Abstract
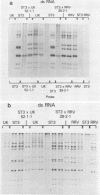
A series of reassortants was isolated from coinfection of cell cultures with wild-type bovine rotavirus (UK strain [serotype 6]) or rhesus rotavirus (strain MMU18006 [serotype 3]) and a tissue culture-adapted human rotavirus strain, ST3 (serotype 4). Monospecific antiserum or a set of monoclonal antibodies to the major outer capsid neutralization glycoprotein, VP7, of the animal rotavirus parent was used to select for reassortants with human rotavirus serotype 4 neutralization specificity. The majority of reassortants contained only gene 9 of the human rotavirus parent, ST3, whereas the remaining genes were derived from the animal rotavirus parent. These single human rotavirus gene substitution reassortants were neutralized to high titer by hyperimmune serum directed at ST3, thus demonstrating that gene 9 of ST3 codes for the major neutralization protein of this strain. Moreover, these single gene substitution, reassortants were also neutralized to low titer by antiserum directed at their animal rotavirus parent, probably because they derived gene 4, which codes for another outer capsid protein, VP3, from their animal rotavirus parent. None of the reassortants derived gene 4, which had previously been shown to be responsible for host range restriction of human rotaviruses in tissue culture, from ST3, despite the fact that the ST3 strain used for gene reassortment had been tissue culture adapted.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias C. F., López S., Bell J. R., Strauss J. H. Primary structure of the neutralization antigen of simian rotavirus SA11 as deduced from cDNA sequence. J Virol. 1984 May;50(2):657–661. doi: 10.1128/jvi.50.2.657-661.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banatvala J. E., Chrystie I. L. Rotaviral infections in human neonates. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):527–530. [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Woode G. N. Neonatal calf diarrhoea: identification of a reovirus-like (rotavirus) agent in faeces by immunofluorescence and immune electron microscopy. Br Vet J. 1975 Sep-Oct;131(5):528–535. [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Sequence homology between human and animal rotavirus serotype-specific glycoproteins. Nucleic Acids Res. 1984 May 11;12(9):3973–3982. doi: 10.1093/nar/12.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Dyall-Smith M. L., Holmes I. H., Azad A. A. Nucleotide sequence of the gene encoding the serotype-specific glycoprotein of UK bovine rotavirus. Nucleic Acids Res. 1983 Jul 25;11(14):4689–4701. doi: 10.1093/nar/11.14.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Greenberg H. B., Myslinski J., Kalica A. R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Use of transcription probes for genotyping rotavirus reassortants. Virology. 1982 Sep;121(2):288–295. doi: 10.1016/0042-6822(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Glass R. I., Keith J., Nakagomi O., Nakagomi T., Askaa J., Kapikian A. Z., Chanock R. M., Flores J. Nucleotide sequence of the structural glycoprotein VP7 gene of Nebraska calf diarrhea virus rotavirus: comparison with homologous genes from four strains of human and animal rotaviruses. Virology. 1985 Mar;141(2):292–298. doi: 10.1016/0042-6822(85)90260-0. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Greenberg H. B., Kalica A. R., Kim H. W., Brandt C. D., Rodriguez W. J., Parrott R. H., Chanock R. M. Approaches to immunization of infants and young children against gastroenteritis due to rotaviruses. Rev Infect Dis. 1980 May-Jun;2(3):459–469. doi: 10.1093/clinids/2.3.459. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Levine M. M., Black R. E., Greenberg H. B., Flores J., Kalica A. R., Hoshino Y., Chanock R. M. Studies in volunteers with human rotaviruses. Dev Biol Stand. 1983;53:209–218. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata L., Simhon A., Urrutia J. J., Kronmal R. A., Fernández R., García B. Epidemiology of rotaviruses in a cohort of 45 Guatamalan Mayan Indian children observed from birth to the age of three years. J Infect Dis. 1983 Sep;148(3):452–461. doi: 10.1093/infdis/148.3.452. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricciani J. C., Hopps H. E., Lorenz D. E. Subhuman primate diploid cells: possible substrates for production of virus vaccines. Science. 1971 Dec 3;174(4013):1025–1027. doi: 10.1126/science.174.4013.1025. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Iwamoto A., Ikegami N., Nomoto A., Furuichi Y. Nucleotide sequence of the gene encoding the serotype-specific antigen of human (Wa) rotavirus: comparison with the homologous genes from simian SA11 and UK bovine rotaviruses. J Virol. 1984 Sep;51(3):860–862. doi: 10.1128/jvi.51.3.860-862.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Sutcliffe J. G., Green N., Lerner R. A. Synthetic peptide immunogens as vaccines. Annu Rev Microbiol. 1983;37:425–446. doi: 10.1146/annurev.mi.37.100183.002233. [DOI] [PubMed] [Google Scholar]
- Stuker G., Oshiro L. S., Schmidt N. J. Antigenic comparisons of two new rotaviruses from rhesus monkeys. J Clin Microbiol. 1980 Feb;11(2):202–203. doi: 10.1128/jcm.11.2.202-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., D'Hondt E., André F. E., Zissis G. Immunogenicity and safety of live oral attenuated bovine rotavirus vaccine strain RIT 4237 in adults and young children. Lancet. 1983 Oct 8;2(8354):807–811. doi: 10.1016/s0140-6736(83)90734-1. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Kapikian A. Z., Greenberg H. B., Kalica A. R., Flores J., Hoshino Y., Chanock R. M., Levine M. M. Development of vaccines against rotavirus disease. Prog Food Nutr Sci. 1983;7(3-4):189–192. [PubMed] [Google Scholar]