Abstract
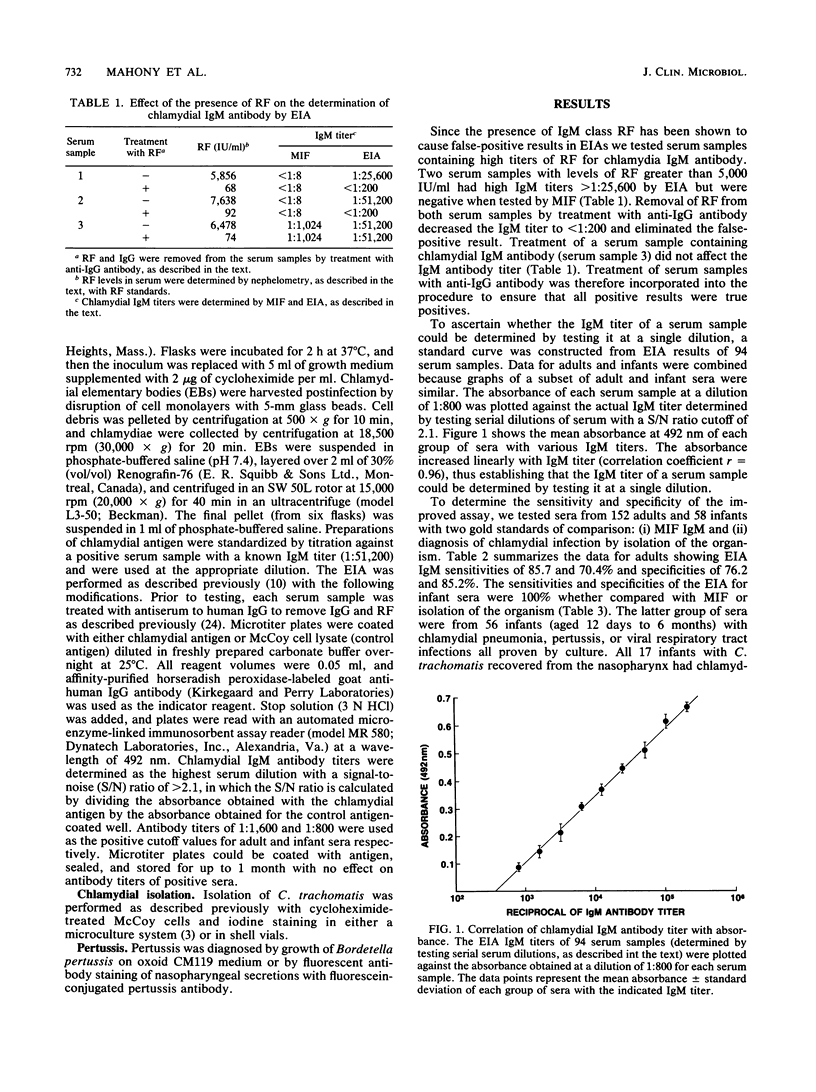
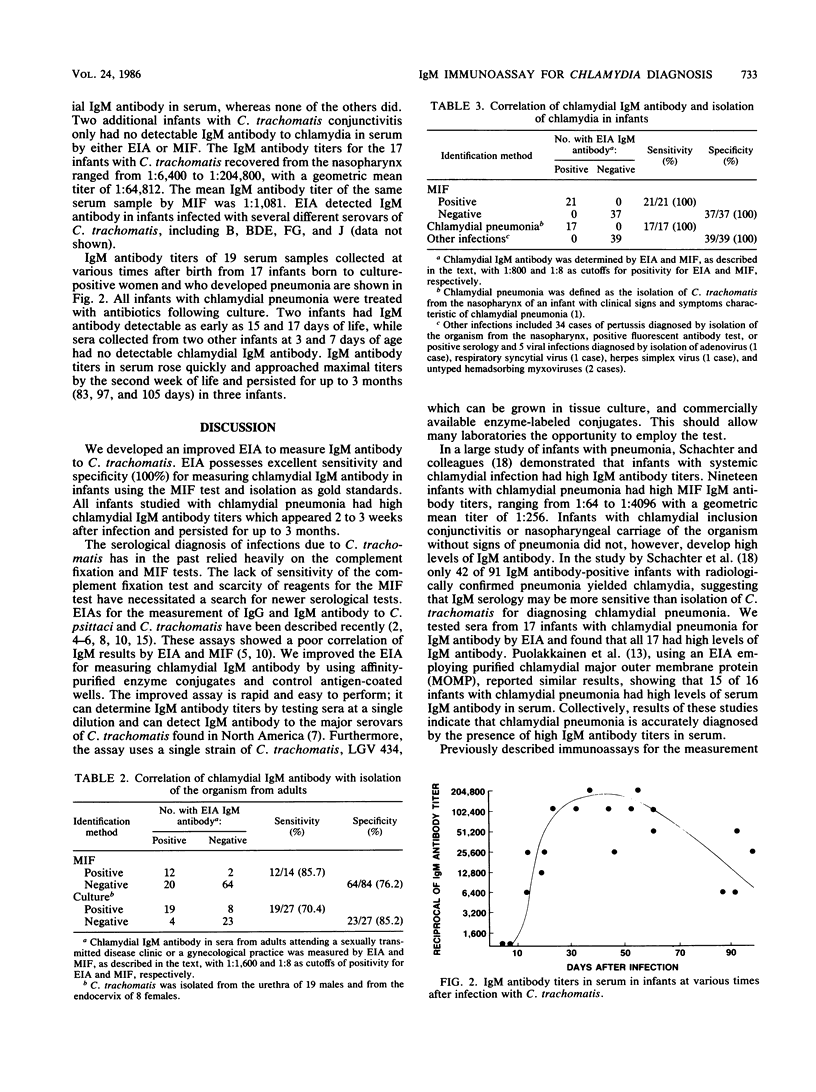
An improved solid-phase enzyme immunoassay (EIA) with Chlamydia trachomatis L2 434/Bu elementary bodies was developed for the measurement of immunoglobulin M (IgM) antibody to C. trachomatis in serum. Comparison of EIA and microimmunofluorescence IgM antibody titers of 156 serum samples revealed an EIA sensitivity and specificity of 100% for infants, but reduced sensitivity (85%) and specificity (76%) for sera from adults. Sera containing IgM class rheumatoid factor produced false-positive IgM results which could easily be eliminated by pretreatment of the sera with anti-human IgG. Analysis of sera from infants with chlamydial infections revealed that 17 of 17 infants with C. trachomatis pneumonia had high IgM antibody titers (geometric mean titer, 1:64,812), whereas two infants with conjunctivitis only lacked detectable IgM antibody. EIA detected IgM antibody to several serovar groups in serum, including serovars B, BDE, FG, and J. IgM antibody to C. trachomatis in serum was detected as early as 5 days after the infection that was acquired at delivery and persisted for 3 months. The availability of an EIA possessing good sensitivity and specificity for the detection of IgM antibody to C. trachomatis may permit more laboratories to diagnose perinatal chlamydial infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beem M. O., Saxon E. M. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977 Feb 10;296(6):306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- Cevenini R., Sarov I., Rumpianesi F., Donati M., Melega C., Varotti C., La Placa M. Serum specific IgA antibody to Chlamydia trachomatis in patients with chlamydial infections detected by ELISA and an immunofluorescence test. J Clin Pathol. 1984 Jun;37(6):686–691. doi: 10.1136/jcp.37.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. T., Taylor-Robinson D. Development and evaluation of an enzyme-linked immunosorbent assay (ELISA), using chlamydial group antigen, to detect antibodies, to Chlamydia trachomatis. J Clin Pathol. 1982 Oct;35(10):1122–1128. doi: 10.1136/jcp.35.10.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn M. P., Ohlin A., Schachter J. Enzyme-linked immunosorbent assay for immunoglobulin G and M antibodies to Chlamydia trachomatis in human sera. J Clin Microbiol. 1983 May;17(5):848–852. doi: 10.1128/jcm.17.5.848-852.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B., Bruins S. C., Newhall W. J., 5th Comparison of reticulate and elementary body antigens in detection of antibodies against Chlamydia trachomatis by an enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Mar;17(3):466–471. doi: 10.1128/jcm.17.3.466-471.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Holmes K. K., Grayston J. T. Immunotypes of Chlamydia trachomatis isolates in Seattle, Washington. Infect Immun. 1983 Aug;41(2):865–868. doi: 10.1128/iai.41.2.865-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis V. J., Thacker W. L., Mitchell S. H. Enzyme-linked immunosorbent assay for chlamydial antibodies. J Clin Microbiol. 1977 Nov;6(5):507–510. doi: 10.1128/jcm.6.5.507-510.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony J. B., Chernesky M. A. Effect of swab type and storage temperature on the isolation of Chlamydia trachomatis from clinical specimens. J Clin Microbiol. 1985 Nov;22(5):865–867. doi: 10.1128/jcm.22.5.865-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony J. B., Schachter J., Chernesky M. A. Detection of antichlamydial immunoglobulin G and M antibodies by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Aug;18(2):270–275. doi: 10.1128/jcm.18.2.270-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A. An overview of infectious agents of salpingitis, their biology, and recent advances in methods of detection. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):933–951. doi: 10.1016/0002-9378(80)91084-4. [DOI] [PubMed] [Google Scholar]
- Puolakkainen M., Saikku P., Leinonen M., Nurminen M., Vänänen P., Mäkelä P. H. Chlamydial pneumonitis and its serodiagnosis in infants. J Infect Dis. 1984 Apr;149(4):598–604. doi: 10.1093/infdis/149.4.598. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Goodell S. E., Mkrtichian E., Schuffler M. D., Wang S. P., Stamm W. E., Holmes K. K. Chlamydia trachomatis proctitis. N Engl J Med. 1981 Jul 23;305(4):195–200. doi: 10.1056/NEJM198107233050404. [DOI] [PubMed] [Google Scholar]
- Saikku P., Paavonen J., Vänänen P., Vaheri A. Solid-phase enzyme immunoassay for Chlamydial antibodies. J Clin Microbiol. 1983 Jan;17(1):22–27. doi: 10.1128/jcm.17.1.22-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Schachter J., Cles L., Ray R., Hines P. A. Failure of serology in diagnosing chlamydial infections of the female genital tract. J Clin Microbiol. 1979 Nov;10(5):647–649. doi: 10.1128/jcm.10.5.647-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Grossman M., Azimi P. H. Serology of Chlamydia trachomatis in infants. J Infect Dis. 1982 Oct;146(4):530–535. doi: 10.1093/infdis/146.4.530. [DOI] [PubMed] [Google Scholar]
- Schachter J., Grossman M., Holt J., Sweet R., Goodner E., Mills J. Prospective study of chlamydial infection in neonates. Lancet. 1979 Aug 25;2(8139):377–380. doi: 10.1016/s0140-6736(79)90400-8. [DOI] [PubMed] [Google Scholar]
- Terho P., Meurman O. Chlamydial serum IgG, IgA and local IgA antibodies in patients with genital-tract infections measured by solid-phase radioimmunoassay. J Med Microbiol. 1981 Feb;14(1):77–87. doi: 10.1099/00222615-14-1-77. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Alexander E. R., Holmes K. K. Simplified microimmunofluorescence test with trachoma-lymphogranuloma venereum (Chlamydia trachomatis) antigens for use as a screening test for antibody. J Clin Microbiol. 1975 Mar;1(3):250–255. doi: 10.1128/jcm.1.3.250-255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata M., Chernesky M., Mahony J. Indirect immunofluorescence staining of Chlamydia trachomatis inclusions in microculture plates with monoclonal antibodies. J Clin Microbiol. 1984 Jun;19(6):937–939. doi: 10.1128/jcm.19.6.937-939.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata M., Mahony J. B., Chernesky M. A. Measurement of BK papovavirus IgG and IgM by radioimmunoassay (RIA). J Med Virol. 1984;14(2):101–114. doi: 10.1002/jmv.1890140204. [DOI] [PubMed] [Google Scholar]


