Abstract
Enhancer of Zeste Homolog 2 (EZH2) is a critical component of the Polycomb Repressive Complex 2 (PRC2), which is involved in gene silencing and histone H3 lysine 27 methylation. EZH2 plays a master regulatory role in controlling such processes as stem cell differentiation, cell proliferation, early embryogenesis, and X chromosome inactivation. While benign epithelial cells express very low levels of EZH2, increased levels of EZH2 have been observed in aggressive solid tumors such as those of the prostate, breast and bladder. The mechanism by which EZH2 mediates tumor aggressiveness is unclear. Here, we demonstrate that EZH2 mediates transcriptional silencing of the tumor suppressor gene E-cadherin by trimethylation of H3 lysine 27. Histone deacetylase inhibitors can prevent EZH2- mediated repression of E-cadherin and attenuate cell invasion, suggesting a possible mechanism that may be useful for the development of therapeutic treatments. Taken together, these observations provide a novel mechanism of E-cadherin regulation and establish a functional link between dysregulation of EZH2 and repression of E-cadherin during cancer progression.
Keywords: Polycomb group protein, Histone, EZH2, E-cadherin, Epigenetics
Introduction
Tumor invasion and metastasis are the major catalysts of morbidity and mortality in cancer patients (Haybittle et al., 1982; Rosen & Groshen, 1990). The initial stages of tumor invasion are characterized by the disruption of cell-cell adhesion, and decreased E-cadherin expression characterizes the invasive phenotype. E-cadherin is a Ca2+-dependent, transmembrane receptor that mediates cell-cell adhesion at adherent junctions via homophilic binding, thus maintaining epithelial cellular adhesion and integrity (Damsky et al., 1983). There is compelling evidence that E-cadherin expression is repressed in cancer, which suggests that it may play a critical role in the malignant progression of epithelial tumors (Day et al., 1999; Frixen & Nagamine, 1993; Pierceall et al., 1995). It has been implicated as a tumor suppressor via negative regulation during the course of invasion (Frixen et al., 1991; Hirohashi & Kanai, 2003). While many epithelial cancer cell lines that lack E-cadherin expression were invasive, administration of exogenous E-cadherin to these cells prevented invasion, suggesting a critical role for this receptor in the invasive process (Frixen et al., 1991). E-cadherin forms dimers, and the cytoplasmic domain of E-cadherin is complexed with catenins that are linked to the actin cytoskeleton network of the cells (Wijnhoven et al., 2000). The interaction between these molecules regulate the cell-cell adhesion (Halbleib & Nelson, 2006).
Reduced E-cadherin expression has been linked to metastasis. Numerous studies have demonstrated that aberrant expression of E-cadherin is associated with the development of metastases in breast cancer (Moll et al., 1993; Oka et al., 1993) and gastric cancer (Wu et al., 2005) among others. A number of mechanisms have been suggested for the repression of E-cadherin function during cancer progression including promoter methylation, mutations, transcriptional repression by snail and slug, ubiquitination and degradation of the E-cadherin, and lysosomal targeting of the E-cadherin for degradation (Batlle et al., 2000; Fujita et al., 2002; Hajra et al., 2002; Palacios et al., 2005; Peinado et al., 2004; Saito et al., 2004; Takeno et al., 2004).
Recent studies have shown that histone H3 lysine 27 trimethylation, which is mediated by EZH2 at the promoters of the gene, leads to silencing of gene expression (Chen et al., 2005; Koyanagi et al., 2005; Yu et al., 2007b). As part of a multi-protein complex with the other members of PRC2 (Satijn & Otte, 1999), EZH2 trimethylates histone H3 tails at lysine 27 (Cao et al., 2002; Kirmizis et al., 2004). This epigenetic modification is also known to be responsible for X-inactivation (Plath et al., 2003). Previously, we demonstrated that EZH2 is up-regulated in aggressive prostate and breast tumors (Kleer et al., 2003; Varambally et al., 2002). Several reports have also shown that EZH2 is over-expressed in other aggressive tumors including bronchial cancer (Breuer et al., 2004) melanoma (Bachmann et al., 2006), bladder cancer (Weikert et al., 2005) liver cancer (Sudo et al., 2005), as well as in vitro cancer cell lines such as SKBR3, MDA-MB-231, T47D breast cell lines (Tan et al., 2007), and the prostate cell lines DU145 and LNCaP (Beke et al., 2007).
EZH2 is a transcriptional repressor that plays a crucial role in maintaining the delicate homeostatic balance between gene expression and repression, the disruption of which may lead to oncogenesis (Jacobs & van Lohuizen, 1999; Jacobs & van Lohuizen, 2002; Sparmann & van Lohuizen, 2006). Recent studies revealed that EZH2 can physically recruit DNA methyltransferases (DNMTs) to certain target genes and silence them, suggesting cross-talk between the two distinct epigenetic silencing mechanisms (Taghavi & van Lohuizen, 2006; Vire et al., 2006). Cancer cells that contain DNA-methylated genes are specifically packaged in nuclesomes with the histone H3K27 trimethylation (Schlesinger et al., 2007). Reports also suggest that stem cell polycomb group targets are more likely to exhibit cancer-specific promoter DNA hypermethylation and histone H3 trimethylation of Lys27 relative to non-targets (Ohm et al., 2007; Widschwendter et al., 2007). In human and mouse embryonic stem cells, as well as in Drosophila, Polycomb Group (PcG) proteins contribute to pluripotency and plasticity via repression of developmental transcriptional factors that normally promote differentiation (Boyer et al., 2006; Bracken et al., 2006; Lee et al., 2006; Tolhuis et al., 2006).
In this study, we explored the role of histone methylation mediated by PRC2 in the silencing of E-cadherin during cancer progression and provide evidence of a functional link between dysregulation of EZH2 and repression of E-cadherin during cancer development.
Results
Alteration in EZH2 expression changes the invasive phenotype of the cells
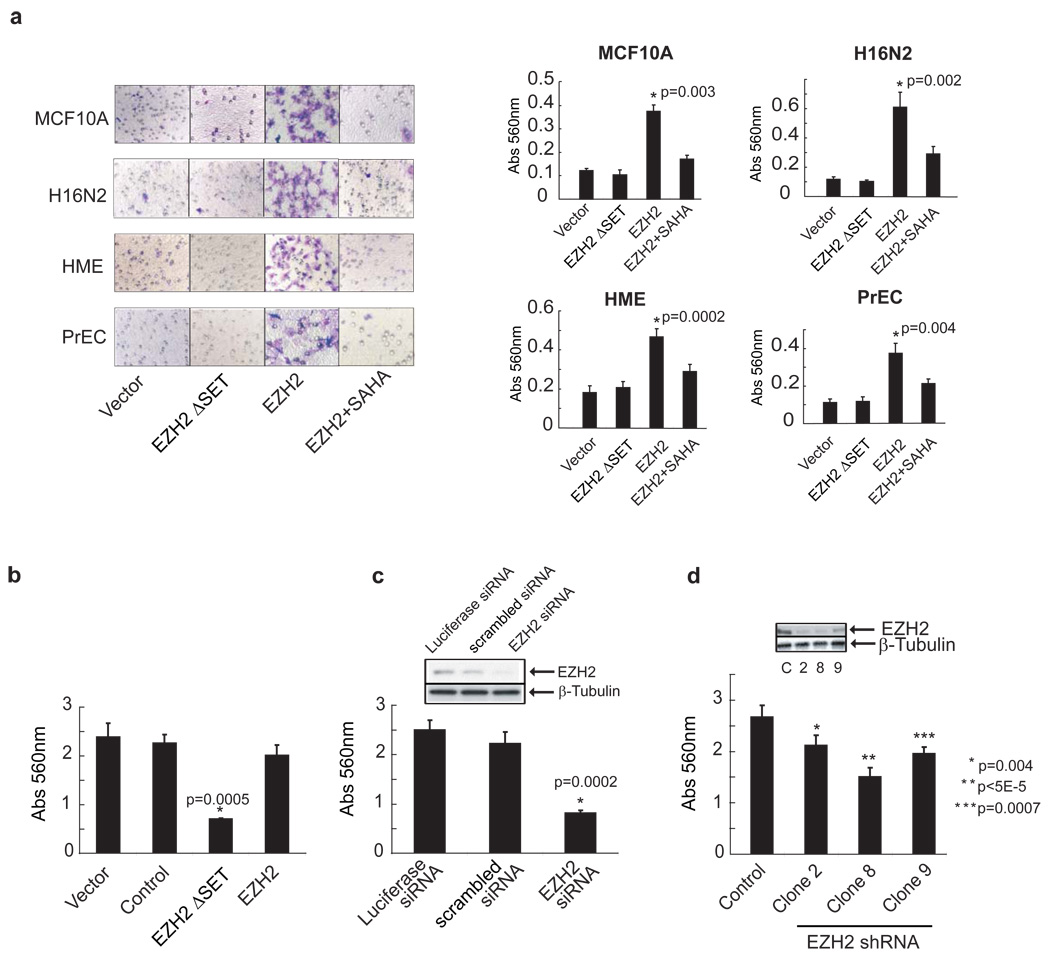
We have reported previously that EZH2 expression is increased in aggressive prostate and breast cancer (Kleer et al., 2003; Varambally et al., 2002). Herein, we evaluated the effect of EZH2 overexpression in multiple primary and non-invasive prostate and breast cells. A modified Boyden chamber assay was used to determine if primary prostate epithelial cells and immortalized breast cell lines (with very low endogenous EZH2 expression) undergo invasion upon ectopic over-expression of EZH2. The epithelial cell lines displayed an invasive phenotype only when infected with an EZH2-encoding adenovirus, and not a control adenovirus (Figure 1a). Importantly, a truncated mutant version of EZH2 EZH2ΔSET (missing the C-terminal SET domain that is required for methyltransferase activity) failed induce invasion. Additionally, EZH2-mediated invasion could be attenuated by incubating cells with the histone deacetylase (HDAC) inhibitor, SAHA, across all of the primary cultures and cell lines tested (Figure 1a). This suggests a role for histone deacetylation in EZH2-mediated effects.
Figure 1. Overexpression of EZH2 enhances invasion.
(a) Ectopic expression of EZH2 induces invasion of primary prostate epithelial cells and benign immortalized breast cell lines. A reconstituted basement membrane invasion chamber assay (Boyden chamber assay) was used to assess the invasive potential of primary prostate and benign breast epithelial cell lines infected with EZH2, EZH2ΔSET or control adenovirus. EZH2 infected cells were also treated with the histone deacetylase inhibitor SAHA (500nM). Representative fields of invaded and stained cells are shown (left). Invasion was quantitated using colorimetry (absorbance at 560 nm, right). All p values were calculated between EZH2 and vector treated samples. (b) The SET domain mutant of EZH2 inhibits cancer cell invasion. DU145 cells, which express high levels of endogenous EZH2, were infected with EZH2, EZH2ΔSET, and control adenoviruses. Invasion was quantitated using colorimetry. The p value was calculated between EZH2ΔSET and vectors. (c) Cell invasion is attenuated by EZH2 knockdown. Boyden chamber invasion assay using DU145 cells treated with siRNA duplexes targeting EZH2. Inset demonstrates knockdown of EZH2 protein by RNA interference. All p values were calculated between control and EZH2 knockdown clones. (d) Stable knockdown of EZH2 decreases invasiveness of DU145 cells. DU145 cells were stably transfected with EZH2 shRNA and assessed by invasion assay. Three stable clones exhibiting knockdown of EZH2 are shown. Inset demonstrates knockdown of EZH2 protein by RNA interference.
To expand our investigations, we explored whether perturbation of endogenous EZH2 would affect the invasiveness of cancer cell lines. For these studies, we employed the highly invasive prostate cancer cell line DU145. Over-expression of EZH2ΔSET in DU145 cells markedly reduced their invasive potential (Figure 1b), suggesting that this mutant version of EZH2 functioned as a dominant negative. Similarly, when EZH2 levels were transiently depleted using siRNA duplexes (Figure 1c and Supplementary data, Figure S1a, left panel) or stable knockdown using shRNA (Figure 1d and Supplementary data, Figure S1a, right panel), there was marked attenuation of DU145 invasive potential.
EZH2 regulates E-cadherin transcript and protein expression
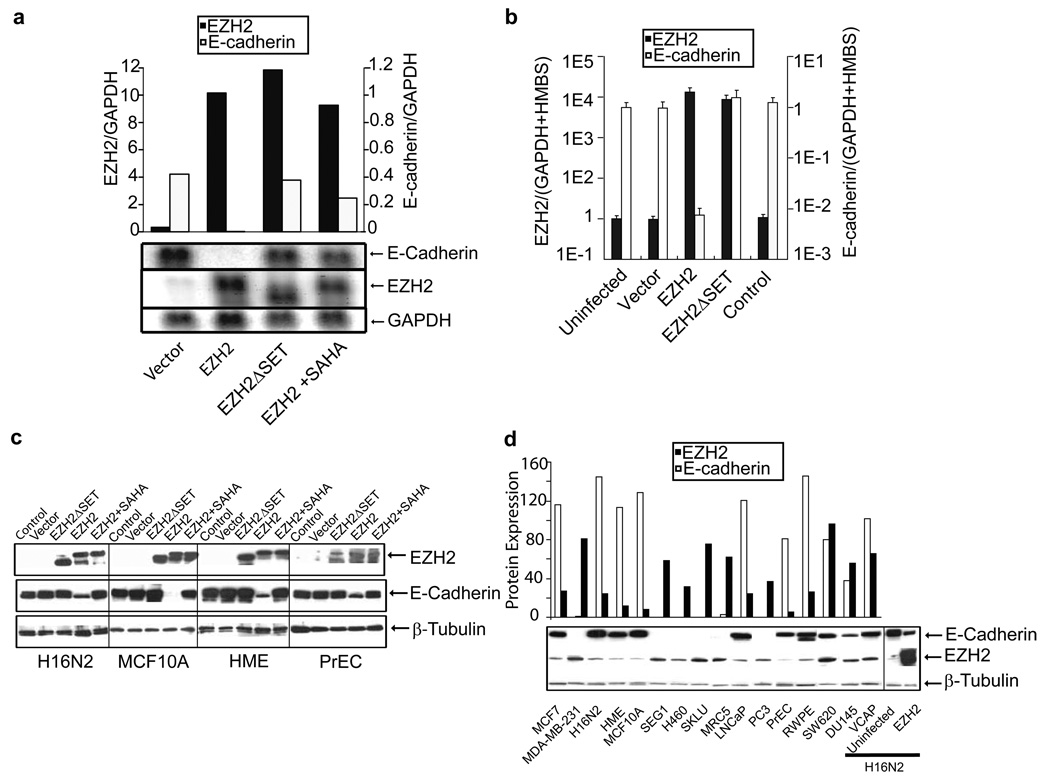
Earlier studies from our group suggested an inverse relationship between EZH2 and E-cadherin expression in prostate cancer (Rhodes et al., 2003), we hypothesized that EZH2 might regulate E-cadherin in the neoplastic process. We infected an immortalized benign breast epithelial cell line, H16N2, with EZH2, EZH2ΔSET, and control adenoviruses to determine whether EZH2 represses expression of the E-cadherin mRNA transcript. As hypothesized, EZH2 overexpression resulted in abrogation of E-cadherin transcripts as confirmed by two independent methods; Northern blot analysis (Figure 2a) and quantitative PCR (Figure 2b). Mutant EZH2 (EZH2ΔSET) or EZH2-infected cells treated with 500nM SAHA did not show down regulation of E-cadherin, indicating the importance of the SET domain of EZH2 as well as HDAC activity. The effect of EZH2 overexpression on E-cadherin protein was examined in four cell lines or primary cultures (H16N2, HME, MCF10A, and PrEC). We observed marked attenuation of E-cadherin protein levels by EZH2 overexpression, but not EZH2ΔSET, nor when EZH2 overexpressing cells were treated with HDAC inhibitor SAHA (Figure 2c). There was dose dependent inhibition of EZH2 mediated E-cadherin repression when cells were treated with HDAC inhibitors SAHA and trichostatin A (Supplementary data, Figure S1b). Immunoblot analysis also showed that E-cadherin repression is dependent on the expression of EZH2; higher EZH2 expression resulting in increased E-cadherin repression (Supplementary data, Figure S2). Interestingly, a panel of breast and prostate cell lines showed an inverse correlation of EZH2 and E-cadherin protein expression (Figure 2d), suggesting that PRC2 may be regulating E-cadherin levels in vivo. Similarly, this inverse association between EZH2 and E-cadherin protein levels was recapitulated in situ in both H16N2 breast epithelial cells (Figure 3a) as well as in breast tumors (Figure 3b).
Figure 2. EZH2 mediates repression of E-cadherin transcript and protein.
(a) Northern blot analyses of the E-cadherin gene in EZH2 over expressing cells. Northern blot analysis was carried out using the RNA from H16N2 cells infected with EZH2 and control adenovirus as well as EZH2 infected cells treated with 500nM SAHA for 48 hours. EZH2, E-cadherin and GAPDH probes were labeled with p32dCTP and hybridized to the blots. Note that uninfected H16N2 cells do not express EZH2, while E-cadherin is expressed at high levels in these cells. (b) Quantitative SYBR green RT-PCR of EZH2 transcript in cell lines infected with EZH2 and control adenoviruses. RT-PCR on each sample was performed in duplicate, and a ratio was calculated relative to the housekeeping genes GAPDH and hydroxymethylbilane synthase (HMBS). (c) Immunoblot analysis of EZH2 and E-cadherin in breast cell lines H16N2, MCF10A, HME and primary prostate cell PrEC infected with EZH2, EZH2ΔSET mutant, and control adenovirus infected cells as well as EZH2 infected cells treated with 500nM SAHA for 48 hours. β-tubulin was included as a loading control. Experiments were performed three times and a representative immunoblot is shown. (d) Immunoblot analysis of EZH2 and E-cadherin in a panel of breast and prostate cell lines. The cultured lines include both invasive and non-invasive cells. β-tubulin was included as a loading control. Semi-quantitation of EZH2 and E-cadherin in multiple cell lines is represented in a graphical format (top panel).
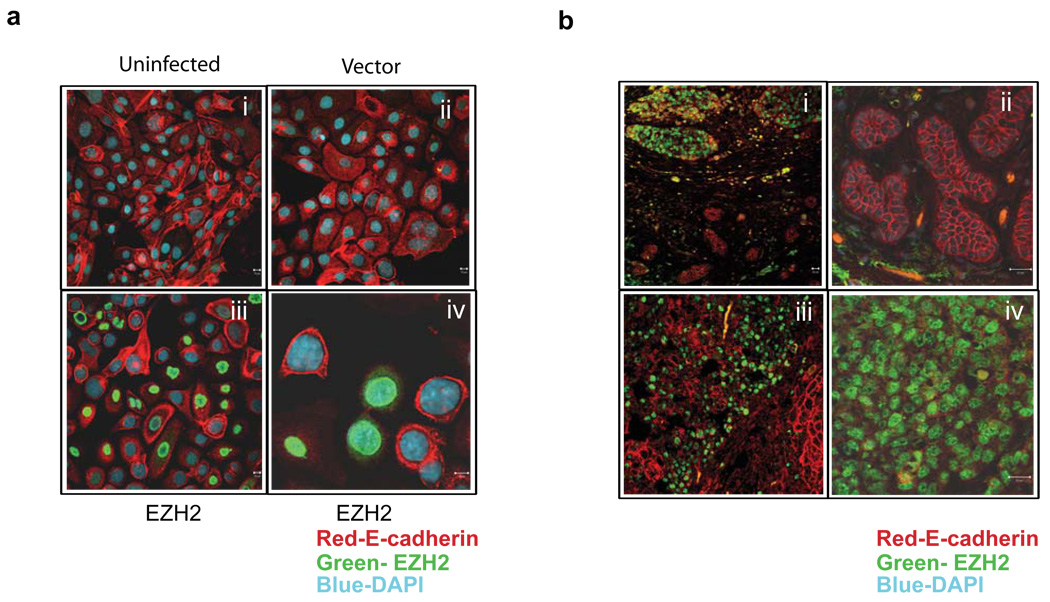
Figure 3. Co-Immunostaining indicates an inverse correlation between EZH2 and E-cadherin in cell lines and tumors.
(a) Immunostaining of the breast cell line H16N2 infected with EZH2 and control adenovirus (i, ii, iii and iv). Green staining represents EZH2 protein, red staining represents E-cadherin, and blue represents nuclear staining with DAPI. Lower right panel (iv) shows a higher magnification image. (b) Association between EZH2 and E-cadherin protein levels in human breast tumors by immunofluorescence. Upper left panel (i) shows invasive carcinoma of the breast with high EZH2 protein expression in the nuclei (green) and low E-cadherin expression (red), evidenced by a decrease in the membrane staining. A normal lobule is present in the lower part of the figure. Top right panel (ii) shows the higher magnification of the normal lobule shown with crisp membrane staining for E-cadherin. EZH2 staining is absent in this region. The lower left panel (iii) shows invasive carcinoma with foci of high EZH2 expression. The lower right panel (iv) shows higher magnification of a focus of invasive carcinoma with high EZH2 expression and nearly absent E-cadherin staining.
E-cadherin expression can rescue EZH2 mediated invasion
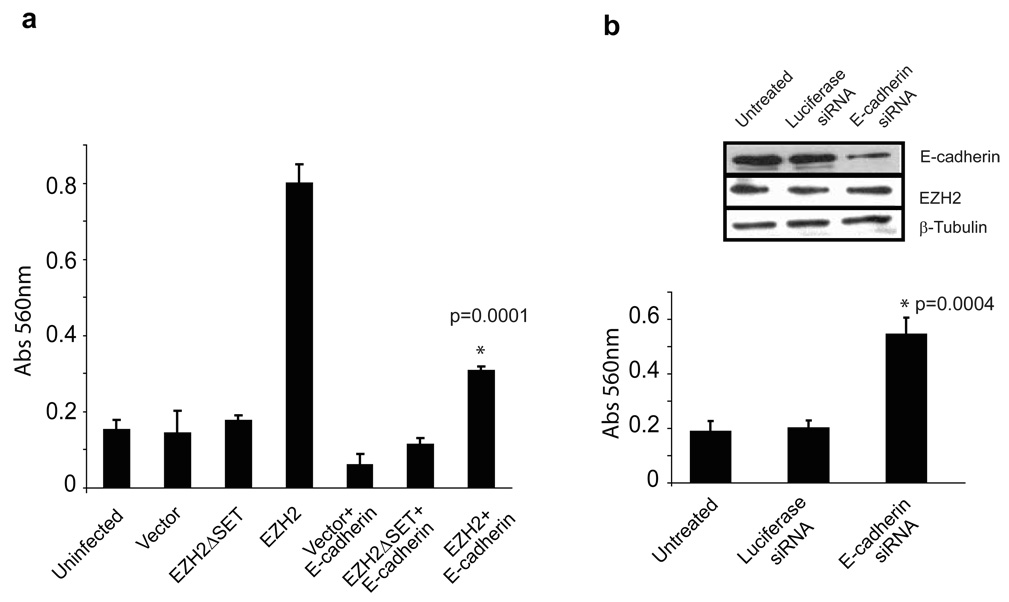
To determine if E-cadherin loss is a significant factor in the downstream regulation of EZH2-mediated invasion, we re-introduced E-cadherin under the regulation of a CMV promoter. We assessed the possibility that this might counteract the effects of EZH2-mediated silencing of E-cadherin. While H16N2 cells infected with EZH2 adenovirus were highly invasive and exhibited strong repression of E-cadherin (Figure 1a and 2a), this was attenuated by overexpression of E-cadherin under a non-EZH2 repressible promoter (i.e., CMV) (Figure 4a). To confirm that the loss of E-cadherin was a critical step in conferring invasiveness to H16N2 cells, E-cadherin was depleted using siRNA duplexes. H16N2 cells treated with siRNA against E-cadherin acquired invasive potential (Figure 4b), while control siRNA did not show this phenotype.
Figure 4. E-cadherin over-expression attenuates EZH2-mediated cell invasion.
(a) H16N2 cells were transfected with E-cadherin or vector alone. Transfected cells were infected with EZH2, EZH2ΔSET, and control adenovirus. Cell invasion was assessed by Boyden chamber assay, and p values were calculated between EZH2 and EZH2+E-cadherin samples. (b) E-cadherin knockdown in H16N2 cells was carried out using siRNA duplex. siRNA targeting luciferase served as a control. The p values were calculated between Luciferease RNAi and EZH2 RNAi samples. The inset demonstrates knockdown of E-cadherin protein by RNA interference.
EZH2 regulates the E-cadherin expression by methylating the histone H3 lysine 27 at the promoter region
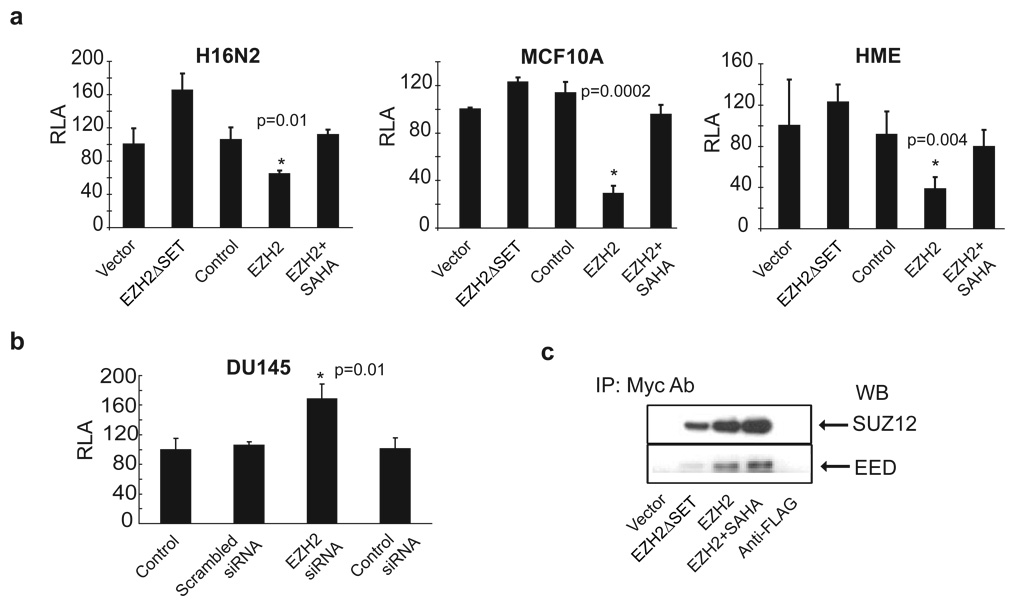
To determine if EZH2 can repress E-cadherin promoter activity, we performed a luciferase assay with an E-cadherin promoter-luciferase reporter construct that contained an endogenous 1.4 KB upstream regulatory region of E-cadherin (Hajra et al., 1999). As predicted, EZH2 inhibited the activity of the transfected E-cadherin promoter-reporter across all three cell lines tested (Figure 5a). EZH2-mediated repression of the E-cadherin promoter was blocked by 500nM SAHA, highlighting the role of histone deacetylation during EZH2-mediated E-cadherin regulation. Interestingly, the E-cadherin promoter-luciferase reporter was slightly induced by expression of EZH2ΔSET (Figure 5a), which suggested a dominant, negative effect. Knockdown of EZH2 in DU145 cells led to increased activity of the transfected E-cadherin promoter-reporter construct (Figure 5b). Similarly, when the E-cadherin promoter-reporter construct was transfected into stable EZH2 knockdowns or control DU145 cells, the E-cadherin promoter activity was significantly higher in stable EZH2 knockdowns showing an inverse correlation with reduced EZH2 expression (Supplementary data, Figure S3a).
Figure 5. EZH2 regulates E-cadherin promoter activity.
(a) Benign breast cell lines H16N2, MCF10A and HME were transfected with an E-cadherin-luciferase promoter construct and infected with either EZH2, EZH2ΔSET or control adenovirus. EZH2 infected cells were also incubated with 500nM SAHA. Relative luciferase activity (RLA) was assessed. (b) Knockdown of EZH2 in DU145 cells induces E-cadherin promoter activity. EZH2 expression was inhibited by RNA interference in DU145 cells that were transfected with the E-cadherin promoter-luciferase construct. The p value was calculated between EZH2 RNAi and scrambled RNAi samples. (c) Ectopically expressed EZH2 functions in a complex with endogenous PRC2 components SUZ12 and EED. H16N2 cells were infected with myc-tagged EZH2 and control adenovirus as well as EZH2 infected cells treated with 500nM SAHA for 48 hours. Immunoprecipitation was carried out using anti-myc antibody, and subsequent Western blotting performed with either SUZ12 or EED antibody.
In order to determine the minimal region of the E-cadherin promoter required for EZH2-mediated repression, we tested mutant E-cadherin promoter-luciferase reporters (Hajra et al., 2002) including Ecad-EboxA.MUT-luc (mutated Ebox A), Ecad-EboxC.MUT-luc (mutated Ebox C), Ecad-EboxABC.MUT-luc (all the three E-boxes, A, B and C are mutated) as well as wild-type E-cadherin promoter-luciferase reporter. EZH2 repressed the wild-type E-cadherin promoter activity and not the E-boxes mutants, indicating the importance of E-box regions in EZH2 mediated E-cadherin repression (Supplementary data, Figure S3b).
Ectopically overexpressed, myc-tagged EZH2 assembles endogenous PRC2 components including SUZ12 and EED, as demonstrated by their presence in anti-myc immunoprecipitates (Figure 5c). Addition of 500nM SAHA did not inhibit the binding of PRC2 complex members, indicating that the HDAC inhibitors do not inhibit PRC2 protein-protein interactions. Furthermore, when we performed immunoprecipitation of endogenous EZH2 and HDAC1, we observed that both EZH2 and HDAC1 interacted with EED (Supplementary data, Figure S4a), which confirmed previous finding that EED could interact with HDAC1(van der Vlag & Otte, 1999) and HDAC activity is essential for PRC2. Immunoblot evaluation suggested that the expression of EZH2, EED or HDAC1 did not change in the presence of HDAC inhibitor SAHA (Supplementary data, Figure S4b).
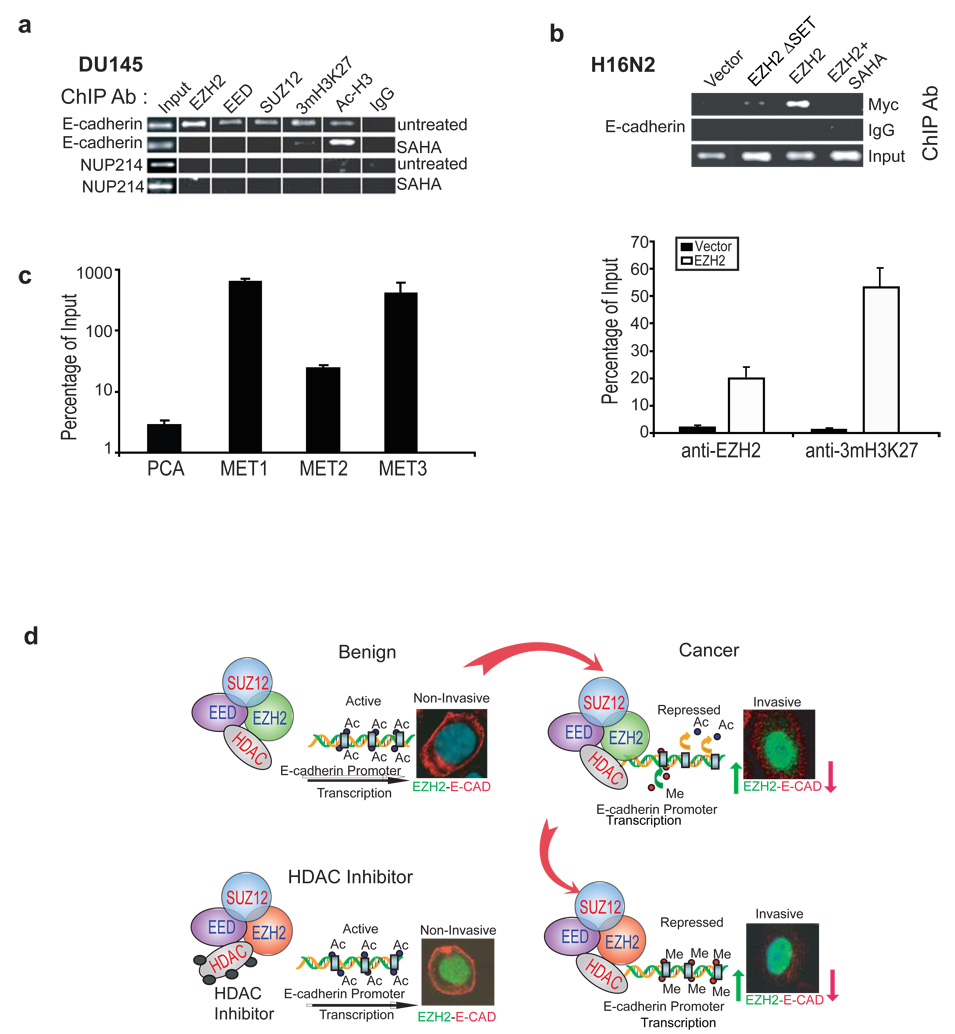
The polycomb group proteins are known to bind to a selected group of target genes and inhibit transcription (Boyer et al., 2006; Lee et al., 2006). To determine whether the endogenous PRC2 complex binds to the E-cadherin gene promoter, we carried out chromatin immunoprecipitation (ChIP) assay using antibodies specific to the PRC2 components and to the histone modifications. The invasive prostate cancer cell line DU145, which expresses high level of EZH2 (Figure 2d), was used to test complex formation by endogenous EZH2 and other PRC2 complex members. These investigations indicated binding of EZH2, SUZ12, and EED to the E-cadherin promoter (Figure 6a). Additionally, histone H3 was found to be trimethylated at lysine 27 on the E-cadherin promoter. Of particular importance was the finding that the HDAC inhibitor SAHA, while increasing histone acetylation as expected, markedly reduced PRC2 occupancy and H3K27 trimethylation on the E-cadherin promoter. To preclude nonspecific enrichment by ChIP, multiple negative controls were used, including the IgG antibody control, NUP214 negative gene control, as well as relative controls for the same antibody enrichment between SAHA-treated and untreated samples. Furthermore, we observed that HDAC1 was recruited to the promoter region of E-cadherin as well, indicating a role for HDAC1 in regulating the promoter activity. The presence of HDAC1 inhibitor SAHA significantly reduced HDAC1 occupancy on E-cadherin promoter region (Supplementary data, Figure S4c) suggesting that histone deacetylation may be a prerequisite for EZH2 mediated repression of E-cadherin expression.
Figure 6. EZH2 and PRC2 members are localized at E-cadherin promoter.
(a) The endogenous PRC2 complex is recruited to the E-cadherin promoter. ChIP was carried out using antibodies against EZH2, EED, SUZ12, trimethyl-histone H3-Lys27 (3mH3K27), acetyl histone H3 (Ac-H3) and IgG control. Addition of 500nM SAHA curtails the recruitment of these complexes to the E-cadherin promoter, while acetylated histone levels increase at the E-cadherin promoter. Each ChIP experiment was repeated at least three times and a representative experiment is shown. (b) Ectopically expressed EZH2 binds the E-cadherin promoter and leads to H3K27 trimethylation. ChIP was carried out in H16N2 cells infected with EZH2 or control adenovirus and assayed by PCR analysis. The upper panel shows in the cells overexpressing myc tagged EZH2 constructs, EZH2 was found to bind the E-cadherin promoter. The lower panel showed by qPCR that EZH2 and 3mH3K27 co-occupy the E-cadherin promoter in the EZH2-overexpressing H16N2 cells. The enrichments in EZH2-overexpressing cells are significantly (p<0.001) higher than those in the vector-treated cells. Error bar: n = 3, mean ± SEM. (c) The E-cadherin promoter contains significantly (p<0.001) more H3K27 trimethylation in metastatic prostate cancer tissues (MET) relative to localized prostate cancer (PCa). ChIP experiments were performed using anti-3mH3K27 antibody in one localized prostate tumor and three independent metastatic prostate tumors. ChIP-enriched DNA and the input DNA were first amplified through ligation-mediated PCR. Equal amounts (50ng) of amplified ChIP DNA and the input DNA were then subjected to PCR, and enrichment by ChIP was assessed relative to the input DNA and normalized to the level of GAPDH. Error bar: n = 3, mean ± SEM. (d) A model depicting the mechanism of EZH2 mediated E-cadherin repression. In benign cells the E-cadherin promoter is not occupied by PRC2 complex. In cancer, the increased expression of PRC2 leads to tight binding to the promoter of E-cadherin, followed by deacetylation of histone H3 and subsequent trimethylation of lysine 27. This leads to repression of E-cadherin expression. Addition of HDAC inhibitors prevents the first step of histone deacetylation, and hence the EZH2 complex cannot methylate histone H3.
As ectopic EZH2 assembles the PRC2 complex (Figure 5c), we explored the possibility that it might recruit the PRC2 complex proteins to the E-cadherin promoter. The H16N2 immortalized breast epithelial cell line, which has low level of endogenous EZH2, was infected with either vector control or EZH2 adenovirus and examined for PRC2 occupancy on the E-cadherin promoter. Using an antibody against myc epitope, tagged at both EZH2 and mutant EZH2 constructs, we confirmed by ChIP that ectopically expressed EZH2, but not the vector or mutant EZH2, binds to the E-cadherin promoter (Figure 6b and Supplementary data, Figure S4d). This binding can be mitigated by the HDAC inhibitor SAHA. Concordantly, ChIP-PCR demonstrated significant (p<0.001) enrichment of EZH2 binding and H3K27 trimethylation on the E-cadherin promoter by EZH2 overexpression (Figure 6b and Supplementary data, Figure S4d).
Next we attempted to examine H3K27 trimethylation on the E-cadherin promoter in vivo in EZH2-high metastatic prostate tumors. ChIP combined with ligation-mediated PCR, as described earlier(Yu et al., 2007b), was used to detect the enrichment of target genomic region by an antibody against H3K27 trimethylation, relative to the input DNA. Remarkably, hundred-fold enrichment of H3K27 trimethylation on the E-cadherin promoter was detected (Figure 6c). This enrichment was also detected in a previously characterized PRC2 target gene WNT1, but not in the NUP214 negative control gene (Figure S4e). By contrast, analysis of an EZH2-low localized prostate tumor showed significantly less enrichment (p<0.001) of the E-cadherin promoter for H3K27 trimethylation.
Subsequently, we attempted to test if DNA methylation has any role in EZH2 mediated E-cadherin repression. E-cadherin promoter DNA methylation analysis using EZH2 over-expressing cells by bisulfite modification and methylation-specific PCR as well as pyrosequencing did not exhibit E-cadherin promoter DNA methylation (Supplementary data, Figure S5a, b, c, d and e).
Recently, it has been shown that DNA methyltransferases (DNMTs) and EZH2 cooperate in silencing genes such as MYT1, WNT1, KCNA1 and CNR1 (Vire et al., 2006). Additional H3K9 methylation may lead to promoter CpG island DNA methylation (Ohm et al., 2007). However our study indicated that there was no alteration in the DNA methylation status of E-cadherin promoter upon EZH2 overexpression. This indicated that histone trimethylation-mediated by EZH2 plays a key role in the silencing of E-cadherin.
Discussion
In the present study we describe a novel mechanism by which E-cadherin is down-regulated in EZH2-overexpressing cells through histone H3K27 trimethylation at the E-cadherin promoter. While EZH2 expression was low in benign epithelial tissues, the expression of EZH2 increased with tumor progression. EZH2 expression became dysregulated concurrently with increased HDAC activity, which resulted in trimethylation of histone H3 lysine 27 at the E-cadherin promoter with subsequent repression of expression (Figure 6d). This enzymatic activity was inhibited, however, when the cells were treated with HDAC inhibitor despite overexpression of EZH2.
A large body of evidence suggests that loss of E-cadherin expression is associated with the acquisition of invasiveness and advanced tumor stage for cancers of epithelial origin including prostate (Umbas et al., 1994; Umbas et al., 1997), gastric (Mayer et al., 1993), colon (Dorudi et al., 1995), and breast cancer (Oka et al., 1993; Palacios et al., 1995; Rasbridge et al., 1993). EZH2 is also known to increase the proliferation and invasiveness of prostate cells(Bryant et al., 2007) and our study indicates the role of E-cadherin in EZH2 mediated cell invasion. While several mechanisms have been proposed for the downregulation of E-cadherin, our data suggest a novel mechanism whereby increased levels of EZH2 in aggressive tumors silence E-cadherin expression through histone H3K27 trimethylation. EZH2 regulates E-cadherin transcription by physically binding to its promoter. The expression of EZH2 enhances HDAC activity (Kleer et al., 2003) and we hypothesize that increased HDAC activity leads to the removal of the acetyl group from the histone H3K27 at the promoter region of E-cadherin. This enables EZH2 to exert its histone methyltransferase enzymatic activity. Recent studies by Fujii and Ochiai (Fujii & Ochiai, 2008) indicate that in gastric cancer, EZH2 down regulates E-cadherin expression by histone modification. Tri-methylation of histone H3 on lysine 27 leads to compaction of chromatin and blocks transcription factors from binding and initiating transcription. EZH2 may mediate increased invasiveness and metastasis by silencing a number of downstream targets in addition to E-cadherin including beta adrenergic receptor ADRB2(Yu et al., 2007a). Interestingly, our observations indicate that DNA methylation does not play a role in EZH2 mediated repression of E-cadherin expression. A recent study by Kondo et al., (Kondo et al., 2008) suggests that gene silencing by histone H3 lysine K27 tri-methylation is independent of promoter DNA methylation.
Further, we demonstrated that HDAC inhibitors inhibited the activity of EZH2 and prevented the EZH2 mediated downregulation of E-cadherin, as well as reduced the invasion, suggesting a mechanism for these anti-cancer drugs. We propose that EZH2 acts on deacetylated histone to methylate the lysine residue. However, inhibition of deacetylases prevents the removal acetyl groups from lysine residues of histones. Acetylated lysine residues in histones will not serve as substrate for methylation by EZH2. Additionally, our findings suggest that EZH2 may be a viable target for therapeutic inhibition in aggressive tumors of epithelial origin. Our findings identify a molecular mechanism by which EZH2 mediates transcriptional repression of E-cadherin and provide insight into EZH2 mediated invasion and metastasis.
Materials and Methods
Basement Membrane Matrix Invasion Assay
For invasion assays, the breast cell lines H16N2, HME, and MCF10A (ATCC, Manassas, VA), as well as normal prostate epithelial cells (PrEC, Cambrex, East Rutherford, NJ), were infected with vector, EZH2 and EZH2ΔSET adenovirus. Forty-eight hours post-infection, cells were seeded onto the basement membrane matrix (EC matrix, Chemicon, Temecula, CA) present in the insert of a 24-well culture plate. Fetal bovine serum was added to the lower chamber as a chemoattractant with or without 500nM HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) (Biovision Inc., Mountain View, CA). After 48 hours, the non-invading cells and EC matrix were gently removed with a cotton swab. Invasive cells located on the lower side of the chamber were stained with crystal violet, air dried and photographed. They were then enumerated microscopically using multiple representative areas. For colorimetric assays, the inserts were treated with 150 µl of 10% acetic acid and the absorbance measured at 560nm using a spectrophotometer (GE Healthcare Life Sciences, Piscataway, NJ).
RNA interference
The knockdown of EZH2 was accomplished with either siRNA duplex (Dharmacon, Lafayette, CO) as previously described (Varambally et al., 2002) or shRNA expression vectors (Open Biosystems, Huntsville, AL). E-cadherin knockdown was performed using siRNA duplex. (Dharmacon)
Immunoblot Analyses
The breast cell lines H16N2, HME, and MCF10A, as well as normal prostate epithelial cells, were grown to 60% confluency and infected with either EZH2, vector control, or EZH2ΔSET adenovirus for 48 hours. An additional set of cells were infected with EZH2 adenovirus and treated with 500nM HDAC inhibitor SAHA (Biovision, Mountain View, CA) for 48 hours. Cells were homogenized in NP40 lysis buffer (50 mM Tris-HCl, 1% NP40, pH 7.4, Sigma, St. Louis, MO), and complete proteinase inhibitor mixture (Roche, Indianapolis, IN). Ten micrograms of each protein extract were boiled in sample buffer, separated by SDS-PAGE, and transferred onto Polyvinylidene Difluoride membrane (GE Healthcare). The membrane was incubated for one hour in blocking buffer [Tris-buffered saline, 0.1% Tween (TBS-T), 5% nonfat dry milk] and incubated overnight at 4°C with the following: anti-EZH2 mouse monoclonal (1:1000, 1:5000 in dilution buffer, BD Biosciences, San Jose, CA, #612666), anti-E-CAD mouse monoclonal antibodies (1:1000, 1:5000 in dilution buffer, BD Biosciences, #610405), anti-EED rabbit polyclonal antibody (1:1000 in dilution buffer, Upstate, Charlottesville, VA, #07–368), anti-SUZ12 rabbit polyclonal antibody (1:1000 in dilution buffer, kind gift from Prof. Otte), and HDAC1 monoclonal antibody (1:1000 in dilution buffer, Millipore, #05–614). Following a wash with TBS-T, the blot was incubated with horseradish peroxidase-conjugated secondary antibody and the signals visualized by enhanced chemiluminescence system as described by the manufacturer (GE Healthcare). The blot was re-probed with β-tubulin for confirmation of equal loading.
Northern blot analyses
Total RNA was isolated from H16N2 cells that were infected with either vector, EZH2, or EZH2ΔSET adenovirus for 48 hours. An additional set of cells were infected with EZH2 adenovirus and were treated with 500nM HDAC inhibitor SAHA (Biovision, Mountain View, CA) for 48 hours. Twenty micrograms of total RNA from each condition were resolved on a denaturing-formaldehyde agarose gel and subsequently transferred onto a Hybond-NX membrane (Amersham Biosciences, Piscataway, NJ). EZH2, E-cadherin, and GAPDH probes were labeled with p32dCTP (GE Healthcare) and hybridized to the blots. The signal was visualized and quantified using a Typhoon Scanner 9000B and Image Quant Software (Amersham Biosciences). E-cadherin and EZH2 signals were normalized to that of GAPDH.
SYBR Green Quantitative Real-Time PCR
Total RNA was isolated from H16N2 cells that were infected either with vector, EZH2, or EZH2ΔSET adenovirus. Quantitative PCR (QPCR) was performed using SYBR Green dye on an Applied Biosystems 7300 Real Time PCR system (Applied Biosystems, Foster City, CA). Briefly, 1 µg of total RNA was reverse transcribed into cDNA using SuperScript III (Invitrogen, Carlsbad, CA) in the presence of random hexamers and oligo dT primers (Invitrogen). All reactions were performed in duplicate with SYBR Green Master Mix (Applied Biosystems) plus 25 ng of both the forward and reverse primer according to the manufacturer’s recommended thermocycling conditions, and then subjected to melt curve analysis. Threshold levels for each experiment were set during the exponential phase of the QPCR reaction using Sequence Detection Software version 1.2.2 (Applied Biosystems). The DNA in each sample was quantified by interpolation of its threshold cycle (Ct) value from a standard curve of Ct values, which were created from a serially diluted cDNA mixture of all samples. The calculated quantity of the target gene for each sample was divided by the average sample quantity of the housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and hydroxymethylbilane synthase (HMBS) to obtain the relative gene expression. All oligonucleotide primers were synthesized by Integrated DNA Technologies (Coralville, IA). Primers for HMBS and GAPDH were as described (Vandesompele et al., 2002). Primers for CDH1 were: CDH1-F, 5’-GGAGGAGAGCGGTGGTCAAA-3’; CDH1-R, 5’-TGTGCAGCTGGCTCAAGTCAA-3’.
Immunofluorescence
H16N2 cells were grown using chamber slides (Nunc, Rochester, NY) and infected with either control or EZH2 virus for cell line co-immunostaining with EZH2 and E-cadherin antibody. Forty-eight hours post-infection, the slides were washed with PBS, and were fixed using ice cold methanol. Following an additional PBS wash, the slides were blocked for two hours using 5% donkey serum in PBS-T (phosphate buffered saline, 0.05% Tween-20). A mixture of rabbit anti-E-cadherin antibody (Labvision, Fremont, CA, #RB-9214-P) and mouse anti-EZH2 antibody (BD Biosciences, San Jose, CA) were added to the slides at 1:250 and 1:100 dilutions, respectively, and incubated overnight at 4° C. Following an additional wash, the slides were incubated with Alexa 555-conjugated goat, anti-rabbit antibody and Alexa 488-conjugated goat, anti-rabbit secondary antibody (Invitrogen) for one hour in the dark at room temperature. After washing, the slides were mounted using Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingam, CA).
Breast tissues samples were collected with informed consent and prior institutional review board approval. To prepare for tissue section staining, paraffin-embedded breast tissue slides were soaked in xylene for one hour removal of paraffin. Slides were placed in citrate buffer (pH 6.0) and heated under pressure for 15 minutes for antigen retrieval. They were then blocked in PBS-T with 5% normal donkey serum for one hour. A mixture of rabbit anti-E-cadherin antibody (Labvision) and mouse anti-EZH2 antibody (BD Biosciences) was added to the slides at 1:250 and 1:100 dilutions respectively and incubated overnight at 4° C. Slides were then incubated with secondary antibodies for one hour (anti-mouse IgG horse radish peroxidase conjugate and anti-rabbit Alexa 555, both at 1:1000 dilution). Following a wash, fluorescently-labeled tyramide (Aelxa Fluor 488, Invitrogen) was added and the slides incubated for 10 minutes at room temperature. They were washed and then mounted using Vectashield mounting medium. Confocal images were taken with a Zeiss LSM510 META imaging system using Argon and Helium Neon 1 and Helium Neon 2 light source (Carl Zeiss, Thornwood, NY). The color images were exported as TIFF images.
Luciferase Assay
E-cadherin regulation by EZH2 was examined using the E-cadherin promoter luciferase reporter gene and transient transfection assays were performed. The breast cell lines H16N2, MCF10A and HME were transfected with wild-type or E-box mutant E-cadherin luciferase construct (kind gift of Dr. Eric Fearon) as well as pRL-TK vector as internal control for luciferase activity, then subsequently infected with either EZH2 or control viruses. Following two days of incubation, the cells were lysed and luciferase assays conducted using the dual luciferase assay system (Promega, Madison, WI). Each experiment was performed in triplicate. Using siRNA duplex, an EZH2 knockdown was performed in the invasive prostate cell line DU145. Both were simultaneously transfected with E-cadherin promoter-luciferase reporter constructs, and the luciferase activity was measured after two days as previously described.
Chromatin immunoprecipitation (ChIP) Assay
ChIP experiments were carried out as described by Yu, et al.(Yu et al., 2007a). For each ChIP assay, 5ug of antibodies were used; EZH2 (BD Biosciences, #612666), SUZ12 (Abcam, Cambridge, MA, #ab12201), EED (Upstate, #07–368), trimethyl H3-Lys27 (Upstate, #07–449) and acetyl H3 (Upstate, #06–599), Myc (Abcam, #ab32), HDAC1 (Milipore, # 17–608) or IgG control (Santa Cruz, #12–370). Approximately 2–5 ul of ChIP-enriched chromatins were subjected to a standard ChIP-PCR reaction, and the enrichment of specific genomic regions was assessed relative to either control IgG or control cells. Each ChIP experiment was repeated at least three times. For ChIP with human tissues, ChIP-enriched DNA and input DNA were amplified through ligation-mediated PCR. Equal amounts (50ng) of amplified ChIP DNA and input DNA were subjected to PCR. Enrichment by ChIP was assessed relative to the input DNA and normalized to the level of GAPDH. The primers used in the ChIP experiments were designed to flank the promoter regions of CDH1 and the WNT1 positive control gene, as well as the intragenic region of the NUP214 negative control. The sequences of the primers were: CDH1-pF,TAGAGGGTCACCGCGTCTAT; CDH1-pR, TCACAGGTGCTTTGCAGTTC; WNT1-pF1, ACCCGTCAGCTCTCGGCTCA; WNT1-pR1, TGCAGTTGCGGCGACTTTGG; NUP214_pF1, CAGTGAGGTCTCAGCATCAGCA; NUP214_pR1, CTGGAGGCTATGGGGGTACTTG.
Supplementary Material
Acknowledgments
We thank Professor Eric Fearon for providing the E-cadherin promoter-reporter constructs. We thank Jill Granger for critically reading the manuscript and thoughtful suggestions. We thank R. Kunkel for help in figure preperation and the staff of the Microscopy and Image Analyses laboratory at the University of Michigan for their assistance in the microscopic analyses employed in this study. We thank the University of Michigan Vector Core for virus generation.
Financial Support: A.M.C. is supported by a Burroughs Welcome Foundation Award in Clinical Translational Research. S.A.T. is supported by the Medical Scientist Training Program and a Rackham Pre-doctoral Award. This research was supported in part by National Institutes of Health Grant RO1 CA97063 (to A.M.C); U01 CA111275 (to A.M.C); P50 CA69568 (to A.M.C); Department of Defense Grants PC040517 (to R.M.), PC051081 (to A.M.C. and S.V.), PC060266 (to J.Y.) and R01CA107469 (to CGK).
References
- Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Beke L, Nuytten M, Van Eynde A, Beullens M, Bollen M. The gene encoding the prostatic tumor suppressor PSP94 is a target for repression by the Polycomb group protein EZH2. Oncogene. 2007;26:4590–4595. doi: 10.1038/sj.onc.1210248. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer RH, Snijders PJ, Smit EF, Sutedja TG, Sewalt RG, Otte AP, et al. Increased expression of the EZH2 polycomb group gene in BMI-1-positive neoplastic cells during bronchial carcinogenesis. Neoplasia. 2004;6:736–743. doi: 10.1593/neo.04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RJ, Cross NA, Eaton CL, Hamdy FC, Cunliffe VT. EZH2 promotes proliferation and invasiveness of prostate cancer cells. Prostate. 2007;67:547–556. doi: 10.1002/pros.20550. [DOI] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Chen H, Tu SW, Hsieh JT. Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J Biol Chem. 2005;280:22437–22444. doi: 10.1074/jbc.M501379200. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Richa J, Solter D, Knudsen K, Buck CA. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983;34:455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Day ML, Zhao X, Vallorosi CJ, Putzi M, Powell CT, Lin C, et al. E-cadherin mediates aggregation-dependent survival of prostate and mammary epithelial cells through the retinoblastoma cell cycle control pathway. J Biol Chem. 1999;274:9656–9664. doi: 10.1074/jbc.274.14.9656. [DOI] [PubMed] [Google Scholar]
- Dorudi S, Hanby AM, Poulsom R, Northover J, Hart IR. Level of expression of E-cadherin mRNA in colorectal cancer correlates with clinical outcome. Br J Cancer. 1995;71:614–616. doi: 10.1038/bjc.1995.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen UH, Nagamine Y. Stimulation of urokinase-type plasminogen activator expression by blockage of E-cadherin-dependent cell-cell adhesion. Cancer Res. 1993;53:3618–3623. [PubMed] [Google Scholar]
- Fujii S, Ochiai A. Enhancer of zeste homolog 2 downregulates E-cadherin by mediating histone H3 methylation in gastric cancer cells. Cancer Sci. 2008;99:738–746. doi: 10.1111/j.1349-7006.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- Hajra KM, Ji X, Fearon ER. Extinction of E-cadherin expression in breast cancer via a dominant repression pathway acting on proximal promoter elements. Oncogene. 1999;18:7274–7279. doi: 10.1038/sj.onc.1203336. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Haybittle JL, Blamey RW, Elston CW, Johnson J, Doyle PJ, Campbell FC, et al. A prognostic index in primary breast cancer. Br J Cancer. 1982;45:361–366. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575–581. doi: 10.1111/j.1349-7006.2003.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JJ, van Lohuizen M. Cellular memory of transcriptional states by Polycomb-group proteins. Semin Cell Dev Biol. 1999;10:227–235. doi: 10.1006/scdb.1999.0304. [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, van Lohuizen M. Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta. 2002;1602:151–161. doi: 10.1016/s0304-419x(02)00052-5. [DOI] [PubMed] [Google Scholar]
- Kirmizis A, Bartley SM, Kuzmichev A, Margueron R, Reinberg D, Green R, et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18:1592–1605. doi: 10.1101/gad.1200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Baguet A, Martens J, Margueron R, Jenuwein T, Bix M. EZH2 and histone 3 trimethyl lysine 27 associated with Il4 and Il13 gene silencing in Th1 cells. J Biol Chem. 2005;280:31470–31477. doi: 10.1074/jbc.M504766200. [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, et al. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–1695. [PubMed] [Google Scholar]
- Moll R, Mitze M, Frixen UH, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143:1731–1742. [PMC free article] [PubMed] [Google Scholar]
- Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53:1696–1701. [PubMed] [Google Scholar]
- Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol. 2005;25:389–402. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios J, Benito N, Pizarro A, Limeres MA, Suarez A, Cano A, et al. Relationship between ERBB2 and E-cadherin expression in human breast cancer. Virchows Arch. 1995;427:259–263. doi: 10.1007/BF00203392. [DOI] [PubMed] [Google Scholar]
- Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierceall WE, Woodard AS, Morrow JS, Rimm D, Fearon ER. Frequent alterations in E-cadherin and alpha-and beta-catenin expression in human breast cancer cell lines. Oncogene. 1995;11:1319–1326. [PubMed] [Google Scholar]
- Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, Wang H, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300:131–135. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- Rasbridge SA, Gillett CE, Sampson SA, Walsh FS, Millis RR. Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma. J Pathol. 1993;169:245–250. doi: 10.1002/path.1711690211. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Sanda MG, Otte AP, Chinnaiyan AM, Rubin MA. Multiplex biomarker approach for determining risk of prostate-specific antigen-defined recurrence of prostate cancer. J Natl Cancer Inst. 2003;95:661–668. doi: 10.1093/jnci/95.9.661. [DOI] [PubMed] [Google Scholar]
- Rosen PP, Groshen S. Factors influencing survival and prognosis in early breast carcinoma (T1N0M0-T1N1M0). Assessment of 644 patients with median follow-up of 18 years. Surg Clin North Am. 1990;70:937–962. doi: 10.1016/s0039-6109(16)45190-x. [DOI] [PubMed] [Google Scholar]
- Saito T, Oda Y, Kawaguchi K, Sugimachi K, Yamamoto H, Tateishi N, et al. E-cadherin mutation and Snail overexpression as alternative mechanisms of E-cadherin inactivation in synovial sarcoma. Oncogene. 2004;23:8629–8638. doi: 10.1038/sj.onc.1207960. [DOI] [PubMed] [Google Scholar]
- Satijn DP, Otte AP. Polycomb group protein complexes: do different complexes regulate distinct target genes? Biochim Biophys Acta. 1999;1447:1–16. doi: 10.1016/s0167-4781(99)00130-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet. 2007;39:232–236. doi: 10.1038/ng1950. [DOI] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- Sudo T, Utsunomiya T, Mimori K, Nagahara H, Ogawa K, Inoue H, et al. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer. 2005;92:1754–1758. doi: 10.1038/sj.bjc.6602531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi P, van Lohuizen M. Developmental biology: two paths to silence merge. Nature. 2006;439:794–795. doi: 10.1038/439794a. [DOI] [PubMed] [Google Scholar]
- Takeno S, Noguchi T, Fumoto S, Kimura Y, Shibata T, Kawahara K. E-cadherin expression in patients with esophageal squamous cell carcinoma: promoter hypermethylation, Snail overexpression, and clinicopathologic implications. Am J Clin Pathol. 2004;122:78–84. doi: 10.1309/P2CD-FGU1-U7CL-V5YR. [DOI] [PubMed] [Google Scholar]
- Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B, de Wit E, Muijrers I, Teunissen H, Talhout W, van Steensel B, et al. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat Genet. 2006;38:694–699. doi: 10.1038/ng1792. [DOI] [PubMed] [Google Scholar]
- Umbas R, Isaacs WB, Bringuier PP, Schaafsma HE, Karthaus HF, Oosterhof GO, et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54:3929–3933. [PubMed] [Google Scholar]
- Umbas R, Isaacs WB, Bringuier PP, Xue Y, Debruyne FM, Schalken JA. Relation between aberrant alpha-catenin expression and loss of E-cadherin function in prostate cancer. Int J Cancer. 1997;74:374–377. doi: 10.1002/(sici)1097-0215(19970822)74:4<374::aid-ijc2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23:474–478. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- Weikert S, Christoph F, Kollermann J, Muller M, Schrader M, Miller K, et al. Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med. 2005;16:349–353. [PubMed] [Google Scholar]
- Widschwendter M, Fiegl H, Egle D, Mueller-Holzner E, Spizzo G, Marth C, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- Wu ZY, Zhan WH, Li JH, He YL, Wang JP, Lan P, et al. Expression of E-cadherin in gastric carcinoma and its correlation with lymph node micrometastasis. World J Gastroenterol. 2005;11:3139–3143. doi: 10.3748/wjg.v11.i20.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007a;12:419–431. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Yu J, Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res. 2007b;67:10657–10663. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.