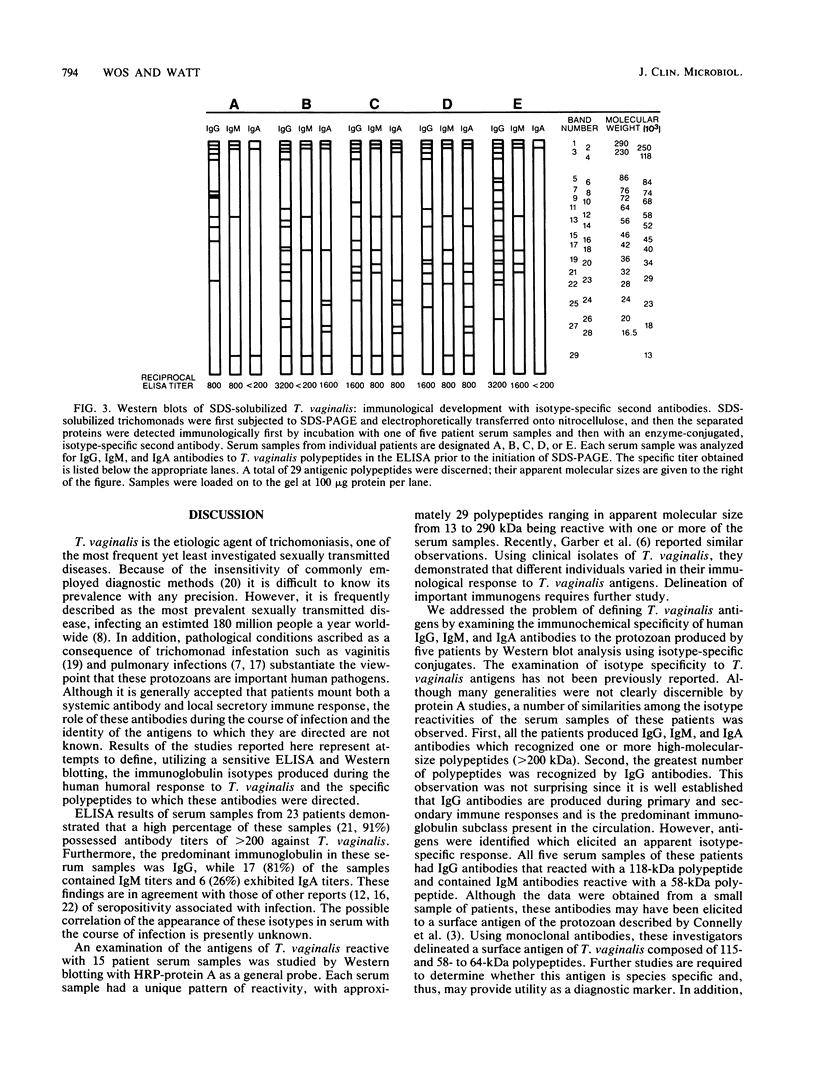
Abstract
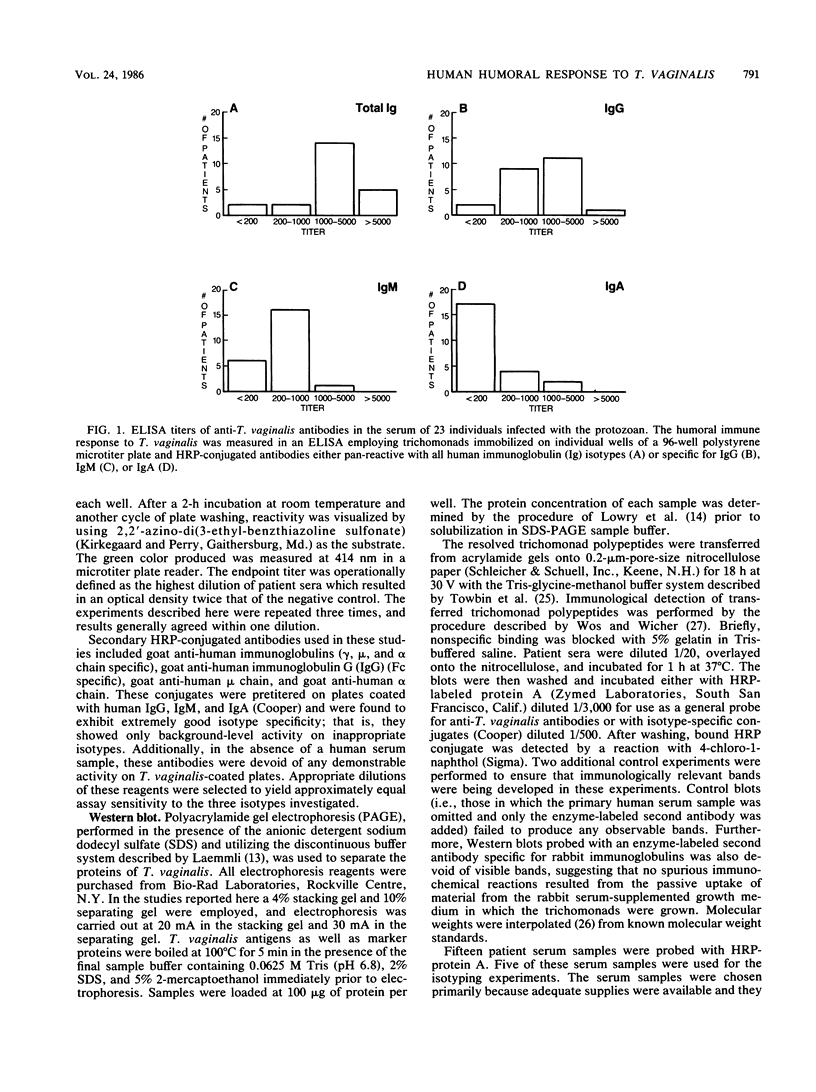
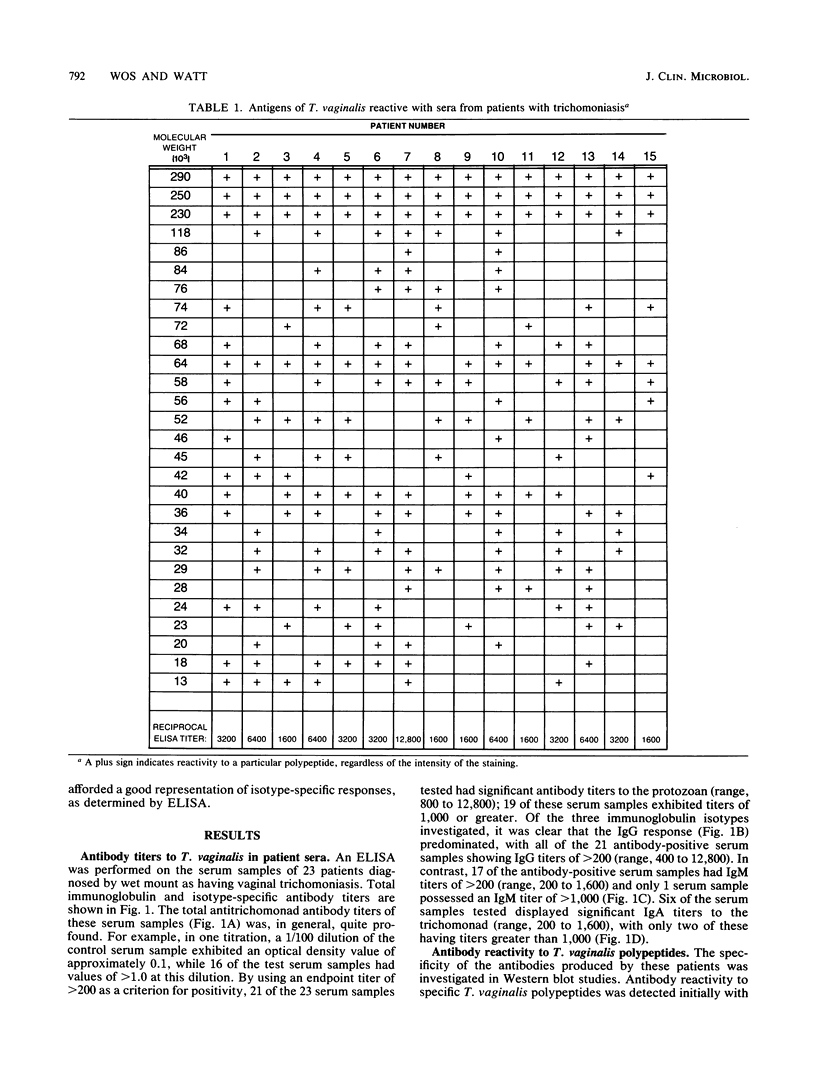
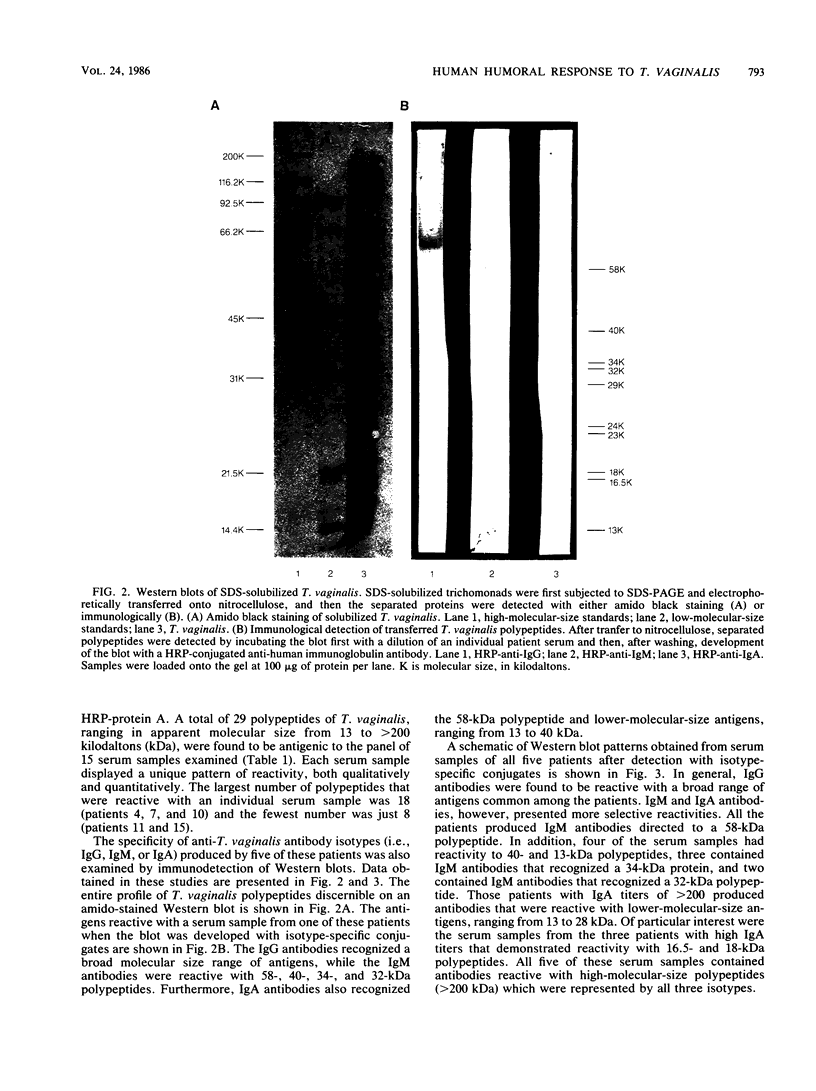
Studies of anti-Trichomonas vaginalis antibodies in patients with vaginal trichomoniasis were undertaken in attempts to identify the predominant antibody isotype produced and to delineate clinically significant antigens. The total antibody content of serum samples from 23 patients was determined by an enzyme-linked immunosorbent assay (ELISA) that employed anti-human immunoglobulin and isotype-specific antisera. The immunochemical reactivity of these antibodies was examined by Western blot analysis. The anti-T. vaginalis titer of all but two of these serum samples was greater than 200 (range, greater than 200 to 12,800). By using an ELISA titer of at least 200 as a criterion, 21 of the serum samples contained antibodies of the immunoglobulin G (IgG) isotype, 17 contained IgM antibodies, and 6 contained IgA antibodies directed to the protozoan. Western blot analyses of these serum samples revealed approximately 29 antigenic trichomonad polypeptides, with apparent molecular sizes ranging from 14 to greater than 100 kilodaltons and with individual serum samples possessing different patterns of reactivity. These results add to the current understanding of the serological and secretory immune responses to T. vaginalis, as well as define potential antigens for use in immunodiagnostics.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Enzyme linked immunosorbent assay for detecting antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br J Vener Dis. 1984 Jun;60(3):164–170. doi: 10.1136/sti.60.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L., Smith J., Spence M. Heterogeneity of Trichomonas vaginalis and discrimination among trichomonal isolates and subpopulations with sera of patients and experimentally infected mice. Infect Immun. 1985 Sep;49(3):463–468. doi: 10.1128/iai.49.3.463-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly R. J., Torian B. E., Stibbs H. H. Identification of a surface antigen of Trichomonas vaginalis. Infect Immun. 1985 Aug;49(2):270–274. doi: 10.1128/iai.49.2.270-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Felman Y. M., Nikitas J. A. Trichomoniasis, candidiasis, and Corynebacterium vaginale vaginitis. N Y State J Med. 1979 Sep;79(10):1563–1566. [PubMed] [Google Scholar]
- Garber G. E., Proctor E. M., Bowie W. R. Immunogenic proteins of Trichomonas vaginalis as demonstrated by the immunoblot technique. Infect Immun. 1986 Jan;51(1):250–253. doi: 10.1128/iai.51.1.250-253.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh S. M. Pulmonary trichomoniasis and Trichomonas tenax. J Med Microbiol. 1985 Aug;20(1):1–10. doi: 10.1099/00222615-20-1-1. [DOI] [PubMed] [Google Scholar]
- KOTT H., ADLER S. A serological study of Trichomonas sp. parasitic in man. Trans R Soc Trop Med Hyg. 1961 Jul;55:333–344. doi: 10.1016/0035-9203(61)90102-x. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Holmes K. K., Spence M. R., Rein M. F., McCormack W. M., Tam M. R. Geographic variation among isolates of Trichomonas vaginalis: demonstration of antigenic heterogeneity by using monoclonal antibodies and the indirect immunofluorescence technique. J Infect Dis. 1985 Nov;152(5):979–984. doi: 10.1093/infdis/152.5.979. [DOI] [PubMed] [Google Scholar]
- Kuberski T. Evaluation of the indirect hemagglutination technique for study of Trichomonas vaginalis infections, particularly in men. Sex Transm Dis. 1978 Jul-Sep;5(3):97–102. doi: 10.1097/00007435-197807000-00004. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mason P. R., Patterson B. A. Proliferative response of human lymphocytes to secretory and cellular antigens of Trichomonas vaginalis. J Parasitol. 1985 Jun;71(3):265–268. [PubMed] [Google Scholar]
- Mathews H. M., Healy G. R. Evaluation of two serological tests for Trichomonas vaginalis infection. J Clin Microbiol. 1983 May;17(5):840–843. doi: 10.1128/jcm.17.5.840-843.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren L. C., Davis L. E., Healy G. R., James C. G. Isolation of Trichomonas vaginalis from the respiratory tract of infants with respiratory disease. Pediatrics. 1983 Jun;71(6):888–890. [PubMed] [Google Scholar]
- Rein M. F., Chapel T. A. Trichomoniasis, candidiasis, and the minor venereal diseases. Clin Obstet Gynecol. 1975 Mar;18(1):73–88. doi: 10.1097/00003081-197503000-00008. [DOI] [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Street D. A., Taylor-Robinson D., Ackers J. P., Hanna N. F., McMillan A. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibody to Trichomonas vaginalis in sera and vaginal secretions. Br J Vener Dis. 1982 Oct;58(5):330–333. doi: 10.1136/sti.58.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K. E. Antibody to Trichomonas vaginalis in human cervicovaginal secretions. Infect Immun. 1982 Sep;37(3):852–857. doi: 10.1128/iai.37.3.852-857.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wos S. M., Wicher K. Antigenic evidence for host origin of exudative fluids in lesions of Treponema pallidum-infected rabbits. Infect Immun. 1985 Jan;47(1):228–233. doi: 10.1128/iai.47.1.228-233.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano A., Yui K., Aosai F., Kojima S., Kawana T., Ovary Z. Immune response to Trichomonas vaginalis. IV. Immunochemical and immunobiological analyses of T. vaginalis antigen. Int Arch Allergy Appl Immunol. 1983;72(2):150–157. [PubMed] [Google Scholar]