Abstract
Interactions between natural selection and environmental change are well recognized and sit at the core of ecology and evolutionary biology. Reciprocal interactions between ecology and evolution, eco-evolutionary feedbacks, are less well studied, even though they may be critical for understanding the evolution of biological diversity, the structure of communities and the function of ecosystems. Eco-evolutionary feedbacks require that populations alter their environment (niche construction) and that those changes in the environment feed back to influence the subsequent evolution of the population. There is strong evidence that organisms influence their environment through predation, nutrient excretion and habitat modification, and that populations evolve in response to changes in their environment at time-scales congruent with ecological change (contemporary evolution). Here, we outline how the niche construction and contemporary evolution interact to alter the direction of evolution and the structure and function of communities and ecosystems. We then present five empirical systems that highlight important characteristics of eco-evolutionary feedbacks: rotifer–algae chemostats; alewife–zooplankton interactions in lakes; guppy life-history evolution and nutrient cycling in streams; avian seed predators and plants; and tree leaf chemistry and soil processes. The alewife–zooplankton system provides the most complete evidence for eco-evolutionary feedbacks, but other systems highlight the potential for eco-evolutionary feedbacks in a wide variety of natural systems.
Keywords: eco-evolutionary feedbacks, intraspecific variation, niche construction, evolution, community ecology, ecosystem ecology
At every moment natural selection is operating to change the genetic composition of populations in response to the momentary environment, but as that composition changes it forces a concomitant change in the environment itself. Thus organisms and environments are both causes and effects in a coevolutionary process.
(Lewontin 2000, p. 126)
1. Introduction
At the core of both ecology and evolutionary biology is a fundamental interest in biological diversity, although it is often approached from very different perspectives. Evolutionary biology is singularly focused on the processes that generate diversity. Ecology, particularly community and ecosystem ecology, has focused on the maintenance and implications of biological diversity. At the interface is the growing interest in eco-evolutionary interactions (Laland et al. 1999; Odling-Smee et al. 2003; Hairston et al. 2005; Carroll et al. 2007; Fussmann et al. 2007; Kinnison & Hairston 2007). This area of research has been addressed in a variety of ways, but we believe eco-evolutionary feedbacks—reciprocal interactions between the ecology of populations, communities and ecosystems, and the evolution of organismal traits—may be the most rewarding and challenging component of this growing area of research. Eco-evolutionary feedbacks dynamically link the functional role of organisms within their environment to the evolution of organism function, and, therefore, they may sit at the centre of many adaptive radiations (Grant 1986; Losos 1994; Losos et al. 1998; Schluter 2001; Grant & Grant 2006; Calsbeek et al. 2007), where feedbacks emerge from and probably intensify the intraspecific differences that ultimately result in new species (Habets et al. 2006; Palkovacs & Post 2008). Eco-evolutionary feedbacks may also strongly affect community and ecosystem processes by altering the ecological role of differentiated populations (Bailey et al. 2006; Whitham et al. 2006; Post et al. 2008).
We define eco-evolutionary feedbacks as the cyclical interaction between ecology and evolution such that changes in ecological interactions drive evolutionary change in organismal traits that, in turn, alter the form of ecological interactions, and so forth. This interaction has been appreciated over the very long time-scales of evolution of organisms on the Earth (Lewontin 2000; Brodie 2005), but its importance in shaping ecological and evolutionary diversity at shorter (contemporary) time-scales is often overlooked (Lewontin 2000). It is the two-way interaction between ecology and evolution that characterizes eco-evolutionary feedbacks, not simply the need to incorporate ecology into evolution or evolution into ecology. Following Lewontin (2000) and Odling-Smee et al. (2003), we describe eco-evolutionary feedbacks as
| (1.1) |
| (1.2) |
where evolution of the organismal traits (dO/dt) is a function of the present state of the organism (O) and the environment (E), and changes in the environment (dE/dt) are a function of the present state of the environment and the organism. We define the environment (E) broadly as ‘…any property outside the organisms under consideration’ (Hutchinson 1957), or its n-dimensional niche. This includes all of the biological and physiochemical conditions (external to the organism) that might influence evolution. This formulation makes explicit the observation—central to much of modern ecology—that organisms can shape their environment, and the observation—at the centre of evolutionary biology—that the environment shapes the subsequent evolution of an organism.
Here, we present the general requirements for eco-evolutionary feedbacks in community and ecosystem ecology. We review the limited theoretical research on this topic and then present five empirical systems that demonstrate both important requirements of eco-evolutionary feedbacks and the full scope for feedbacks in natural systems. Our research on alewife populations, in particular, highlights the potential for eco-evolutionary feedbacks to drive evolutionary changes in traits that can substantially impact community structure and ecosystem function in natural ecosystems. Finally, we draw parallels to and outline differences among our eco-evolutionary perspective and other frameworks for synthesizing contemporary evolution with community and ecosystem ecology, including niche construction (sensu Laland et al. 1999; Odling-Smee et al. 2003), community genetics (Whitham et al. 2006), evolving metacommunities (Urban et al. 2008) and the geographic mosaic of coevolution (Thompson 2005).
2. Requirements for eco-evolutionary feedbacks
We start by making explicit the requirements for eco-evolutionary feedbacks in community and ecosystem ecology, which we will expand upon in §3. First, there must be a strong effect of the phenotype on the environment—organisms must structure or construct their environment (Odling-Smee et al. 2003). This requires that the population of interest has strong interactions with its environment. Second, the constructed environment must cause the subsequent evolution of the population in question. This requires that changes in the environment cause selection on the population (directional or disruptive), and that the population has sufficient genetic capacity to evolve in response to changes in its environment. Implicit in these two requirements is the key observation that the time-scales for the ecological and evolutionary responses need to be congruent (Laland et al. 1999; Hairston et al. 2005). Theoretical results indicate that eco-evolutionary feedbacks can emerge even in the presence of external environmental or evolutionary drivers (Laland et al. 1999). Finally, although not necessary for eco-evolutionary feedbacks, population-level (intraspecific) genetic and phenotypic variations (e.g. Palkovacs & Post 2008; Post et al. 2008) are important for testing the importance of eco-evolutionary dynamics in empirical systems (Laland et al. 1999).
The ecological literature is replete with examples of species that strongly impact the communities and ecosystems in which they reside (table 1), which we will call niche construction. Major mechanisms for niche construction include consumption, nutrient excretion (both inputs and recycling) and physical habitat alteration (table 1). Strongly interacting species are often well recognized as keystone species (Brooks & Dodson 1965; Paine 1966; Power et al. 1996), ecosystem engineers (Jones et al. 1994), foundation or dominant species (Whitham et al. 2006) and species that alter nutrient cycles through translocation or recycling (Jefferies et al. 1994; Post et al. 1998; Naiman et al. 2002; Vanni 2002; Schindler et al. 2003). All these types of species have strong impacts on the communities and ecosystems in which they reside, and, therefore, have the potential for niche construction. Species that have little or no impact on their community or ecosystem, either because their per capita interactions are weak (Paine 1992) or because they are rare members of the community, are less likely to produce eco-evolutionary feedbacks within their community or ecosystem. It is also important to note that effect size and duration may be important. Large, short-term effects on an ecosystem or community may not be sufficient to produce selection and subsequent evolution (see §3b below).
Table 1.
Well-studied examples of organisms that strongly shape their environment through nutrient cycling or translocation, consumption or habitat modification. (These organisms and others similar to them are candidates for eco-evolutionary feedbacks.)
| taxonomic group | mechanism | sources |
|---|---|---|
| plants | ||
| big bluestem (Andropogon gerardii) | habitat modification | Smith & Knapp (2003) |
| nutrient cycling | ||
| dominant tree species (e.g. Populus spp., Acer spp., Quercus spp.) | habitat modification | Likens et al. (1970), Madritch & Hunter (2002) and Whitham et al. (2006) |
| nutrient cycling | ||
| Eurasian water milfoil (Myriophyllum spicatum) | habitat modification | Carpenter (1980) and Carpenter & Lodge (1986) |
| nutrient cycling | ||
| Myrica faya | nutrient cycling | Vitousek et al. (1987) |
| invertebrates | ||
| crayfish (e.g. Orconectes rusticus) | consumption | Lodge & Lorman (1987), Lodge et al. (1994) and Covich et al. (1999) |
| habitat modification | ||
| nutrient cycling | ||
| earthworms (e.g. Lumbricus spp.) | consumption | Bohlen et al. (2004) |
| habitat modification | ||
| nutrient cycling | ||
| spiders (e.g. Phidippus rimator) | consumption | Schmitz (2008) |
| starfish (Pisaster ochraceus) | consumption | Paine (1966) and Menge et al. (1994) |
| zebra mussel (Dreissena polymorpha) | consumption | Arnott & Vanni (1996), MacIsaac (1996) and Strayer et al. (1999) |
| habitat modification | ||
| nutrient cycling and translocation | ||
| fish | ||
| alewife (Alosa pseudoharengus) | consumption | Brooks & Dodson (1965), Durbin et al. (1979), Kraft (1993) and Post et al. (2008) |
| habitat modification (indirect) | ||
| nutrient cycling and translocation | ||
| gizzard shad (Dorosoma cepedianum) | consumption | Devries & Stein (1992), Stein et al. (1995) and Schaus & Vanni (2000) |
| nutrient cycling | ||
| largemouth bass (Micropterus salmoides) | consumption | Carpenter et al. (1987), Mittelbach et al. (1995) and Post et al. (1997) |
| Pacific salmon (Oncorhynchus spp.) | nutrient cycling and translocation | Donaldson (1969), Schindler (1992), Bilby et al. (1996), Finney et al. (2002), Naiman et al. (2002) and Schindler et al. (2005) |
| peacock bass (Cichla ocellaris) | consumption | Zaret & Paine (1973) |
| reptiles and amphibians | ||
| Anolis lizards (Anolis spp.) | consumption | Schoener & Spiller (1996) and Schoener & Spiller (1999) |
| brown tree snake (Boiga irregularis) | consumption | Fritts & Rodda (1998) |
| birds | ||
| double-crested cormorants (Phalacrocorax auritus) | consumption | Madenjian & Gabrey (1995), Mills et al. (2003), Rudstam et al. (2004) and Dalton et al. (in press) |
| seabirds (e.g. Larus spp., Phalacrocorax spp.) | consumption | Bosman & Hockey (1986), Bosman et al. (1986), Wootton (1991) and Wootton (1995) |
| nutrient cycling and translocation | ||
| snow geese (Chen caerulescens) | consumption | Bazely & Jefferies (1985), Jefferies et al. (1994), Post et al. (1998) and Kitchell et al. (1999) |
| nutrient cycling | ||
| mammals | ||
| beaver (Castor canadensis) | consumption | Naiman et al. (1988), Jones et al. (1994) and Wright et al. (2002) |
| habitat modification | ||
| nutrient cycling | ||
| pocket gopher | habitat modification | Huntly & Inouye (1988) |
| nutrient cycling | ||
| sea otter (Enhydra lutris) | consumption | Estes & Palmisan (1974) |
| habitat modification (indirect) | ||
| elephants (Loxodonta africana) | habitat modification | Naiman (1988) and Jones et al. (1994) |
The definition of a species as a strong interactor often depends upon the ecological context being considered (Menge et al. 1994; Norkko et al. 2006). For example, the seastar Pisaster is the archetypal keystone species (Paine 1966), but its impact on intertidal food webs is much less in wave-protected sites than along wave-exposed shorelines (Menge et al. 1994). Likewise, dominant or foundational species in one habitat may be rare in another habitat, species moving nutrients into low-nutrient environments are likely to have greater effects on ecosystem function than species moving nutrients into high-nutrient environments, and species that have strong effects on communities and ecosystems when found in low-diversity communities may have much less impact on communities or ecosystems when found in high-diversity communities.
Our use of the term niche construction differs from that of Laland et al. (1999) and Odling-Smee et al. (2003) because it includes cases where an organism changes its environment (niche), even where that change does not feed back to influence its subsequent evolution (similar to Dawkins' (2004) use of niche change). By separating the effect of an organism on its environment (niche construction sensu D.M.P. and E.P.P.) from the evolutionary response of the organism, we hope to make it clear that eco-evolutionary feedbacks result from the convergence of the two processes, both of which often operate independently. We believe this addresses the criticism that niche construction theory (sensu Odling-Smee et al. 2003) is overly broad because it implies that all processes that shape the environment cause subsequent evolution (Dawkins 2004; Brodie 2005). Niche construction is therefore not limited to the active engineering of the environment but includes all of the by-products of living (eating, excreting, nutrient uptake and mineralization, etc.), and, as we discuss below, eco-evolutionary feedbacks at the community and ecosystem level can emerge from both direct engineering and the by-products of living (contrary to the arguments of Dawkins (2004) and Brodie (2005)).
In order for the eco-evolutionary feedbacks to occur, populations must not only shape their environment but also possess the ability to evolve in response to selection caused by the changes in the environment. Factors that might prevent a population from responding to niche construction are the same factors that constrain adaptive evolution generally. These factors include genetic constraints, including a lack of genetic variation and low heritability in the traits under selection, and demographic or ecological constraints, such as the swamping effects of genetic drift and gene flow and strong selection from extrinsic environmental drivers.
Finally, for eco-evolutionary feedbacks to emerge, both niche construction and evolution need to occur at congruent time-scales. This does not imply that evolution must be rapid or ecological change must be slow, but rather that the time-scale of change is sufficiently similar that it allows the dynamic feedback between evolutionary and ecological changes (Laland et al. 1999; Lewontin 2000). There is rapidly growing evidence for widespread rapid evolution among many traits and many organisms (Thompson 1998; Hairston et al. 1999; Hendry & Kinnison 1999; Hendry et al. 2000), and strong evidence for congruent time-scales in a few potential eco-evolutionary systems (Hairston et al. 2005). However, we stress that evolution does not have to be rapid for eco-evolutionary feedbacks to emerge. Slow niche construction caused by slow rates of evolution (and the reciprocal) are as likely to create eco-evolutionary feedbacks as rapid evolution and rapid niche construction. We also stress that it is not generation time per se that determines temporal congruence (although it might be related), but rather the rates of evolution and ecological change (or duration of niche construction), which need to be congruent. For example, niche construction must last long enough to cause evolution (Odling-Smee et al. (2003) call this ‘ecological inheritance’), and evolution must occur fast enough to feed back and influence the niche. A discontinuity in the time-scale of ecological and evolutionary responses is one of the probable disruptions to the complete eco-evolutionary feedback.
Intraspecific variation (Whitham et al. 2006; Post et al. 2008) is not a requisite for eco-evolutionary feedbacks, but it is critical for testing the importance of eco-evolutionary feedbacks for ecological and evolutionary dynamics in most natural systems. Variation among populations or experimental units provides the opportunity to break apart the dynamics of the feedback and to test the importance of the feedback for ecological interactions and evolutionary dynamics (e.g. Yoshida et al. 2003; Palkovacs & Post 2008; Post et al. 2008). For example, as we outline in more detail below, intraspecific variation in migratory behaviour and the strength of niche construction among populations of alewives were central to documenting the importance of eco-evolutionary feedbacks for alewife populations (Palkovacs & Post 2008; Palkovacs et al. 2008; Post et al. 2008), and variation in evolvability among experimental populations was used to test the importance of eco-evolutionary interactions in a rotifer–algae predator–prey system (Yoshida et al. 2003). Intraspecific variation in traits related to niche construction may also represent the initial stages in ecological speciation (Knox et al. 2001; Calsbeek et al. 2007), suggesting that studies of the origin and ecological implications of intraspecific variation (Bailey et al. 2006; Whitham et al. 2006; Post et al. 2008) may be of critical importance to understanding the origin of species diversity.
3. Empirical examples
Here, we summarize evidence for potential eco-evolutionary feedbacks in community and ecosystem ecology in five empirical systems: algal–rotifer chemostats (Yoshida et al. 2003); alewife–zooplankton communities in eastern North American lakes (Brooks & Dodson 1965; Palkovacs & Post 2008; Post et al. 2008); guppies in the streams of Trinidad (Reznick et al. 1997; Palkovacs et al. 2009); Darwin's finches of the Galápagos Islands (Grant 1986; Hairston et al. 2005; Grant & Grant 2006); and poplar trees of western North America (Whitham et al. 2006). Using these examples, we outline evidence for niche construction, the evolutionary response to niche construction and the processes that break apart eco-evolutionary feedbacks. In all five examples, the whole eco-evolutionary feedback is likely, but perhaps not fully documented, producing a mosaic of evidence for feedbacks in natural systems.
(a) Algal–rotifer chemostats
Using algal–rotifer chemostats, Yoshida et al. (2003) demonstrated experimentally that rapid evolution can alter predator–prey dynamics, consistent with some theoretical predictions (Abrams 2000). The experiment highlights a key requirement for eco-evolutionary feedbacks. Yoshida et al. (2003) compared the dynamics of algal–rotifer systems where evolution could occur (cultures initiated with multiple algal clones) with the dynamics in systems where evolution was not possible over the time-scale of the experiment (cultures initiated with a single clone). The lack of genetic variation in the single-clone treatment prevented evolution and provided the critical control for testing the importance of rapid evolution in modifying predator–prey dynamics. The lack of potential for adaptive evolution, either because of limited genetic variation among traits under selection, strong external selection (but see Laland et al. 1999) or strong gene flow from other populations, will prevent eco-evolutionary feedbacks. Subsequent research has shown that variation in anti-predator defence among algal genotypes can strongly influence rotifer growth rates and densities, which feed back to influence gene frequencies in algal populations (Yoshida et al. 2004; Meyer et al. 2006).
(b) Alewife: migration, foraging traits and zooplankton communities
The eco-evolutionary feedback in the alewife system revolves around interactions between young-of-the-year (YOY) alewives and zooplankton, their primary prey (Palkovacs & Post 2008; Post et al. 2008). The key players are anadromous alewives, which move between freshwater and marine habitats, landlocked alewives that spend their entire life in freshwater and the zooplankton upon which both prey. Migratory differences between landlocked and anadromous alewives are set up by differences in spatial openness among lakes. Lakes connected to the ocean contain anadromous alewife populations, while lakes isolated from the ocean contain either landlocked alewife populations or no alewives (Post et al. 2008). Key traits of alewife populations include the duration of residence in freshwater (approx. six months for anadromous alewives and year-round for landlocked alewives) and morphology related to feeding on zooplankton prey (mouth gape and gill raker spacing).
Anadromous alewives spawn in coastal lakes, ponds and streams from South Carolina, USA, to Nova Scotia, Canada (Scott & Crossman 1973). The adults spawn in March–May and YOY spend their first summer of life in freshwater before migrating to the ocean (Post et al. 2008). Adults remain resident in the spawning lakes and ponds for only a few weeks (Cooper 1961; Kissil 1974), and are not thought to feed during their spawning migration.
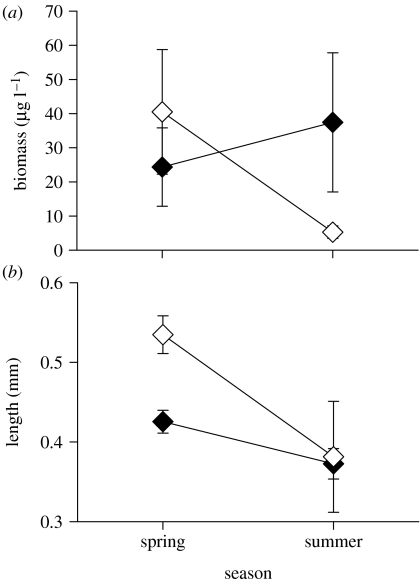
The zooplankton communities in lakes with anadromous alewives go through pronounced seasonal changes driven by predation by YOY (figure 1; Post et al. 2008). In the spring, just after ice out, the zooplankton community is dominated by a large biomass of large-bodied zooplankton (e.g. Daphnia spp. and Mesocyclops edax; Post et al. 2008). Once YOY become large enough to prey upon large-bodied zooplankton in mid-July (Palkovacs & Post 2008), they rapidly extirpate large-bodied zooplankton from the water column (Post et al. 2008). Through the rest of the summer and autumn, predation by alewives is sufficient to keep large-bodied zooplankton from reinvading and the zooplankton community is dominated by a low biomass of small-bodied zooplankton (figure 1; Post et al. 2008). Except in early summer when gape-limited, anadromous alewives always prey upon the largest zooplankton in the water column (positively size selective; Palkovacs & Post 2008). Once the final YOY emigrate in the autumn, large-bodied zooplankton re-establish over the winter and early spring, and by the time adults return to spawn the zooplankton community is once again dominated by a high biomass of large-bodied zooplankton (figure 1; Post et al. 2008). In anadromous populations, alewives have a very strong impact on zooplankton community structure (Post et al. 2008), but the rapid ecological recovery of the zooplankton communities, most probably from resting eggs in the sediments, means that the effects of strong niche construction do not persist to affect the evolution of subsequent generations (Palkovacs & Post 2008).
Figure 1.
Mean crustacean (a) biomass and (b) length in spring (late March to April) and summer (July and August) in lakes with anadromous alewives (unfilled diamonds) and landlocked alewives (filled diamonds). Error bars are 1 s.e. Data modified from Post et al. (2008).
The small streams through which alewives migrate to spawn were probably regularly blocked by beaver dams (Naiman et al. 1988) and wind throws. Alewives are able to move through very narrow gaps in a dam but are not able to jump over a complete blockage (D. M. Post 2007, personal observation); thus, an active beaver dam or large wind throw could have easily blocked migration into and out of coastal watersheds (e.g. Havey 1973). YOY alewives isolated from the ocean would have to overwinter in freshwater until access was restored to the ocean. While there are no good estimates of the duration of wind throws and beaver dams as a blockage to alewife migration, the average duration of beaver ponds on the landscape (Naiman et al. 1988) suggests that beaver dams and wind throws could block migration for years to decades. Thus, throughout their evolutionary history, some populations of anadromous alewives probably became landlocked for short periods of time (years to decades) behind these natural barriers.
Beaver dams and wind throws would have represented a greater obstacle for returning adults than for emigrating juveniles. Thus, these blockages may have served mainly to disrupt gene flow between the genotypes within a population that display the tendency for landlocking and those that display the tendency for anadromy. Evidence from salmonids suggests that various aspects of anadromous migratory behaviour have a heritable genetic component (Hendry et al. 2004). Therefore, the disruption of gene flow in the context of natural stream blockages may have played an important role in the evolution of landlocked alewife populations.
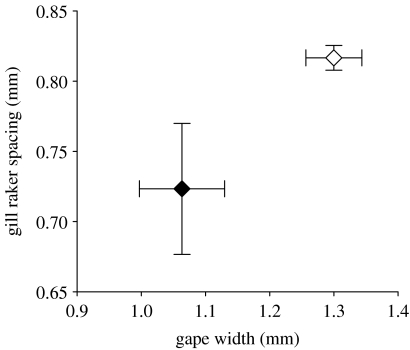
In New England, the barrier to migration between freshwater and the ocean formed by beaver dams and wind throws has been replaced in most coastal watersheds by dams built by humans. This has created landlocked populations isolated from the coastal ocean for much longer periods of time than previously experienced (Palkovacs et al. 2008). Across the landscape, landlocked populations derive either from human stocking efforts or naturally from anadromous ancestors (Palkovacs et al. 2008). The interaction between alewives and their zooplankton prey in lakes is fundamentally altered by the change in migratory behaviour, which changes the duration of residence of alewives in freshwater (Palkovacs & Post 2008; Post et al. 2008). Intense year-round predation pressure by landlocked alewives eliminates large-bodied prey and produces a zooplankton community of relatively low biomass of small-bodied zooplankton throughout the year (figure 1; Palkovacs & Post 2008; Post et al. 2008). The constant exposure of landlocked alewives to only small-bodied zooplankton (the newly constructed niche) leads to strong selection for traits related to foraging on small-bodied zooplankton (Palkovacs & Post 2008; Post et al. 2008). As a result, contemporary landlocked populations have smaller gape and narrower spacing between gill rakers than ancestral anadromous populations (figure 2; Palkovacs & Post 2008). Furthermore, while anadromous alewife populations are positively size selective for zooplankton prey, landlocked alewives are not size selective and prey upon the most abundant prey (typically, small-bodied zooplankton; Palkovacs & Post 2008).
Figure 2.
Mean (±1 s.e.) size-standardized gape width (mm) and gill raker spacing (mm) for anadromous (unfilled diamond) and landlocked alewives (filled diamond). Data are from three landlocked alewife populations (Pattagansett, Quonnipaug and Rogers) and three anadromous populations (Bride, Dodge and Gorton) in Connecticut, USA. Data modified from Palkovacs & Post (2008) and Post et al. (2008).
Thus, in the alewife system, access to the ocean (spatial openness) mediates migratory behaviour and the duration of residence in freshwater. Both anadromous and landlocked alewives structure zooplankton communities (Post et al. 2008), but the outmigration of anadromous alewives allows zooplankton communities to recover each year, preventing niche construction from influencing the evolution of subsequent generations and disrupting eco-evolutionary feedbacks (Palkovacs & Post 2008). Eco-evolutionary feedbacks are disrupted because the time-scale of niche construction (less than 1 year) is short relative to the time-scale required for selection to act upon foraging traits (multiple generations; Palkovacs & Post 2008; Post et al. 2008). By contrast, landlocked alewives permanently structure the zooplankton community (Post et al. 2008), which then selects for traits that improve feeding performance on small-bodied zooplankton (gape, gill raker and prey size preferences; Palkovacs & Post 2008). The divergence in morphology and prey selectivity between anadromous and landlocked populations alter the ecological role of alewife in lakes, where phenotypic differences create different zooplankton communities and alter the strength of the trophic cascade caused by alewives (Post et al. 2008; Palkovacs & Post 2009).
(c) Trinidadian guppies: life-history traits and nutrient cycling
In the streams of Trinidad's Northern Range Mountains, guppies exist either in the presence or the absence of large predatory fish. Guppy populations show divergence in life-history traits that are associated with differences in predation pressure. Compared to guppies in low-predation environments, guppies in high-predation environments display earlier age and smaller size at maturity and give birth to more frequent clutches of smaller offspring (Reznick 1982; Reznick & Endler 1982; Reznick & Bryga 1996; Reznick et al. 1996). It is likely that life-history shifts have important consequences for population dynamics (Kokko & López-Sepulcre 2007). Guppy populations at sites lacking predators reach higher densities than populations at sites with predators; these density differences may influence resource availability, the strength of intraspecific competition and subsequent guppy evolution (Grether et al. 2001; Reznick et al. 2001). Shifts in guppy life-history traits also directly influence the body size distribution of the guppy population at a given locality. Under conditions of equal biomass, a population dominated by more, smaller individuals (in this case, a high predation population) is expected to drive higher nutrient fluxes than a population dominated by fewer, larger individuals (in this case, low-predation populations; Vanni 2002; Hall et al. 2007). Higher nutrient fluxes may increase rates of primary production (Vanni & Layne 1997; Flecker et al. 2002).
A mesocosm experiment was performed to examine the effects of guppy life-history evolution on ecosystem processes (Palkovacs et al. 2009). The results show that high-predation guppy populations contributed approximately double the amount of N and P to the total nutrient pool via excretion compared to low-predation populations. Relative to nutrient levels in source water from the adjacent stream, nutrient contributions to mesocosms via guppy excretion drove an 11 per cent increase in N and an 83 per cent increase in P for high-predation populations compared with a 6 per cent increase in N and a 46 per cent increase in P for low-predation populations. This difference in excretion contributed to a significant increase in algal biomass in the high-predation treatment. Algal biomass may have been further influenced by evolutionary divergence in other guppy traits, including trophic morphology and dietary preferences. In turn, overall changes in algal biomass may influence subsequent guppy evolution. For example, male guppy colour patterns (which are under both natural and sexual selection) are sensitive to the amount of algae-derived carotenoids available in the environment (Grether 2000; Grether et al. 2005). Therefore, evolutionary changes caused by biotic factors, such as predators, may have ecological effects at multiple levels of organization and may feed back to influence evolution in relatively unexpected ways.
(d) Avian seed predators: foraging traits and seed availability
Darwin's finches are famous for diverse beak sizes and shapes, which enable different populations and species to exploit different food resources across the Galápagos Islands (Lack 1947; Grant 1986). Within populations, beak size and shape can evolve rapidly in response to changes in seed availability (Grant & Grant 1995, 2002). Seed availability changes in response to rainfall patterns, but the link between seeds and rainfall may be mediated by finch predation. During periods of drought, finches deplete favoured seeds first, driving selection on finch traits that enable the exploitation of larger, harder seeds (Grant & Grant 2006). Thus, rather than rainfall driving finch evolution directly, it is likely that rainfall mediates the ability of finches to construct their niche. When rainfall is abundant, favoured seeds are plentiful and finches are unable to influence the overall distribution of seed sizes in the environment. However, during droughts, favoured seeds become limiting and finches can influence the abundance of different types of seeds. Thus, abundant rainfall may decouple the eco-evolutionary feedback between finches and seeds. The scenario outlined above is speculative—the degree to which finches can shape the size distribution of seeds and the effect this might have on the long-term composition of the plant community is not well known. However, information from another avian seed predator, the red crossbill, suggests that feedbacks between seed predators and plants may be common.
Red crossbills are specialist feeders that consume the seeds of lodgepole pines. In some isolated habitats, reciprocal selection between crossbills and pines is strong, and these two species co-evolve in an arms race (Benkman 1999; Benkman et al. 2001, 2003). This arms race takes subtly different forms in different localities and is thought to have contributed to the adaptive radiation of crossbills (Edelaar & Benkman 2006; Smith & Benkman 2007). Thus, crossbills influence the trajectory of their own evolution by shaping the availability of seed resources. However, this pattern is disrupted by the presence of another seed predator, the red squirrel. Where present, red squirrels, not crossbills, serve as the primary agent of selection on lodgepole pine cones (Benkman 1999; Benkman et al. 2001, 2003). Thus, the presence of red squirrels can decouple the co-evolutionary feedback between crossbills and pines.
From the standpoint of eco-evolutionary feedbacks, these examples illustrate two main points. First, co-evolutionary interactions may be viewed as a special case of eco-evolutionary interactions. Coevolution is the reciprocal evolutionary interaction between populations of two species, while eco-evolutionary feedbacks emerge from the reciprocal interaction between a population and its environment (which could be a population of another species). Second, the strength of feedbacks may be contingent on abiotic or biotic factors. In the case of the finches, the existence of an eco-evolutionary feedback may depend on the amount of rainfall in a given year. In the case of the crossbills, the existence of coevolution depends on the presence or absence of a competitor species. Abiotic and biotic factors that shape the strength of feedbacks may create the landscape-scale templates that shape environmental (i.e. ecological) and organismal (i.e. evolutionary) diversity. This perspective extends the scope of geographic mosaics from coevolving systems (Thompson 2005) to eco-evolutionary systems, as we will discuss further. The ability of spatial structure to promote ecological and evolutionary diversity in the context of eco-evolutionary feedbacks is supported by experimental work with microbial systems (Habets et al. 2006) and our work with alewives (Palkovacs & Post 2008; Post et al. 2008). An emerging frontier in the study of eco-evolutionary feedbacks is the examination of the importance of spatial structure in natural ecosystems.
(e) Populus: leaf tannins and soil processes
Foundation species, such as trees of the genus Populus, can control community and ecosystem processes through their chemical effects on leaf litter (Whitham et al. 2006; Schweitzer et al. 2008b). Intraspecific phenotypic variation in leaf chemistry has been shown to drive variation in soil processes in multiple terrestrial plant species (Treseder & Vitousek 2001; Madritch & Hunter 2002; Schweitzer et al. 2004). In Populus, condensed tannin levels in leaves, which are under genetic control, strongly impact decomposition rates, nitrogen mineralization rates and microbial community composition of riparian forest in western North America (Schweitzer et al. 2004, 2005a,b, 2008a; Madritch et al. 2006). Trees create their own soil microhabitats; therefore, genotypes with higher concentrations of foliar condensed tannins (which impede nutrient release) must cope with decreased nutrient availability in the soil. If eco-evolutionary feedbacks are important, Populus genotypes with high foliar tannin levels would be expected to display adaptations to cope with limited nutrients. Indeed, there is a strong positive correlation between leaf tannin levels and the production of fine roots, providing indirect evidence for an eco-evolutionary feedback in Populus driven by soil processes and nutrient availability (Fischer et al. 2006). Theoretical results also support the hypothesis that eco-evolutionary feedbacks may be important for the ecology and evolution of plant litter–nutrient uptake systems (Kylafis & Loreau 2008). However, the ability of a plant genotype to shape its nutrient environment is influenced by ecological interactions, including the presence of other tree species (Madritch & Hunter 2004), herbivores (Madritch et al. 2007) and nutrient loading (Madritch et al. 2006). Thus, similar to the example of the red crossbills, the potential for and strength of feedbacks in Populus are probably mediated by interactions among species and the strength of external drivers such as nutrient loading.
4. Complementary perspectives
The perspective we outline here complements other emerging frameworks for synthesizing contemporary evolution with community and ecosystem ecology, including niche construction (sensu Odling-Smee et al. 2003), community genetics, evolving metacommunities and the geographic mosaic of coevolution (reviewed in Johnson & Stinchcombe 2007; Urban et al. 2008). These frameworks share features, such as the recognition that contemporary evolution influences species' interactions; however, they differ in the specific patterns and processes they seek to explain. The niche construction perspective (sensu Laland et al. 1999; Odling-Smee et al. 2003) explicitly recognizes the ability of organisms to shape the biotic and abiotic attributes of their environments, and the potential for those changes to influence subsequent adaptive evolution. The community genetics approach focuses on the impact of genetic variation in foundation species on the structure of ecological communities (Bailey et al. 2006; Whitham et al. 2006). The evolving metacommunity approach focuses on the ecological and evolutionary mechanisms that promote species' coexistence (Urban & Skelly 2006). The geographical mosaic theory seeks to understand why two-way species' interactions vary across the landscape as a function of ecological and evolutionary processes (Thompson 2005). In developing our eco-evolutionary feedbacks framework, we have integrated aspects of all these approaches. Similar to the geographical mosaic of coevolution, we focus on understanding what structures biodiversity across the landscape as a function of reciprocal interactions and recognize the importance of eco-evolutionary ‘hot spots’ and ‘cold spots’ driven by landscape-scale variation in abiotic and biotic interactions. However, our focus is not strictly on reciprocal evolutionary interactions, but on reciprocal interactions between evolutionary and ecological processes. Similar to community genetics, we are interested in how evolutionary changes in strongly interacting species influence community structure (Bailey et al. 2006; Whitham et al. 2006). However, we are also interested in how changes to ecological communities feed back to influence the trajectory of evolution.
Finally, we draw heavily from the niche construction perspective of Laland et al. (1999) and Odling-Smee et al. (2003), but here we explicitly separate the process of structuring the environment (niche construction sensu D.M.P. and E.P.P.) from the process of evolution by natural selection, producing a framework that reflects the independence of the two processes required for eco-evolutionary feedbacks (Brodie 2005). Ultimately, understanding what drives changes in community structure involves understanding the ecological and evolutionary mechanisms mediating coexistence, which itself involves understanding how coevolution operates in multi-species communities.
The various frameworks emerging to integrate contemporary evolution into community and ecosystem ecology complement each other by seeking to understand the same core processes from slightly different perspectives.
5. Conclusions and future directions
We believe that the future direction of eco-evolutionary dynamics is in the study of feedbacks between ecology and evolution. Such feedbacks, where they exist, have the potential to alter the direction of evolution and strongly modify the role of species in ecosystems. In the alewife system, ecological isolation of lakes from the ocean has altered the direction of evolution, and phenotypic differentiation in feeding morphology and prey selectivity has fundamentally altered the ecological role of alewives in coastal lakes. Likewise, life-history evolution in Trinidadian guppies has altered their ecological role in streams, which has the potential to feed back and influence the evolution of guppy traits. In each of our empirical examples, the eco-evolutionary feedback (or potential for feedback) has fundamentally altered both the ecological role of the organism and the trajectory of evolution.
Despite these compelling examples, there currently exists a handful of empirical systems where the effects of phenotypic (or genetic) differences on community and ecosystem ecology are well resolved (alewife, Populus) or where the evolution of phenotypic differences are well resolved (guppies and finches), but not a single system where the evidence of dynamic feedbacks is without gaps. Furthermore, the constraints on eco-evolutionary feedbacks have not been well tested. Future research should work towards providing better evidence for the occurrence of eco-evolutionary feedbacks in a variety of systems and better tests of the conditions under which feedbacks may be important. More complete evidence for feedbacks in empirical systems requires documenting (i) the strength of niche construction, (ii) the strength of selection and the direction of evolution in the constructed environment, and (iii) the strength of niche construction after evolution. Strong external drivers may be able to overwhelm eco-evolutionary feedbacks, but there is some theoretical evidence that even strong donor control of resources or high rates of gene flow can still allow for niche construction and selection (Laland et al. 1999). Future research should explore the range of extrinsic environmental factors which allow for niche construction, and the range of resulting selection pressures which allow for local adaptation. Future work should also test the assumption that niche construction and evolution must occur at congruent time-scales. Studies across a range of disparity in time-scales would provide important insights into when feedbacks ultimately break apart and interactions become unidirectional.
There is also a question of where eco-evolutionary feedbacks are most likely to be important. Feedbacks are mostly likely to emerge for species that strongly alter their environment (table 1). Keystone, dominant or foundation species and ecosystem engineers are all likely candidates for eco-evolutionary feedbacks because they interact strongly with their environment, and intraspecific variation among or within populations can alter the role of the species in the ecosystem and alter the strength of the eco-evolutionary feedback. However, species not recognized as having strong effects on their environment in species-rich communities might have stronger effects in species-poor communities. For example, strong eco-evolutionary feedbacks may be initiated when species-poor islands are colonized by populations from species-rich mainlands (e.g. many invasive species). The phenotypic and genetic variations produced by eco-evolutionary feedbacks may be critically important for understanding the early stages of ecological speciation and adaptive radiation (Habets et al. 2006). Clades that exhibit diversification in traits related to foraging, excretion and habitat modification are all likely candidates for study.
We believe that studying eco-evolutionary feedbacks is important because it pushes evolutionary biologists to recognize that organisms can shape their environment in ways that alter the outcome of evolution, and it pushes ecologists to recognize that contemporary evolution creates phenotypic differences that can alter the role of a species in a community or ecosystem at contemporary time-scales. In this way, the study of eco-evolutionary feedbacks focuses attention on the bidirectional interactions that unify ecology and evolution, and highlights the importance of conserving both ecological and evolutionary diversity in nature (e.g. Stockwell et al. 2003; Kinnison & Hairston 2007).
Acknowledgments
We thank the ‘Eco-evolutionary dynamics’ working group hosted by the Centre for Population Biology for discussions that improved this manuscript. J. K. Bailey and J. A. Schweitzer provided important insights on potential eco-evolutionary feedbacks involving Populus, N. G. Hairston provided insight on eco-evolutionary feedbacks in the algae–rotifer system, and M. Festa-Bianchet, T. Ezard and L. M. Puth provided helpful comments and suggestions on a draft of this manuscript. Funding for our work on alewife–zooplankton interactions was provided by the USA National Science Foundation (DEB no. 0717265), the Connecticut Institute for Water Resources and an EPA STAR Graduate Fellowship Award to E.P.P. Work on the guppy system was funded by the USA National Science Foundation FIBR Program (EF 0623632).
Footnotes
One contribution of 14 to a Theme Issue ‘Eco-evolutionary dynamics’.
References
- Abrams P.A. The evolution of predator–prey interactions: theory and evidence. Annu. Rev. Ecol. Syst. 2000;31:79–105. doi:10.1146/annurev.ecolsys.31.1.79 [Google Scholar]
- Arnott D.L., Vanni M.J. Nitrogen and phosphorus recycling by the zebra mussel (Dreissena polymorpha) in the western basin of Lake Erie. Can. J. Fish. Aquat. Sci. 1996;53:646–659. doi:10.1139/cjfas-53-3-646 [Google Scholar]
- Bailey J.K., Wooley S.C., Lindroth R.L., Whitham T.G. Importance of species interactions to community heritability: a genetic basis to trophic-level interactions. Ecol. Lett. 2006;9:78–85. doi: 10.1111/j.1461-0248.2005.00844.x. doi:10.1111/j.1461-0248.2005.00844.x [DOI] [PubMed] [Google Scholar]
- Bazely D.R., Jefferies R.L. Goose feces: a source of nitrogen for plant growth in a grazed salt marsh. J. Appl. Ecol. 1985;22:693–703. doi:10.2307/2403222 [Google Scholar]
- Benkman C.W. The selection mosaic and diversifying coevolution between crossbills and lodgepole pine. Am. Nat. 1999;153:S75–S91. doi: 10.1086/303213. doi:10.1086/303213 [DOI] [PubMed] [Google Scholar]
- Benkman C.W., Holimon W.C., Smith J.W. The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution. 2001;55:282–294. doi: 10.1111/j.0014-3820.2001.tb01293.x. doi:10.1111/j.0014-3820.2001.tb01293.x [DOI] [PubMed] [Google Scholar]
- Benkman C.W., Parchman T.L., Favis A., Siepielski A.M. Reciprocal selection causes a coevolutionary arms race between crossbills and lodgepole pine. Am. Nat. 2003;162:182–194. doi: 10.1086/376580. doi:10.1086/376580 [DOI] [PubMed] [Google Scholar]
- Bilby R.E., Fransen B.R., Bisson P.A. Incorporation of nitrogen and carbon from spawning coho salmon into the trophic system of small streams: evidence from stable isotopes. Can. J. Fish. Aquat. Sci. 1996;53:164–173. doi:10.1139/cjfas-53-1-164 [Google Scholar]
- Bohlen P.J., Groffman P.M., Fahey T.J., Fisk M.C., Suarez E., Pelletier D.M., Fahey R.T. Ecosystem consequences of exotic earthworm invasion of north temperate forests. Ecosystems. 2004;7:1–12. doi:10.1007/s10021-003-0126-z [Google Scholar]
- Bosman A.L., Hockey P.A.R. Seabird guano as a determinant of rocky intertidal community structure. Mar. Ecol. Prog. Ser. 1986;32:247–257. doi:10.3354/meps032247 [Google Scholar]
- Bosman A.L., Dutoit J.T., Hockey P.A.R., Branch G.M. A field experiment demonstrating the influence of seabird guano on intertidal primary production. Estuar. Coast. Shelf Sci. 1986;23:283–294. doi:10.1016/0272-7714(86)90028-4 [Google Scholar]
- Brodie E.D. Caution: niche construction ahead. Evolution. 2005;59:249–251. doi:10.1111/j.0014-3820.2005.tb00914.x [Google Scholar]
- Brooks J.L., Dodson S.I. Predation, body size, and the composition of plankton. Science. 1965;150:28–35. doi: 10.1126/science.150.3692.28. doi:10.1126/science.150.3692.28 [DOI] [PubMed] [Google Scholar]
- Calsbeek R., Smith T.B., Bardeleben C. Intraspecific variation in Anolis sagrei mirrors the adaptive radiation of Greater Antillean anoles. Biol. J. Linn. Soc. 2007;90:189–199. doi:10.1111/j.1095-8312.2007.00700.x [Google Scholar]
- Carpenter S.R. Enrichment of Lake Wingra, Wisconsin, by submersed macrophyte decay. Ecology. 1980;61:1145–1155. doi:10.2307/1936834 [Google Scholar]
- Carpenter S.R., Lodge D.M. Effects of submersed macrophytes on ecosystem processes. Aquat. Bot. 1986;26:341–370. doi:10.1016/0304-3770(86)90031-8 [Google Scholar]
- Carpenter S.R., et al. Regulation of lake primary productivity by food web structure. Ecology. 1987;68:1863–1876. doi: 10.2307/1939878. doi:10.2307/1939878 [DOI] [PubMed] [Google Scholar]
- Carroll S.P., Hendry A.P., Reznick D.N., Fox C.W. Evolution on ecological time-scales. Funct. Ecol. 2007;21:387–393. doi:10.1111/j.1365-2435.2007.01289.x [Google Scholar]
- Cooper, R. A. 1961 Early life history and spawning migration of the alewife, Alosa pseudoharengus MSc thesis, p. 58. University of Rhode Island, Kingston, RI.
- Covich A.P., Palmer M.A., Crowl T.A. The role of benthic invertebrate species in freshwater ecosystems—zoobenthic species influence energy flows and nutrient cycling. Bioscience. 1999;49:119–127. doi:10.2307/1313537 [Google Scholar]
- Dalton, C. M., Ellis, D. & Post, D. M. In press. The impact of double-crested cormorant (Phalacrocorax aurtitus) predation on anadromous alewive (Alosa pseudoharengus) in south-central Connecticut, USA. Can. J. Fish. Aquat. Sci 66, 177–186. (doi:10.1139/F08-198)
- Dawkins R. Extended phenotype—but not too extended. A reply to Laland, Turner and Jablonka. Biol. Philos. 2004;19:377–396. doi:10.1023/B:BIPH.0000036180.14904.96 [Google Scholar]
- Devries D.R., Stein R.A. Complex interactions between fish and zooplankton—quantifying the role of an open-water planktivore. Can. J. Fish. Aquat. Sci. 1992;49:1216–1227. doi:10.1139/f92-137 [Google Scholar]
- Donaldson J.R. University of Washington; Seattle, WA: 1969. Phosphorus budget of Iliama Lake, Alaska, as related to the cyclic abundance of sockeye salmon. [Google Scholar]
- Durbin A.G., Nixon S.W., Oviatt C.A. Effects of the spawning migration of the alewife, Alosa pseudoharengus, on freshwater ecosystems. Ecology. 1979;60:8–17. doi:10.2307/1936461 [Google Scholar]
- Edelaar P., Benkman C.W. Replicated population divergence caused by localized coevolution? A test of three hypotheses in the red crossbill–lodgepole pine system. J. Evol. Biol. 2006;19:1651–1659. doi: 10.1111/j.1420-9101.2006.01113.x. doi:10.1111/j.1420-9101.2006.01113.x [DOI] [PubMed] [Google Scholar]
- Estes J.A., Palmisan J.F. Sea otters—their role in structuring nearshore communities. Science. 1974;185:1058–1060. doi: 10.1126/science.185.4156.1058. doi:10.1126/science.185.4156.1058 [DOI] [PubMed] [Google Scholar]
- Finney B.P., Gregory-Eaves I., Douglas M.S.V., Smol J.P. Fisheries productivity in the northeastern Pacific Ocean over the past 2,200 years. Nature. 2002;416:729–733. doi: 10.1038/416729a. doi:10.1038/416729a [DOI] [PubMed] [Google Scholar]
- Fischer D.G., Hart S.C., Rehill B.J., Lindroth R.L., Keim P., Whitham T.G. Do high-tannin leaves require more roots? Oecologia. 2006;149:668–675. doi: 10.1007/s00442-006-0471-7. doi:10.1007/s00442-006-0471-7 [DOI] [PubMed] [Google Scholar]
- Flecker A.S., Taylor B.W., Bernhardt E.S., Hood J.M., Cornwell W.K., Cassatt S.R., Vanni M.J., Altman N.S. Interactions between herbivorous fishes and limiting nutrients in a tropical stream ecosystem. Ecology. 2002;83:1831–1844. doi:10.2307/3071768 [Google Scholar]
- Fritts T.H., Rodda G.H. The role of introduced species in the degradation of island ecosystems: a case history of Guam. Annu. Rev. Ecol. Syst. 1998;29:113–140. doi:10.1146/annurev.ecolsys.29.1.113 [Google Scholar]
- Fussmann G.F., Loreau M., Abrams P.A. Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 2007;21:465–477. doi:10.1111/j.1365-2435.2007.01275.x [Google Scholar]
- Grant P.R. Princeton University Press; Princeton, NJ: 1986. Ecology and evolution of Darwin's finches. [Google Scholar]
- Grant P.R., Grant B.R. Predicting microevolutionary responses to directional selection on heritable variation. Evolution. 1995;49:241–251. doi: 10.1111/j.1558-5646.1995.tb02236.x. doi:10.2307/2410334 [DOI] [PubMed] [Google Scholar]
- Grant P.R., Grant B.R. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. doi:10.1126/science.1070315 [DOI] [PubMed] [Google Scholar]
- Grant P.R., Grant B.R. Evolution of character displacement in Darwin's finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. doi:10.1126/science.1128374 [DOI] [PubMed] [Google Scholar]
- Grether G.F. Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies (Poecilia reticulata) Evolution. 2000;54:1712–1724. doi: 10.1111/j.0014-3820.2000.tb00715.x. doi:10.1111/j.0014-3820.2000.tb00715.x [DOI] [PubMed] [Google Scholar]
- Grether G.F., Millie D.F., Bryant M.J., Reznick D.N., Mayea W. Rain forest canopy cover, resource availability, and life history evolution in guppies. Ecology. 2001;82:1546–1559. doi:10.2307/2679799 [Google Scholar]
- Grether G.F., Cummings M.E., Hudon J. Countergradient variation in the sexual coloration of guppies (Poecilia reticulata): drosopterin synthesis balances carotenoid availability. Evolution. 2005;59:175–188. doi:10.1554/04-351 [PubMed] [Google Scholar]
- Habets M., Rozen D.E., Hoekstra R.F., de Visser J. The effect of population structure on the adaptive radiation of microbial populations evolving in spatially structured environments. Ecol. Lett. 2006;9:1041–1048. doi: 10.1111/j.1461-0248.2006.00955.x. doi:10.1111/j.1461-0248.2006.00955.x [DOI] [PubMed] [Google Scholar]
- Hairston N.G., Lampert W., Caceres C.E., Holtmeier C.L., Weider L.J., Gaedke U., Fischer J.M., Fox J.A., Post D.M. Rapid evolution revealed by dormant eggs. Nature. 1999;401:446. doi:10.1038/46731 [Google Scholar]
- Hairston N.G., Ellner S.P., Geber M.A., Yoshida T., Fox J.A. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 2005;8:1114–1127. doi:10.1111/j.1461-0248.2005.00812.x [Google Scholar]
- Hall R.O., Koch B.J., Marshall M.C., Taylor B.W., Tronstad L.M. How body size mediates the role of animals in nutrient cycling in aquatic ecosystems. In: Hildrew A., Raffaelli D., Edmonds-Brown R., editors. Body size: the structure and function of aquatic ecosystems. Cambridge University Press; Cambridge, UK: 2007. pp. 286–305. [Google Scholar]
- Havey K.A. Production of juvenile alewives, Alosa pseudoharengus, at Love Lake, Washington County, Maine. Trans. Am. Fish. Soc. 1973;2:434–438. doi:10.1577/1548-8659(1973)102<434:POJAAP>2.0.CO;2 [Google Scholar]
- Hendry A.P., Kinnison M.T. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. doi:10.2307/2640428 [DOI] [PubMed] [Google Scholar]
- Hendry A.P., Wenburg J.K., Bentzen P., Volk E.C., Quinn T.P. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. doi:10.1126/science.290.5491.516 [DOI] [PubMed] [Google Scholar]
- Hendry A.P., Bohlin T., Jonsson B., Berg O.K. Oxford University Press; Oxford, UK: 2004. To sea or not to sea? Anadromy versus non-anadromy in salmonids. [Google Scholar]
- Huntly N., Inouye R. Pocket gophers in ecosystems: patterns and mechanisms. Bioscience. 1988;38:786–793. doi:10.2307/1310788 [Google Scholar]
- Hutchinson G.E. Concluding remarks. Population studies: animal ecology and demography. Cold Spring Harb. Symp. Quant. Biol. 1957;22:415–427. [Google Scholar]
- Jefferies R.L., Klein D.R., Shaver G.R. Vertebrate herbivores and northern plant-communities—reciprocal influences and responses. Oikos. 1994;71:193–206. doi:10.2307/3546267 [Google Scholar]
- Johnson M.T.J., Stinchcombe J.R. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol. Evol. 2007;22:250–257. doi: 10.1016/j.tree.2007.01.014. doi:10.1016/j.tree.2007.01.014 [DOI] [PubMed] [Google Scholar]
- Jones C.G., Lawton J.H., Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. doi:10.2307/3545850 [Google Scholar]
- Kinnison M.T., Hairston N.G. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 2007;21:444–454. doi:10.1111/j.1365-2435.2007.01278.x [Google Scholar]
- Kissil G.W. Spawning of anadromous alewife, Alosa pseudoharengus, in Bride Lake, Connecticut. Trans. Am. Fish. Soc. 1974;103:312–317. doi:10.1577/1548-8659(1974)103<312:SOTAAA>2.0.CO;2 [Google Scholar]
- Kitchell J.F., Schindler D.E., Herwig B.R., Post D.M., Olson M.H., Oldham M. Nutrient cycling at the landscape scale: the role of diel foraging migrations by geese at the Bosque del Apache National Wildlife Refuge, New Mexico. Limnol. Oceanogr. 1999;44:828–836. [Google Scholar]
- Knox A.K., Losos J.B., Schneider C.J. Adaptive radiation versus intraspecific differentiation: morphological variation in Caribbean Anolis lizards. J. Evol. Biol. 2001;14:904–909. doi:10.1046/j.1420-9101.2001.00358.x [Google Scholar]
- Kokko H., López-Sepulcre A. The ecogenetic link between demography and evolution: can we bridge the gap between theory and data? Ecol. Lett. 2007;10:773–782. doi: 10.1111/j.1461-0248.2007.01086.x. doi:10.1111/j.1461-0248.2007.01086.x [DOI] [PubMed] [Google Scholar]
- Kraft C.E. Phosphorus regeneration by Lake Michigan alewives in the mid-1970s. Trans. Am. Fish. Soc. 1993;122:749–755. doi:10.1577/1548-8659(1993)122<0749:PRBLMA>2.3.CO;2 [Google Scholar]
- Kylafis G., Loreau M. Ecological and evolutionary consequences of niche construction for its agent. Ecol. Lett. 2008;11:1072–1081. doi: 10.1111/j.1461-0248.2008.01220.x. doi:10.1111/j.1461-0248.2008.01220.x [DOI] [PubMed] [Google Scholar]
- Lack D. Cambridge University Press; Cambridge, UK: 1947. Darwin's finches. [Google Scholar]
- Laland K.N., Odling-Smee F.J., Feldman M.W. Evolutionary consequences of niche construction and their implications for ecology. Proc. Natl Acad. Sci. USA. 1999;96:10 242–10 247. doi: 10.1073/pnas.96.18.10242. doi:10.1073/pnas.96.18.10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewontin R.C. Harvard University Press; Cambridge, MA: 2000. The triple helix. Gene, organisms, and environment. [Google Scholar]
- Likens G.E., Bormann F.H., Johnson N.M., Fisher D.W., Pierce R.S. Effects of forest cutting and herbicide treatment on nutrient budgets in Hubbard Brook watershed-ecosystem. Ecol. Monogr. 1970;40:23–47. doi:10.2307/1942440 [Google Scholar]
- Lodge D.M., Lorman J.G. Reductions in submersed macrophyte biomass and species richness by the crayfish Orconectes rusticus. Can. J. Fish. Aquat. Sci. 1987;44:591–597. doi:10.1139/f87-072 [Google Scholar]
- Lodge D.M., Kershner M.W., Aloi J.E., Covich A.P. Effects of an omnivorous crayfish (Orconectes rusticus) on a fresh-water littoral food web. Ecology. 1994;75:1265–1281. doi:10.2307/1937452 [Google Scholar]
- Losos J.B. Integrative approaches to evolutionary ecology: Anolis lizards as model systems. Annu. Rev. Ecol. Syst. 1994;25:467–493. doi:10.1146/annurev.es.25.110194.002343 [Google Scholar]
- Losos J.B., Jackman T.R., Larson A., de Queiroz K., Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. doi:10.1126/science.279.5359.2115 [DOI] [PubMed] [Google Scholar]
- MacIsaac H.J. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. Am. Zool. 1996;36:287–299. doi:10.1093/icb/36.3.287 [Google Scholar]
- Madenjian C.P., Gabrey S.W. Waterbird predation on fish in western Lake Erie: a bioenergetics model application. Condor. 1995;97:141–153. doi:10.2307/1368992 [Google Scholar]
- Madritch M.D., Hunter M.D. Phenotypic diversity influences ecosystem functioning in an oak sandhills community. Ecology. 2002;83:2084–2090. doi:10.2307/3072039 [Google Scholar]
- Madritch M.D., Hunter M.D. Phenotypic diversity and litter chemistry affect nutrient dynamics during litter decomposition in a two species mix. Oikos. 2004;105:125–131. doi:10.1111/j.0030-1299.2004.12760.x [Google Scholar]
- Madritch M.D., Donaldson J.R., Lindroth R.L. Genetic identity of Populus tremuloides litter influences decomposition and nutrient release in a mixed forest stand. Ecosystems. 2006;9:528–537. doi:10.1007/s10021-006-0008-2 [Google Scholar]
- Madritch M.D., Donaldson J.R., Lindroth R.L. Canopy herbivory can mediate the influence of plant genotype on soil processes through frass deposition. Soil Biol. Biochem. 2007;39:1192–1201. doi:10.1016/j.soilbio.2006.12.027 [Google Scholar]
- Menge B.A., Berlow E.L., Blanchette C.A., Navarrete S.A., Yamada S.B. The keystone species concept: variation in interaction strength in a rocky intertidal habitat. Ecol. Monogr. 1994;64:249–286. doi:10.2307/2937163 [Google Scholar]
- Meyer J.R., Ellner S.P., Hairston N.G., Jones L.E., Yoshida T. Prey evolution on the time scale of predator–prey dynamics revealed by allele-specific quantitative PCR. Proc. Natl Acad. Sci. USA. 2006;103:10 690–10 695. doi: 10.1073/pnas.0600434103. doi:10.1073/pnas.0600434103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.L., et al. Lake Ontario: food web dynamics in a changing ecosystem (1970–2000) Can. J. Fish. Aquat. Sci. 2003;60:471–490. doi:10.1139/f03-033 [Google Scholar]
- Mittelbach G.G., Turner A.M., Hall D.J., Rettig J.E., Osenberg C.W. Perturbation and resilience: a long-term, whole-lake study of predator extinction and reintroduction. Ecology. 1995;76:2347–2360. doi:10.2307/2265812 [Google Scholar]
- Naiman R.J. Animal influences on ecosystem dynamics. BioScience. 1988;38:750–752. doi:10.2307/1310783 [Google Scholar]
- Naiman R.J., Johnston C.A., Kelley J.C. Alteration of North American streams by beaver. BioScience. 1988;38:753–762. doi:10.2307/1310784 [Google Scholar]
- Naiman R.J., Bilby R.E., Schindler D.E., Helfield J.M. Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems. 2002;5:399–417. doi:10.1007/s10021-001-0083-3 [Google Scholar]
- Norkko A., Hewitt J.E., Thrush S.F., Funnell G.A. Conditional outcomes of facilitation by a habitat-modifying subtidal bivalve. Ecology. 2006;87:226–234. doi: 10.1890/05-0176. doi:10.1890/05-0176 [DOI] [PubMed] [Google Scholar]
- Odling-Smee, F. J., Laland, K. N. & Feldman, M. W. 2003 Niche construction: the neglected process in evolution Monographs in Population Biology. Princeton, NJ: Princeton University Press.
- Paine R.T. Food web complexity and species diversity. Am. Nat. 1966;100:65–75. doi:10.1086/282400 [Google Scholar]
- Paine R.T. Food-web analysis through field measurements of per capita interaction strength. Nature. 1992;355:73–75. doi:10.1038/355073a0 [Google Scholar]
- Palkovacs E.P., Post D.M. Eco-evolutionary interactions between predators and prey: can predator-induced changes to prey communities feed back to shape predator foraging traits? Evol. Ecol. Res. 2008;10:699–720. [Google Scholar]
- Palkovacs E.P., Post D.M. Experimental evidence that phenotypic divergence in predators drives community divergence in prey. Ecology. 2009;90:300–305. doi: 10.1890/08-1673.1. doi:10.1890/08-1673.1 [DOI] [PubMed] [Google Scholar]
- Palkovacs E.P., Dion K.B., Post D.M., Caccone A. Independent evolutionary origin of landlocked alewife populations and rapid parallel evolution of phenotypic traits. Mol. Ecol. 2008;17:582–597. doi: 10.1111/j.1365-294X.2007.03593.x. doi:10.1111/j.1365-294X.2007.03593.x [DOI] [PubMed] [Google Scholar]
- Palkovacs E.P., Marshall M.C., Lamphere B.A., Lynch B.R., Weese D.J., Fraser D.F., Reznick D.N., Pringle C.M., Kinnison M.T. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Phil. Trans. R. Soc. B. 2009;364:1617–1629. doi: 10.1098/rstb.2009.0016. doi:10.1098/rstb.2009.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post D.M., Carpenter S.R., Christensen D.L., Cottingham K.L., Hodgson J.R., Kitchell J.F., Schindler D.E. Seasonal effects of variable recruitment of a dominant piscivore on pelagic food web structure. Limnol. Oceanogr. 1997;42:722–729. [Google Scholar]
- Post D.M., Taylor J.P., Kitchell J.F., Olson M.H., Schindler D.E., Herwig B.R. The role of migratory waterfowl as nutrient vectors in a managed wetland. Conserv. Biol. 1998;12:910–920. doi:10.1046/j.1523-1739.1998.97112.x [Google Scholar]
- Post D.M., Palkovacs E.P., Schielke E.G., Dodson S.I. Intraspecific phenotypic variation in a predator affects community structure and cascading trophic interactions. Ecology. 2008;89:2019–2032. doi: 10.1890/07-1216.1. doi:10.1890/07-1216.1 [DOI] [PubMed] [Google Scholar]
- Power M.E., et al. Challenges in the quest for keystones: identifying keystone species is difficult—but essential to understanding how loss of species will affect ecosystems. BioScience. 1996;46:609–620. doi:10.2307/1312990 [Google Scholar]
- Reznick D.N. The impact of predation on life history evolution in Trinidadian guppies: genetic basis of observed life history patterns. Evolution. 1982;36:1236–1250. doi: 10.1111/j.1558-5646.1982.tb05493.x. doi:10.2307/2408156 [DOI] [PubMed] [Google Scholar]
- Reznick D.N., Bryga H.A. Life-history evolution in guppies (Poecilia reticulata: Poeciliidae). 5. Genetic basis of parallelism in life histories. Am. Nat. 1996;147:339–359. doi:10.1086/285855 [Google Scholar]
- Reznick D.N., Endler J.A. The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata) Evolution. 1982;36:160–177. doi: 10.1111/j.1558-5646.1982.tb05021.x. doi:10.2307/2407978 [DOI] [PubMed] [Google Scholar]
- Reznick D.N., Butler M.J., Rodd F.H., Ross P. Life-history evolution in guppies (Poecilia reticulata). 6. Differential mortality as a mechanism for natural selection. Evolution. 1996;50:1651–1660. doi: 10.1111/j.1558-5646.1996.tb03937.x. doi:10.2307/2410901 [DOI] [PubMed] [Google Scholar]
- Reznick D.N., Shaw F.H., Rodd F.H., Shaw R.G. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. doi:10.1126/science.275.5308.1934 [DOI] [PubMed] [Google Scholar]
- Reznick D.N., Butler M.J., Rodd H. Life-history evolution in guppies. VII. The comparative ecology of high- and low-predation environments. Am. Nat. 2001;157:126–140. doi: 10.1086/318627. doi:10.1086/318627 [DOI] [PubMed] [Google Scholar]
- Rudstam L.G., VanDeValk A.J., Adams C.M., Coleman J.T.H., Forney J.L., Richmond M.E. Cormorant predation and the population dynamics of walleye and yellow perch in Oneida Lake. Ecol. Appl. 2004;14:149–163. doi:10.1890/03-5010 [Google Scholar]
- Schaus M.H., Vanni M.J. Effects of gizzard shad on phytoplankton and nutrient dynamics: role of sediment feeding and fish size. Ecology. 2000;81:1701–1719. doi:10.2307/177318 [Google Scholar]
- Schindler D.E. Nutrient regeneration by sockeye salmon (Oncorhynchus nerka) fry and subsequent effects on zooplankton and phytoplankton. Can. J. Fish. Aquat. Sci. 1992;49:2498–2506. doi:10.1139/f92-276 [Google Scholar]
- Schindler D.E., Scheuerell M.D., Moore J.W., Gende S.M., Francis T.B., Palen W.J. Pacific salmon and the ecology of coastal ecosystems. Front. Ecol. Environ. 2003;1:31–37. doi:10.2307/3867962 [Google Scholar]
- Schindler D.E., Leavitt P.R., Brock C.S., Johnson S.P., Quay P.D. Marine-derived nutrients, commercial fisheries, and production of salmon and lake algae in Alaska. Ecology. 2005;86:3225–3231. doi:10.1890/04-1730 [Google Scholar]
- Schluter D. Ecology and the origin of species. Trends Ecol. Evol. 2001;16:372–380. doi: 10.1016/s0169-5347(01)02198-x. doi:10.1016/S0169-5347(01)02198-X [DOI] [PubMed] [Google Scholar]
- Schmitz O.J. Effects of predator hunting mode on grassland ecosystem function. Science. 2008;319:952–954. doi: 10.1126/science.1152355. doi:10.1126/science.1152355 [DOI] [PubMed] [Google Scholar]
- Schoener T.W., Spiller D.A. Devastation of prey diversity by experimentally introduced predators in the field. Nature. 1996;381:691–694. doi:10.1038/381691a0 [Google Scholar]
- Schoener T.W., Spiller D.A. Indirect effects in an experimentally staged invasion by a major predator. Am. Nat. 1999;153:347–358. doi: 10.1086/303177. doi:10.1086/303177 [DOI] [PubMed] [Google Scholar]
- Schweitzer J.A., Bailey J.K., Rehill B.J., Martinsen G.D., Hart S.C., Lindroth R.L., Keim P., Whitham T.G. Genetically based trait in a dominant tree affects ecosystem processes. Ecol. Lett. 2004;7:127–134. doi:10.1111/j.1461-0248.2003.00562.x [Google Scholar]
- Schweitzer J.A., Bailey J.K., Hart S.C., Whitham T.G. Nonadditive effects of mixing cottonwood genotypes on litter decomposition and nutrient dynamics. Ecology. 2005a;86:2834–2840. doi:10.1890/04-1955 [Google Scholar]
- Schweitzer J.A., Bailey J.K., Hart S.C., Wimp G.M., Chapman S.K., Whitham T.G. The interaction of plant genotype and herbivory decelerate leaf litter decomposition and alter nutrient dynamics. Oikos. 2005b;110:133–145. doi:10.1111/j.0030-1299.2005.13650.x [Google Scholar]
- Schweitzer J.A., Bailey J.K., Fischer D.G., Leroy C.J., Lonsdorf E.V., Whitham T.G., Hart S.C. Plant–soil–microorganism interactions: heritable relationship between plant genotype and associated soil microorganisms. Ecology. 2008a;89:773–781. doi: 10.1890/07-0337.1. doi:10.1890/07-0337.1 [DOI] [PubMed] [Google Scholar]
- Schweitzer J.A., et al. From genes to ecosystems: the genetic basis of condensed tannins and their role in nutrient regulation in a Populus model system. Ecosystems. 2008b;11:1005–1020. doi:10.1007/s10021-008-9173-9 [Google Scholar]
- Scott, W. B. & Crossman, E. J. 1973 Freshwater fishes of Canada Bulletin of the Fisheries Research Board of Canada, no. 184. Ottawa, Canada: Fisheries Research Board of Canada.
- Smith J.W., Benkman C.W. A coevolutionary arms race causes ecological speciation in crossbills. Am. Nat. 2007;169:455–465. doi: 10.1086/511961. doi:10.1086/511961 [DOI] [PubMed] [Google Scholar]
- Smith M.D., Knapp A.K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003;6:509–517. doi:10.1046/j.1461-0248.2003.00454.x [Google Scholar]
- Stein R.A., DeVries D.R., Dettmers J.M. Food-web regulation by a planktivore: exploring the generality of the trophic cascade hypothesis. Can. J. Fish. Aquat. Sci. 1995;52:2518–2526. doi:10.1139/f95-842 [Google Scholar]
- Stockwell C.A., Hendry A.P., Kinnison M.T. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003;18:94–101. doi:10.1016/S0169-5347(02)00044-7 [Google Scholar]
- Strayer D.L., Caraco N.F., Cole J.J., Findlay S., Pace M.L. Transformation of freshwater ecosystems by bivalves—a case study of zebra mussels in the Hudson River. Bioscience. 1999;49:19–27. doi:10.2307/1313490 [Google Scholar]
- Thompson J.N. Rapid evolution as an ecological process. Trends Ecol. Evol. 1998;13:329–332. doi: 10.1016/s0169-5347(98)01378-0. doi:10.1016/S0169-5347(98)01378-0 [DOI] [PubMed] [Google Scholar]
- Thompson J.N. University of Chicago Press; Chicago, IL: 2005. The geographic mosaic of coevolution. [Google Scholar]
- Treseder K.K., Vitousek P.M. Potential ecosystem-level effects of genetic variation among populations of Metrosideros polymorpha from a soil fertility gradient in Hawaii. Oecologia. 2001;126:266–275. doi: 10.1007/s004420000523. doi:10.1007/s004420000523 [DOI] [PubMed] [Google Scholar]
- Urban M.C., Skelly D.K. Evolving metacommunities: toward an evolutionary perspective on metacommunities. Ecology. 2006;87:1616–1626. doi: 10.1890/0012-9658(2006)87[1616:emtaep]2.0.co;2. doi:10.1890/0012-9658(2006)87[1616:EMTAEP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Urban M.C., et al. The evolutionary ecology of metacommunities. Trends Ecol. Evol. 2008;23:311–317. doi: 10.1016/j.tree.2008.02.007. doi:10.1016/j.tree.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Vanni M.J. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Syst. 2002;33:341–370. doi:10.1146/annurev.ecolsys.33.010802.150519 [Google Scholar]
- Vanni M.J., Layne C.D. Nutrient recycling and herbivory as mechanisms in the ‘top-down’ effect of fish on algae in lakes. Ecology. 1997;78:21–40. doi:10.2307/2265976 [Google Scholar]
- Vitousek P.M., Walker L.R., Whiteaker L.D., Muellerdombois D., Matson P.A. Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science. 1987;238:802–804. doi: 10.1126/science.238.4828.802. doi:10.1126/science.238.4828.802 [DOI] [PubMed] [Google Scholar]
- Whitham T.G., et al. A framework for community and ecosystem genetics: from genes to ecosystems. Nat. Rev. Genet. 2006;7:510–523. doi: 10.1038/nrg1877. doi:10.1038/nrg1877 [DOI] [PubMed] [Google Scholar]
- Wootton J.T. Direct and indirect effects of nutrients on intertidal community structure: variable consequences of seabird guano. J. Exp. Mar. Biol. Ecol. 1991;151:139–153. doi:10.1016/0022-0981(91)90121-C [Google Scholar]
- Wootton J.T. Effects of birds on sea urchins and algae: a lower-intertidal trophic cascade. Ecoscience. 1995;2:321–328. [Google Scholar]
- Wright J.P., Jones C.G., Flecker A.S. An ecosystem engineer, the beaver, increases species richness at the landscape scale. Oecologia. 2002;132:96–101. doi: 10.1007/s00442-002-0929-1. doi:10.1007/s00442-002-0929-1 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Jones L.E., Ellner S.P., Fussmann G.F., Hairston N.G. Rapid evolution drives ecological dynamics in a predator–prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. doi:10.1038/nature01767 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Hairston N.G., Ellner S.P. Evolutionary trade-off between defense against grazing and competitive ability in a simple unicellular alga, Chlorelia vulgaris. Proc. R. Soc. B. 2004;271:1947–1953. doi: 10.1098/rspb.2004.2818. doi:10.1098/rspb.2004.2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret T.M., Paine R.T. Species introduction in a tropical lake. Science. 1973;182:449–455. doi: 10.1126/science.182.4111.449. doi:10.1126/science.182.4111.449 [DOI] [PubMed] [Google Scholar]