of stem cells and progenitors in hematopoietic tissues (51–56), were observed in ES-cell-derived progenitors. Because integrins must be activated to be functional, these ES-cell-derived progenitors should be tested with Mn2+ activation (57) before we rule out integrin α4β1 defects in the activation to transplantable HSCs. Taken together, these studies showed that even lymphohematopoietic stem cells derived from genetically unmodified ES cells do not engraft neonatal or adult mice.
Expression of Stem-Cell-Related Markers on ES-Cell-Derived c-Kit+AA4.1+ Cells.
The above-mentioned experiments showed that AA4.1 expression could mark lymphohematopoietic progenitors derived from ES cells. We examined the expression of other stem-cell-related markers in c-Kit+AA4.1+ cells. In addition to the expression of the panhematopoietic marker CD45, c-Kit+AA4.1+ cells expressed CD34 and CD41 (α4IIb integrin and GPIIb) (Fig. 4A), which were previously shown to be expressed on ES-cell-derived hematopoietic progenitors, although their lymphoid potential has not been tested (58, 59). Especially, CD41 is known to be expressed on YS and AGM hematopoietic progenitors and has been used to isolate these progenitors (58–60). We compared the expression pattern of CD41 and AA4.1 in the c-Kit+ cell fraction and found that ≈80% of the CD41+ cells coexpressed AA4.1, but the remaining 20% were unmarked with AA4.1 (Fig. 4B). The results indicated that CD41 expression marks wider cell populations compared with AA4.1. Thus, AA4.1 expression enables the further enrichment of lymphohematopoietic progenitors in differentiating ES cells. We also examined Sca-1 (Ly-6) expression in c-Kit+AA4.1+ cells. Sca-1 is traditionally used to isolate HSCs present in FL and BM (13, 14, 16). However, c-Kit+AA4.1+ cells were negative for Sca-1 expression (Fig. 4A), suggesting that Sca-1 is not expressed in hematopoietic progenitors in the early stage of development.
Fig. 4.
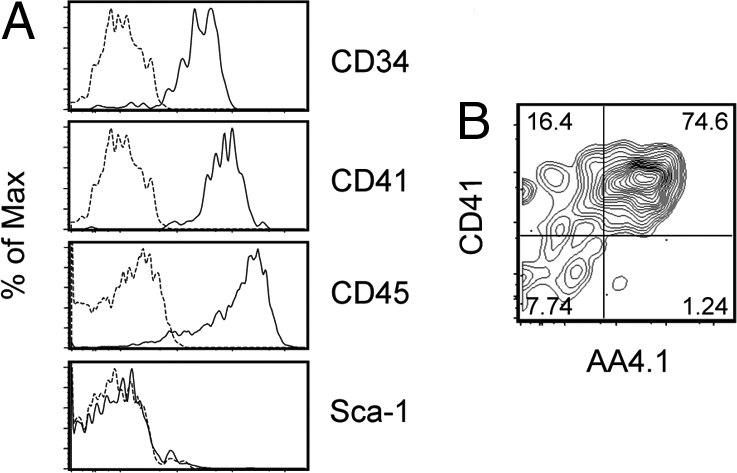
Cell surface antigen expression of embryonic stem (ES)-cell-derived lymphohematopoietic progenitor cell subsets. (A) Cell surface marker expression in c-Kit+AA4.1+ cell subset. (B) CD41 and AA4.1 expression in the c-Kit+ cell fraction. The numbers indicate the percentages within the parent population.
Localization and Characterization of c-Kit+AA4.1+ Cells in Developing Embryos.
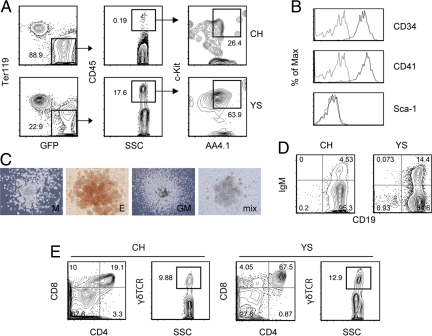
We next searched the in vivo counterpart of ES-cell-derived c-Kit+AA4.1+ cells in E9.5 embryos. Flow cytometric analysis showed that the cell subset was present abundantly in the YS and in small numbers in the CH (including the AGM region) of the embryo proper in a cell fraction deprived of Ter119+ primitive erythrocytes (Fig. 5A). The quantification result showed that ≈20-fold more c-Kit+AA4.1+ cells existed in the YS compared with the CH of the embryos (Table 1), suggesting that the YS is a major site for producing the earliest lymphohematopoietic progenitors (7, 10). The c-Kit+AA4.1+ cells were detected in at least the YS at E8.5 (Fig. S6), although CD45 expression was not detected at that time. Consistent with ES-cell-derived c-Kit+AA4.1+ cells, the cell surface phenotype of c-Kit+AA4.1+ cells in the developing mice was CD34+CD41+Sca-1− (Fig. 5B), and the cells formed myeloid lineage colonies in methylcellulose (Fig. 5C). The c-Kit+AA4.1+ cells, isolated from either the YS or the CH of E9.5 embryos, gave rise to B lymphocytes (Fig. 5D) and αβ/γδ-lineage T lymphocytes when transferred onto OP9 and OP9-DL1 (61) stromal cell lines (Fig. 5E), respectively. The efficiency of these cells in generating lymphocytes was comparable to that of BM c-Kit+Sca-1+Lin− cells. Thus, AA4.1 is a marker of the earliest lymphohematopoietic progenitor of developing embryos.
Fig. 5.
Characterization of c-Kit+AA4.1+ cells present in embryonic day 9.5 (E9.5) mice. (A) Presence of c-Kit+AA4.1+ cells in the yolk sac (YS) and caudal half (CH) of developing embryos. A GFP-Tg male was crossed with non-Tg female to obtain GFP+ embryos to avoid the possible contamination of maternal CD45+ cells. (B) Cell surface marker expression of c-Kit+AA4.1+ cells in the YS. (C) Hematopoietic colony-forming activity of c-Kit+AA4.1+ cells present in the YS. Representative pictures of macrophage (M), erythroid (E), granulocyte–macrophage (GM), and mixed-type (mix) colonies are shown. (D and E) The potential of c-Kit+AA4.1+ cells present in the YS and CH to differentiate into B (D) and T (E) lineage cells was examined using OP9 and OP9DL1 stromal cells, respectively. Representative results from 4 (YS) and 2 (CH) independent experiments each initiated with pooled littermate embryos are shown. The plots shown are pregated on FSC/SSC. The numbers indicate the percentages within the parent population.
Table 1.
Quantification of c-Kit+AA4.1+ cells in E9.5 embryos
| Exp. 1 | Exp. 2 | Exp. 3 | ||
|---|---|---|---|---|
| No. embryos* | 8 | 6 | 7 | |
| No. somite pairs† | 21.1 | 23.8 | 28.2 | |
| No. c-Kit+AA4.1+CD45+Ter119− cells‡ | CH | 20 | 44 | 75 |
| YS | 353 | 920 | 1,419 |
*The number of embryos analyzed. Pooled littermate embryos were used in each experiment.
†The average number of somite pairs for the analyzed embryos.
‡The average number of c-Kit+AA4.1+CD45+Ter119− cells within the indicated location of an embryo estimated from the pooled analysis of caudal half (CH) and yolk sac (YS) cells.
Discussion
We showed that hematopoietic activity from the early differentiation of ES cells resides in c-Kit+Lin− cells. We found that the cell population is further separated into two distinct cell subsets based on AA4.1 expression. We demonstrated that the AA4.1+ cell subset gives rise to both myeloid and lymphoid lineage cells, whereas the differentiation potential of the AA4.1− cell subset is largely restricted to myeloid cell lineages (Figs. 2 and 3C). Although performing clonal analysis is difficult until we improve the culture conditions so that AA4.1+ cells survive at a high efficiency after the cell sorting, experiments must be done in the future to confirm that single cells have both myeloid and lymphoid potential. On the basis of the β-globin expression pattern (Fig. 3A), both AA4.1+ and AA4.1− cell subsets should be classified as definitive-type hematopoietic progenitors. We showed that the c-Kit+AA4.1+ cells gave rise to some c-Kit+AA4.1− cells but not vice versa (Fig. S2). However, whether all of the c-Kit+AA4.1− cells originate from c-Kit+AA4.1+ cells is still unclear, because we detected some c-Kit+AA4.1− cells together with c-Kit+AA4.1+ cells at earlier time points. AA4.1 marks the majority of CD41+ cells; however, 1/5 of the CD41+ cells were AA4.1− (Fig. 3B). Because AA4.1− cells did not give rise to lymphoid cells (Fig. 3C), the cell fractionation method using AA4.1 antibody provides a way to isolate only the most immature hematopoietic cell subset from the ES cell culture. The phenotypic characterization of the first lymphohematopoietic cells in mouse ES cultures should allow the identification of their immediate precursors and which other mesodermal lineage cells can be derived from these precursors (30).
Despite the in vitro potency to generate both myeloid and lymphoid lineage cells, we could not detect in vivo engraftment of the c-Kit+Lin−AA4.1+ cells (Fig. S3 and Fig. S4). The results indicate that even the earliest hematopoietic progenitors derived from ES cells are not competent to engraft mice. Our results also indicate that endogenous HOXB4 expression (Fig. 4B) is not sufficient to provide ES cells with engraftment potency. The transplantation failure is not due to immunological surveillance through T, B, and NK cells nor to a cell survival problem (Fig. S3 and Fig. S4). At least, CXCR4, integrin α4, and integrin β1 were expressed on ES-cell-derived progenitors as homing molecules (Fig. S5). Our results are consistent with those of past studies that reported the inability of genetically unmodified ES-cell-derived progenitors to engraft mice (27). Contrary to our study, Potocnik et al. (62) reported that AA4.1+B220− cells derived from ES cells exhibit some potential for lymphoid engraftment in Rag1-deficient mice. One possible explanation for these contrasting observations is that the cell subset used by Potocnik et al. represents lymphoid-committed cells because these cells were isolated as late as day 15 of differentiation, a time point at which c-Kit+Lin−AA4+ cells were no longer detected in our assay. The expression of cell surface molecules in c-Kit+AA4.1+ cells is consistent with that of embryonic stage HSCs except that the cells do not express Sca-1 antigen (Fig. 4) (14, 16, 43–46, 58, 60, 63). The in vivo counterpart of c-Kit+AA4.1+ cells was found mainly in the YS, with some cells in the CH of the embryo proper (Fig. 5 and Table 1), and the cells gave rise to both myeloid and lymphoid lineages in culture. Further studies are required to determine the exact location of c-Kit+AA4+ cells within the YS and CH of developing mice (64). Yolk sac blood island cells taken from E8 and E9 donors fail to engraft adult irradiated syngeneic or congenic mice (3), yet the same cells transplanted in utero into the YS cavities of E8–E10 haploidentical hosts engraft and give rise to myeloerythroid day 10 spleen colonies and donor-derived T cells in the hosts at all ages tested (7). The cell tracking technique also revealed the contribution of early YS cells to adult hematopoieisis (11). We propose that our ES-cell-derived c-Kit+Lin−AA4.1+ cells and the homologous cells in the YS and CH require signals in vivo to transit to fetal, transplantable HSCs. Sca-1 expression may be acquired during the switch.
In conclusion, we demonstrated that expression of AA4.1 marks the earliest lymphohematopoietic progenitors in ES cell culture and in early embryos. This is the clear demonstration that genetically unmodified ES cells give rise to multipotent hematopoietic progenitors with both myeloid and lymphoid lineage potentials in vitro. Our findings should provide helpful guidance for the therapeutic utilization of human pluripotent stem cells and will facilitate the understanding of how HSCs develop in embryos.
Materials and Methods
Cell Culture.
Mouse D3 ES cells (65), R1 ES cells (28), D3 ES cell lines expressing EGFP under the control of human EF-1α promoter or CAG promoter (provided by Shin-Ichi Hayashi, Tottori University, Japan), D3 ES cells overexpressing human Bcl-2 (22), OP9 stromal cells (36), OP9-DL1 (61), or ST2 stromal cells (34) were maintained as described earlier (24). To induce hematopoietic differentiation of ES cells in vitro, cells were placed on ST2 or OP9 stromal cells in MEM α medium (Gibco) supplemented with 10% or 20% FBS (HyClone), respectively. On day 5 or day 6 of differentiation, colonies were dissociated with 0.25% trypsin and 0.5 mM EDTA (Gibco) and replated on freshly prepared stromal cell lines. For FACS and analysis, cells were collected by dissociating colonies with Hanks-based Cell Dissociation Buffer (Gibco) up to day 6 of differentiation and thereafter by pipetting. For B or T lymphocyte culture, cells were placed and cultured in RPMI medium 1640 supplemented with 5% FBS, 50 μM 2-mercaptoethanol, and 10 ng/mL recombinant murine IL-7 on OP9 or cultured in MEM α medium supplemented with 20% FBS on OP9-DL1, respectively.
Antibodies.
The following monoclonal antibodies were used in this study: AA4.1, 6B2 (anti-B220), KT31.1 (CD3), GK1.5 (CD4), 53–6.7 (CD8), 6D5 (CD19), RAM34 (CD34), MWReg30 (CD41), 30-F11 (CD45), 2B8 (c-Kit), 2B11/CXCR4 (CXCR4), 8C5 (Gr-1), II/41 (IgM), M1/70 (Mac-1), E13–161.7 (Sca-1), H57–597 (TCRβ), Ter119, R1–2 (α4 integrin), Ha2/5 (β1 integrin), GL3 (γδTCR).
Mice.
Mice were purchased from CLEA Japan and maintained in the Institute of Laboratory Animals at Mie University or bred and maintained in Stanford University's Research Animal Facility. All experiments were performed according to the guidelines of the animal committee of Mie University or Stanford Administrative Panel on Laboratory Animal Care.
Cell Preparation from Embryos.
Caudal half and YS obtained from timed-mated C57BL/6J females were incubated with 1 mg/mL collagenase (Wako) in 2% FBS/HBSS for 30 min at 37 °C. A single-cell suspension prepared by pipetting after the incubation was used for the experiments.
Colony-Formation Assay, FACS, and RT-PCR.
Supplementary Material
Acknowledgments.
We thank L. Jerabek and M. Yamada for laboratory management, C. Richter for antibody production, L. Hidalgo for animal care, J. C. Zúñiga-Pflücker for the OP9-DL1 cells, and S. I. Hayashi for the ES cell lines. This work was supported by National Institutes of Health Grants R01HL058770 and R01CA086065, by a gift from the Smith Family Foundation (to I.L.W.), and by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to T.Y.). T.Y. was partly supported by a fellowship from the Uehara Memorial Foundation. N.H. was supported by a fellowship from the Japan Society for the Promotion of Science.
Footnotes
Conflict of interest statement: I.L.W. was formerly a member of the scientific advisory board of Amgen and owns significant Amgen stock; he cofounded and consulted for Systemix, is a cofounder and director of Stem Cells, Inc., cofounded and is a director of Cellerant, Inc. All other authors have no conflicting financial interests.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904090106/DCSupplemental.
References
- 1.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 2.Haar JL, Ackerman GA. A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat Rec. 1971;170:199–223. doi: 10.1002/ar.1091700206. [DOI] [PubMed] [Google Scholar]
- 3.Medvinsky AL, Samoylina NL, Müller AM, Dzierzak EA. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature. 1993;364:64–67. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- 4.Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Delassus S, Cumano A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity. 1996;4:97–106. doi: 10.1016/s1074-7613(00)80302-7. [DOI] [PubMed] [Google Scholar]
- 7.Weissman IL, Papaioannou VE, Gardner RL. Fetal hematopoietic origins of the adult hematolymphoid system. In: Clarkson B, Marks PA, Till JE, editors. Differentiation of Normal and Neoplastic Hematopoietic Cells. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1978. pp. 33–47. [Google Scholar]
- 8.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 9.Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 10.Yoder MC, et al. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 11.Samokhvalov IM, Samokhvalov NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 12.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 13.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 14.Ikuta K, et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 15.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 16.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci USA. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 18.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:633–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Williams RL, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 21.Smith AG, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 22.Yamane T, Dylla SJ, Muijtjens M, Weissman IL. Enforced Bcl-2 expression overrides serum and feeder cell requirements for mouse embryonic stem cell self-renewal. Proc Natl Acad Sci USA. 2005;102:3312–3317. doi: 10.1073/pnas.0500167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 24.Yamane T, et al. Development of osteoclasts from embryonic stem cells through a pathway that is c-fms but not c-kit dependent. Blood. 1997;90:3516–3523. [PubMed] [Google Scholar]
- 25.Wiles MV, Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991;111:259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt TM, et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 27.Müller AM, Dzierzak EA. ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development. 1993;118:1343–1351. doi: 10.1242/dev.118.4.1343. [DOI] [PubMed] [Google Scholar]
- 28.Forrester LM, Bernstein A, Rossant J, Nagy A. Long-term reconstitution of the mouse hematopoietic system by embryonic stem cell-derived fetal liver. Proc Natl Acad Sci USA. 1991;88:7514–7517. doi: 10.1073/pnas.88.17.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Perlingeiro RC, Kyba M, Daley GQ. Clonal analysis of differentiating embryonic stem cells reveals a hematopoietic progenitor with primitive erythroid and adult lymphoid-myeloid potential. Development. 2001;128:4597–4604. doi: 10.1242/dev.128.22.4597. [DOI] [PubMed] [Google Scholar]
- 32.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa M, et al. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J. 1988;7:1337–1343. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 36.Kodama H, Nose M, Niida S, Nishikawa S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol. 1994;22:979–984. [PubMed] [Google Scholar]
- 37.Ogawa M, et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamane T, Hayashi S, Mizoguchi M, Yamazaki H, Kunisada T. Derivation of melanocytes from embryonic stem cells in culture. Dev Dyn. 1999;216:450–458. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<450::AID-DVDY13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Ema H, Takano H, Sudo K, Nakauchi H. In vitro self-renewal division of hematopoietic stem cells. J Exp Med. 2000;192:1281–1288. doi: 10.1084/jem.192.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J Exp Med. 2000;192:1707–1718. doi: 10.1084/jem.192.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang H, Auerbach R. Identification and characterization of hematopoietic stem cells from the yolk sac of the early mouse embryo. Proc Natl Acad Sci USA. 1993;90:10110–10114. doi: 10.1073/pnas.90.21.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan CT, McKearn JP, Lemischka IR. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990;61:953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- 45.Jordan CT, et al. Long-term repopulating abilities of enriched fetal liver stem cells measured by competitive repopulation. Exp Hematol. 1995;23:1011–1015. [PubMed] [Google Scholar]
- 46.Trevisan M, Iscove NN. Phenotypic analysis of murine long-term hemopoietic reconstituting cells quantitated competitively in vivo and comparison with more advanced colony-forming progeny. J Exp Med. 1995;181:93–103. doi: 10.1084/jem.181.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee G, Namen AE, Gillis S, Ellingsworth LR, Kincade PW. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-beta. J Immunol. 1989;142:3875–3883. [PubMed] [Google Scholar]
- 48.Vieira P, Cumano A. Differentiation of B lymphocytes from hematopoietic stem cells. Methods Mol Biol. 2004;271:67–76. doi: 10.1385/1-59259-796-3:067. [DOI] [PubMed] [Google Scholar]
- 49.Goldman JP, et al. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor γ chain. Br J Haematol. 1998;103:335–342. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]
- 50.Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: Overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191:253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawabata K, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci USA. 1999;96:5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 53.Neuhaus H, et al. Cloning and expression of cDNAs for the α subunit of the murine lymphocyte-Peyer's patch adhesion molecule. J Cell Biol. 1991;115:1149–1158. doi: 10.1083/jcb.115.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagers AJ, Allsopp RC, Weissman IL. Changes in integrin expression are associated with altered homing properties of Lin−/loThy1.1loSca-1+c-kit+ hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Exp Hematol. 2002;30:176–185. doi: 10.1016/s0301-472x(01)00777-9. [DOI] [PubMed] [Google Scholar]
- 56.Potocnik AJ, Brakebusch C, Fässler R. Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 57.Elices MJ, Urry LA, Hemler ME. Receptor functions for the integrin VLA-3: Fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J Cell Biol. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitjavila-Garcia MT, et al. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development. 2002;129:2003–2013. doi: 10.1242/dev.129.8.2003. [DOI] [PubMed] [Google Scholar]
- 59.Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 60.Ferkowicz MJ, et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 61.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 62.Potocnik AJ, Kohler H, Eichmann K. Hemato-lymphoid in vivo reconstitution potential of subpopulations derived from in vitro differentiated embryonic stem cells. Proc Natl Acad Sci USA. 1997;94:10295–10300. doi: 10.1073/pnas.94.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sánchez MJ, Holmes A, Miles C, Dzierzak E. Characterization of the first definitive hematopoietic stem cells in the AGM and liver of the mouse embryo. Immunity. 1996;5:513–525. doi: 10.1016/s1074-7613(00)80267-8. [DOI] [PubMed] [Google Scholar]
- 64.Petrenko O, et al. The molecular characterization of the fetal stem cell marker AA4. Immunity. 1999;10:691–700. doi: 10.1016/s1074-7613(00)80068-0. [DOI] [PubMed] [Google Scholar]
- 65.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.