Summary
Safe and effective adjuvants for influenza vaccines that could increase both the levels of neutralizing antibody, including against drifted viral subtypes, and T-cell immunity would be a major advance in vaccine design. The JVRS-100 adjuvant, consisting of DOTIM/cholesterol cationic liposome-DNA complexes, is particularly promising for vaccines that require induction of high levels of antibody and T-cell immunity, including CD8+ cytotoxic T lymphocytes (CTL). Inclusion of protein antigens with JVRS-100 results in the induction of enhanced humoral and cell-mediated (i.e., CD4+, and CD8+ T cells) immune responses. The JVRS-100 adjuvant combined with a split trivalent influenza vaccine (Fluzone® - sanofi pasteur) elicited increased antibody and T-cell responses in mice and non-human primates compared to vaccination with Fluzone® alone. Mice vaccinated with JVRS-100-Fluzone® and challenged with antigenically drifted strains of H1N1 (PR/8/34) and influenza B (B/Lee/40) viruses had higher grade protection, as measured by attenuation of weight loss and increased survival, compared to recipients of unadjuvanted vaccine. The results indicate that the JVRS-100 adjuvant substantially increases immunogenicity and protection from drifted-strain challenge using an existing influenza vaccine.
Keywords: Influenza, Vaccine, Adjuvant
1. Introduction
Antibodies to hemagglutinin (HA) and neuraminidase (NA) were shown more than three decades ago to protect from influenza A infection and disease [1,2] and remain the correlate of protection afforded by inactivated human influenza vaccines [3]. Inactivated influenza vaccine and cold-adapted (live) influenza vaccine (CAIV) are currently licensed as seasonal epidemic influenza vaccines. Both are trivalent and include H3N2, H1N1, and type B-derived antigens or attenuated viral strains of these subtypes. Trivalent inactivated vaccines (TIV) licensed in the United States are split or subunit vaccines, in which HA and NA have been partially purified from other viral components, and none contain an adjuvant. Both TIV and CAIV vaccines are reformulated annually in an attempt to match the vaccine with those strains that will circulate in the subsequent annual epidemic. However, influenza viral variants periodically emerge by antigenic drift or, less commonly, antigenic shift that are distinct from the vaccine strains [4]. This mismatch may result in limited protection since the currently approved vaccine does not induce antibody-mediated cross-neutralization with consequential dramatic increases in influenza-related morbidity and mortality.
The presence of serum anti-HA IgG is a strong correlate of protection from homosubtypic challenge in people vaccinated with TIV [4]. The precise correlates of protection for CAIV are still poorly understood, but in challenge studies, serum hemagglutinin inhibition (HAI) antibody or nasal wash IgA antibody induced by CAIV correlated with significant protection from infection [5,6,7]. Murine and ferret studies also support a critical role for HA-specific IgG in protection from challenge [8]. T-cell mediated immunity, particularly by CD8+ T cells with cytotoxic activity, is important in the control of viral infection in mice lacking neutralizing antibody [9,10], and these observations are in agreement with human studies indicating that influenza A-specific CD8+ T-cell responses are associated with effective clearance of experimental infection [11]. In addition, both CD4+ and CD8+ T-cell responses may play a role in providing some protection against potential pandemic strains such as highly-pathogenic avian influenza (H5N1) [12].
The ability of an influenza vaccine to reliably provide protective immunity to antigenically drifted strains of the same viral subtype as well as heterosubtypic immunity (e.g., immunity to an H3N2 subtype vaccine providing protection against an H5N1 subtype) would be highly desirable. Heterosubtypic immunity could be especially important in a pandemic scenario involving a novel subtype, e.g., avian H5N1 infection, in which virtually all of the population would lack neutralizing antibody. In mice, recent influenza A infection can induce substantial heterosubtypic immunity mediated by CD4+ and CD8+ T cells in the absence of antibody [13]. Alternatively, vaccine-induced antibody can provide heterosubtypic immunity to an otherwise lethal H5N1 challenge in the absence of CD8+ CTL [14]. In humans, some epidemiologic evidence suggests that natural infection by H1N1 can induce significant heterosubtypic immune resistance to H3N2 infection, most likely based on T-cell responses to relatively conserved internal viral proteins [15]. However, clinically relevant and durable heterosubtypic immunity is not routinely induced by natural infection in humans, as observed by the regular emergence of new influenza A subtype viruses in the human population. The relative weakness of heterosubtypic immunity is also supported by the observation that previous immunity from natural infection had little effect on replication of a different subtype of CAIV in humans [16].
A variety of novel vaccine approaches have been pursued in animal models of influenza A (mainly mice or ferrets) to improve or broaden immune protection [17,18,19,20]. These vaccines including a universal vaccine directed at conserved external epitopes of the M2 protein [21], DNA vaccines (single or polyepitope) either alone [22,23] or as part of a prime/boost strategy [24], molecularly attenuated strains deficient in the NS1 protein to overcome antagonism of type I interferon (IFN) responses [25], and the use of novel adjuvants, such as immunostimulating complexes (ISCOMS) [26], E. coli heat labile toxin [27], oil and water type adjuvants such as MF59 [28] and Montanide [29], and CpG oligodeoxynucleotides (ODN) (including those directly conjugated to antigen) [30]. However, the addition of CpG ODN (CpG 7909) as an adjuvant to TIV in human vaccines modestly increased the T-cell immune response (IFN-γ secretion) compared to standard (unadjuvanted) TIV [31]. Furthermore, CpG ODN addition did not enhance the humoral response at either a standard or suboptimal dose of TIV [31].
JVRS-100 is a cationic liposome-DNA complex (CLDC) composed of cationic DOTIM/cholesterol liposomes and plasmid DNA. The addition of peptide or protein antigens to DOTIM/cholesterol has been shown to produce a potent adjuvant effect following vaccination, with induction of enhanced CD4+ and CD8+ T-cell and antibody responses [32]. The nature of these responses suggests that viral vaccines, specifically influenza vaccines, could be greatly improved by administration with a CLDC adjuvant. CLDC-based vaccines have previously been shown to produce a greater CD8+ T-cell specific response than Freund’s complete adjuvant, peptide-pulsed dendritic cells, vaccinia viral vector, and DNA vaccination [32]. Additionally CLDC-based vaccines demonstrated a greater CD4+ T cell-specific response than natural lymphocytic choriomeningitis virus (LCMV) infection and greater protection in a model of aerosol challenge with Mycobacterium tuberculosis (Erdman strain) [32].
JVRS-100 exhibits marked immunostimulatory properties, particularly for the induction of T-dependent antibody, characteristic of T helper 1 (Th1) responses (IFN-γ predominant cytokine secretion pattern) and CD8+ CTL responses (unpublished observations). Given these features, we predicted that a JVRS-100-adjuvanted influenza vaccine would produce a considerably increased level of humoral and T-cell immunity versus unadjuvanted vaccines. The expected robust T-cell and neutralizing antibody response would be advantageous in situations where the seroconversion rate was low (elderly or immunocompromised) and in the case of an influenza pandemic (e.g., H5N1), where fully or partially HA- and NA-matched vaccines may not be available due to incorrect viral strain choices, production issues, or vaccine shortages. We, therefore, tested JVRS-100 as an adjuvant for TIV in both mice and non-human primates, characterized immune responses compared to unadjuvanted vaccine, determined the potential dose-sparing effects of JVRS-100, and evaluated the adjuvant’s ability to protect against challenge with heterologous influenza viruses.
2. Methods
2.1. Preparation of JVRS-100 adjuvant-antigen mixture
JVRS-100 was prepared by mixing a 1:1 ratio of DOTIM/cholesterol liposomes with 0.03% w/v plasmid DNA (pMB75.6) in the presence of lactose followed by lyophilization and storage at 2–8°C. The plasmid lacks a cDNA coding sequence and, thus, was used as an immunostimulant rather than as a means for gene expression. JVRS-100 was reconstituted prior to use by the addition of sterile water for injection. Various concentrations of JVRS-100 were prepared in 5% dextrose in water (D5W) to which Fluzone® vaccine was added and diluted appropriately to administer the indicated dosage. Influenza vaccine (Fluzone®) manufactured by sanofi pasteur (Swiftwater, PA) was purchased from a commercial distributor. The vaccine was used in these studies was the 2006–2007 formulation and contained H1N1 (A/New Caledonia/20/99), H3N2 (A/Wisconsin/67/2005), and B (B/Malaysia/2506/2004)
2.2. Mouse immunization schedule
Groups of 10 CD1 (outbred) mice were immunized via the subcutaneous (SC) route of administration (scruff of neck) with JVRS-100 (20μg) adjuvanted or unadjuvanted Fluzone® (5μg) at days 0 and 14. Mice were sacrificed at day 0, 7, 14, 21, or 28 to assess humoral and cellular immunity as described below, or were challenged at 28 days and followed for survival and weight loss to assess the response to challenge with drifted influenza viral strains.
2.3. Anti-Fluzone® antibody titer ELISA
Fluzone® at 0.5 μg/ml was plated at 4°C overnight on Maxisorp plates (NUNC, Rochester, NY). Plates were washed three times with PBS containing 0.05% Tween-20 and blocked with PBS with 1% BSA for a minimum of 1 hour. Plates were washed and 100 μl of serum dilutions from vaccinated mice were incubated for 2 hours at room temperature. Plates were washed and incubated with a 1:6000 dilution of the appropriate isotype specific antibody conjugated to horseradish peroxidase (Southern Biotech, Birmingham, AL). Plates were visualized with the addition of 100 μl of TMB substrate (Pierce, Rockford, IL) and incubation for 20–30 minutes. Fifty microliters of stop solution (1.0 M H2SO4) was then added and the absorbance was measured at 450 nm and 570 nm. The reading at 570 nm was subtracted from the reading at 450 nm to correct for plate abnormalities and bubbles in the analyte solution. The resulting data for each sample were plotted to obtain a curve of the reciprocal dilution versus the A450–A570 measurement. The antibody titer was determined as the midpoint of the dilution curve as defined by EC50 calculations using Prism statistical software (Graphpad Software, San Diego, CA). The mean of the EC50 for each cohort was determined to be the final antibody titer. Serum pooled from several mice that had been immunized twice with JVRS-100/Fluzone® and characterized by the described assay served as a positive control and internal standard in all assays.
2.4. Hemagglutination determination
Chicken red blood cells (cRBC) in Alsever’s Solution (Colorado Serum Company, Denver, CO) were washed three times with PBS and resuspended to a 10% stock solution. Serial 1:2 dilutions of individual viruses in PBS were added to an equal volume of 0.5% cRBC solution. Following 1 hour incubation at room temperature, the plates were read for hemagglutination. HA titer was determined to be the reciprocal of the highest dilution exhibiting partial or complete hemagglutination.
2.5. Hemagglutination inhibition antibody (HAI) assay
Sera from mice or plasma from non-human primates were pre-treated by incubation overnight at 37°C with 3 parts RDEII enzyme (Denka Seiken, Tokyo, Japan) to eliminate non-specific hemagglutination. After inactivation at 56°C for 30–60 minutes, serial 1:2 dilutions in PBS were mixed with 4.0 HA units per 25 μl of individual viruses and incubated at room temperature for 15 minutes, followed by the addition of an equal volume of 0.5% cRBC. Samples were performed in duplicate and a no antigen control was included to rule out non-specific activity. Plates were read at 1 hour. The HAI titer was determined to be the reciprocal dilution of the last well that showed complete inhibition of hemagglutination.
2.6. Splenic T-cell restimulation assays
Splenocytes isolated from vaccinated and control mice were restimulated with Fluzone®, or a representative H1N1 (PR/8/34), H3N2 (HKx31), or influenza B (B/Lee/40) inactivated virus. Supernatants were collected after 48 hours of stimulation and assayed by ELISA for mouse IFN-γ (R & D Systems). Controls included spleen cells cultured 48 hours without the addition of specific antigens and unvaccinated splenocytes incubated with Fluzone® or inactivated influenza virus.
2.7. Influenza lethal challenge of mice
Cohorts of BALB/c mice (n=5) were injected SC (scruff of neck) with Fluzone® alone (5μg), JVRS-100 alone (20μg), JVRS-100/Fluzone® (20μg/5μg), or diluent at day 0 and 14. At day 28, mice were lightly anesthetized with isoflorane and challenged via intranasal administration of 50μl containing 2 × MLD50 of PR/8/34 (H1N1), HKx31 (H3N1) or B/Lee/40 viral strains, respectively. Prior to determination of MLD50, viruses were successively passaged in mice via lung infection to increase virulence prior to growth of a large seed stock in eggs. The respective MLD50 of a challenge virus was determined by infection of unvaccinated mice with increasing amounts of virus. Successive qualification runs with mice receiving viral challenge around the MLD50 demonstrated a minimal threshold challenge dose for lethality, beyond which resulted in total mortality of the challenged groups. The mice were monitored for weight loss and mortality for 14–20 days following infection.
2.8. Non-human primate adjuvant dose-ranging study
Groups of rhesus macaques (Macaca mulatta, n=4) were vaccinated intramuscularly (IM) on week 0 and 2 with graded doses (0, 2.5, 7.5, and 22.5 μg) of JVRS-100 adjuvant mixed with a fixed pediatric dose (22.5 μg) of Fluzone®. The 0 and 2-week immunization schedule used in the mouse and non-human primate studies is a relatively short cycle compared to what is current clinical practice. The intent of closely spacing the vaccinations was to model a vaccination schedule that may be necessary in case of an ongoing pandemic, where immunity to unmatched strains may need to reach a minimal protective level in a relatively short period of time. It could be that the adjuvant impact may have been more pronounced if a longer interval had been used to ensure plateau and decline in the primary response, prior to boosting. Plasma was collected at weeks 0, 1, 2, 3, 4, 6, and 9 for HAI analysis and peripheral blood mononuclear cells (PBMCs) were collected at weeks 0, 2, 4, and 9 for assessment of intracellular cytokine staining following antigen restimulation as described below.
2.9. Non-human primate microneutralization
Microneutralization antibody titers were performed by cell-culture and ELISA-based assay previously described [39]. Briefly, plasma from non-human primates was heat-inactivated at 56°C for 30 minutes. Two-fold serial dilutions of plasma were incubated with 100 TCID50/50 μl of individual influenza viruses for 1 hour at 37°C. 1.5 × 106 Madin-Darby Canine Kidney (MDCK) cells (kindly provided by K. Hancock, Centers for Disease Control and Prevention) were added to serum/virus mixtures and incubated overnight at 37°C. After fixing with 80% acetone, the presence of influenza nucleoprotein was detected by ELISA using influenza A or B-specific anti-nucleoprotein antibody (Millipore, Bellerica, MA). Neutralizing end-point titers were defined as the reciprocal of the last dilution that fell below the calculated 50% specific-signal value.
2.10. Intracellular cytokine staining of influenza-specific rhesus macaque T cells
For intracellular staining to detect influenza specific T cells in rhesus macaque PBMCs, a modification of a previously reported protocol was used [34]. Briefly, PBMCs were prepared by Ficoll-Hypaque density gradient centrifugation and cryopreserved in 10% DMSO-containing medium for later assay. Cryopreserved samples were thawed and rested overnight at 37°C, in 5% CO2 atmosphere, in complete RPMI-1640 media containing 10% FCS. The next day, cells were adjusted to a concentration of 5 × 106/ml, and incubated with anti-CD28 and anti-CD49d antibodies (1.0 μg/ml final concentration; BD-Biosciences, San Jose, CA) as co-stimulatory molecules in a total volume of 200 μl complete RPMI-1640/10% FCS. In all experiments, the following samples were prepared: pediatric (preservative-free) Fluzone® 1.0 μg/ml, background controls containing co-stimulatory molecules alone, and a positive control stimulated with staphylococcal enterotoxin B (SEB; 0.2 μg/ml, Sigma, Inc., St Louis, MO). Cells were incubated for 16 hours at 37°C, brefeldin A (Sigma) and monensin (GolgiStop®; BD Biosciences) were added for the last 10 hours. Following incubation, cells were washed and surface stained with anti-CD3-Pacific Blue®, anti-CD4-PerCP-Cy5.5, and anti-CD8-APC-Cy7. A marker to exclude dead cells (7-AAD; Molecular Probes, Eugene, OR) was added, then samples were fixed (1% paraformaldehyde), permeabilized (0.5% saponin) and stained intracellularly with IFN-γ-APC (clone B27), TNF-α-PE-Cy7 (clone Mab11), IL-2-PE (clone MQ1-17H12) for 20 minutes at room temperature. All monoclonal antibodies were from Pharmingen/Becton-Dickinson, unless otherwise specified. After washing with permeabilizing buffer, cells were fixed in PBS containing 1% paraformaldehyde. Data was acquired using a FACSAria flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Treestar, Inc., Ashland, OR). At least 100,000 events in the FSC/SSC lymphocyte gate were acquired. The background level of cytokine staining varied from sample to sample, but was typically <0.05% of the unstimulated CD8+ T cells. All data are reported after subtraction of the medium control cultures. For each chart, the percentage of cells (and proportion) responding is indicated below a chart. Mean frequency (± SEM) of each response is shown as a number in the middle of a chart; only animals with a positive response are included. Each portion of a chart indicates the percentage of Fluzone®-specific T cells that responded with one, two, three, or four functions; and the arcs around the charts show the function or combination of functions to which the specific response corresponds (see color legend).
3. Results
3.1. Increased antibody response in mice to JVRS-100/Fluzone® vaccine compared with Fluzone® alone
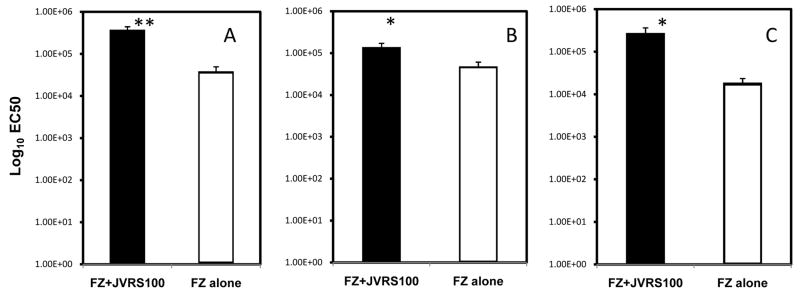
The presence of influenza specific serum IgG is a strong correlate of protection from homosubtypic challenge in people vaccinated with TIV [4]. To test the increase in the Fluzone®-specific IgG response following vaccination with JVRS-100/Fluzone®, cohorts of mice were administered Fluzone® with and without JVRS-100 at day 0 and 14 and the Fluzone®-specific IgG antibody titer was determined by ELISA at 28 days. As shown in Figure 1A, mice vaccinated with Fluzone® adjuvanted with JVRS-100 showed a consistently 10-fold higher level of Fluzone®-specific IgG compared to animals immunized with Fluzone® alone (P<0.005, unpaired two-tailed t test)).
Fig. 1.
Vaccine-specific antibody isotype titers following vaccination with Fluzone®/JVRS-100 versus Fluzone®, alone. Groups of CD1 mice (n=10) were vaccinated subcutaneously with 5.0 μg of Fluzone® alone or combined with 20.0 μg of JVRS-100 at day 0 and 14. At day 28, serum was collected and the Fluzone®-specific antibody isotype profile was determined. The total Fluzone®-specific IgG(A) response was enhanced using JVRS-100 by approximately 10-fold (**P<0.005), Fluzone®-specific IgG1(B) was enhanced by approximately 5-fold (*P<0.05), and Fluzone®-specific IgG2a(C)was enhanced by approximately 50-fold (*P<0.05) compared to that obtained with Fluzone® vaccination alone. P values were calculated using an unpaired, two-tailed Student’s t-test.
Certain antibody isotypes, such as IgG2a, may provide greater protection in vivo against influenza by their superior ability to bind Fc receptors [35] or to activate complement [36] compared to other isotypes, such as IgG1. To examine whether the anti-Fluzone® antibody isotype response was biased toward IgG2a compared to IgG1, Fluzone®-specific antibody responses were analyzed for these antibody isotypes by ELISA. There were slightly increased levels of IgG1 and an approximately 50-fold greater increase in IgG2a antibody (P<0.05, two-tailed unpaired t test; Figure 1B–1C) using the JVRS-100-Fluzone® vaccine. These results demonstrated a selectively enhanced IgG2a immune response to the JVRS-100 adjuvanted Fluzone® when compared with Fluzone® alone, corresponding to an IgG2a/IgG1 ratio of approximately 1.0 for JVRS-100 adjuvanted Fluzone® compared to 0.125 for Fluzone® alone.
The established standard surrogate measurement of efficacy for vaccination against influenza is demonstrated increases in hemagglutination inhibition (HAI) antibody titer. To test antibody-mediated homosubtypic reactivity, sera from vaccinated mice (previous study) were tested in the HAI assay using the individual Fluzone® vaccine components [i.e., H1N1 (A/New Caledonia/20/99), H3N2 (A/Wisconsin/67/2005), and B (B/Malaysia/2506/2004). The HAI titer was increased in the JVRS-100/Fluzone® group as early as day 14 (i.e., H3N2) and showed an increase at Day 21 and 28 for all three of the viral types contained in the Fluzone® vaccine (Figure 2). The JVRS-100/Fluzone® vaccine showed a similar HAI response following a single vaccination (i.e., day 14 serum testing) compared to the Fluzone® alone group after two vaccinations (i.e., day 21 and 28 serum testing).
Fig. 2.
Kinetics of the antibody response to vaccination with Fluzone® plus JVRS-100 adjuvant. Groups (n=5) of CD1 mice immunized twice by the subcutaneous route at day 0 and 14. Serum was collected on days 7, 14, 21, and 28 and tested for influenza strain-specific HAI titer. Panel A – H1N1 (A/New Caledonia/20/99)-specific titers (*p<0.05); Panel B – H3N2 (A/Wisconsin/67/2005)-specific titers (*p<0.05); Panel C – B/Malaysia/2506/2004. P values were calculated using an unpaired, two-tailed Student’s t-test.
3.2 Antigen-dependent splenocyte response
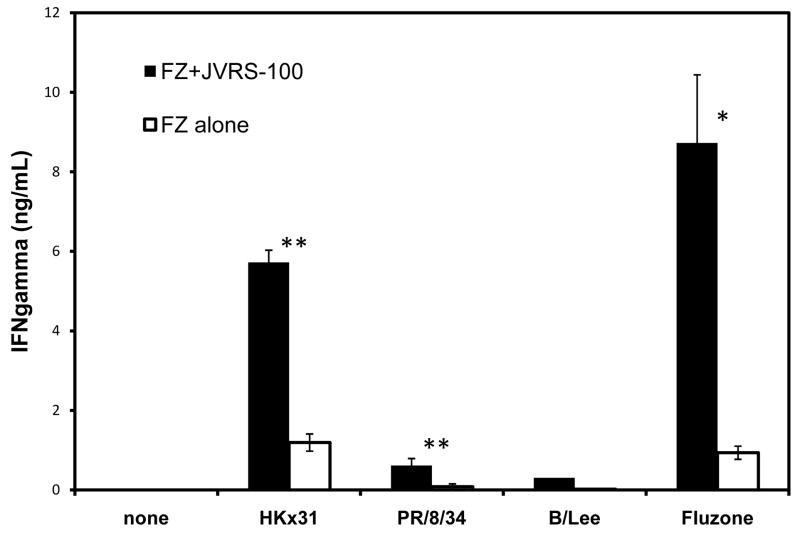
In cases of insufficient neutralizing antibody or a mismatch of the influenza vaccine strain versus the circulating strain, protection would likely depend on the T-cell response [9,10]. To measure the level of antigen-specific CD4+ and CD8+ homosubtypic- and antigenically-drifted T-cell responses, splenocytes from immunized mice were collected and restimulated in vitro with antigen. To evaluate the homosubtypic T-cell response, splenocytes were restimulated with the intact Fluzone® preparation. For evaluation of the antigenically-drifted T-cell response, splenocytes were restimulated with individual viruses representative of each vaccine subtype; PR/8/34 (H1N1), HKx31 (H3N2), or B/Lee/40 inactivated virus isolates. Restimulation with Fluzone® vaccine or whole inactivated virus may include responses not relevant to protection such as reaction to egg-based proteins or other viral contaminants; therefore, the Fluzone® vaccine and whole virus were purified to minimize the presence of non-influenza proteins. JVRS-100-Fluzone® vaccination resulted in an approximately 10-fold increase in antigen-specific splenocyte response when compared with Fluzone® alone as measured by IFN-γ production (P<0.05, Figure 3) following Fluzone® (i.e., homosubtypic) restimulation. Furthermore, the level of antigenically-drifted splenocyte response following restimulation with representative mismatched H1N1 (PR/8/34) and H3N2 (HKx31) viral isolates was also increased in the JVRS-100-Fluzone® vaccinated groups compared to Fluzone® alone (P<0.005, Figure 3).
Fig. 3.
IFN-γ production by T cells following vaccination with Fluzone® plus JVRS-100 versus Fluzone® alone. Groups of CD1 mice (n=10) were vaccinated subcutaneously with 5.0 μg of Fluzone® combined with 20.0 ug of JVRS-100 at day 0 and 14. At day 28, splenocytes were prepared and incubated with Fluzone® or a representative heat-inactivated H1N1 (PR/8/34) virus, H3N2 (HKx31) virus, or influenza B (B/Lee/40) virus. Forty-eight hours after stimulation, the supernatant was collected and analyzed for IFN-γ. The IFN-γ content was significantly higher following Fluzone® plus JVRS-100 vaccination compared to Fluzone alone after stimulation of splenocytes in vitro with Fluzone (P<0.05); HKx31 (P<0.005); and PR/8/34 (P<0.005). The response was also higher, but not statistically significant when splenocytes were restimulated with B/Lee/40. P values were calculated using an unpaired, two-tailed Student’s t-test.
3.3 Efficacy of JVRS-100 adjuvanted Fluzone® against mismatched lethal influenza virus challenge
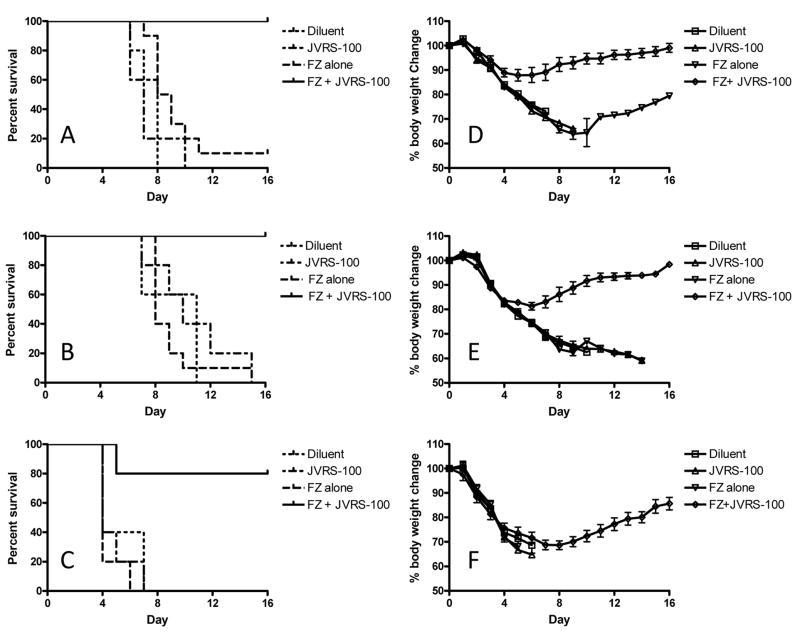
The robust antibody and T-cell response to JVRS-100-Fluzone suggests that this adjuvant may induce cross-protection to drifted influenza strains. To test the effectiveness of vaccination against drifted strains, mice were vaccinated twice (day 0 and 14) with 5.0 μg Fluzone® with or without JVRS-100 (20 μg) and subsequently challenged intranasally at day 28 with 2xLD50 of PR/8/34 (H1N1), 2xLD50 of an adapted HKx31 (H3N2) or 2xLD50 of B/Lee/40 (influenza B) viruses. Mice were monitored daily post-challenge for body weight and survival (Figure 4).
Fig. 4.
Fluzone® plus JVRS-100 immunization provides considerable cross-protection directed against influenza viral heterosubtypes. BALB/cJ mice (n=10 per group) were vaccinated with Fluzone® on Day 0 and 14 and challenged intranasally on day 28 with 2xLD50 of H1N1 (PR/8/34), H3N2 (HKx31), or influenza B (B/Lee/40) viruses. The groups were monitored for survival (panels A-C) and weight loss (panels D-F). Mice vaccinated with JVRS-100-Fluzone® compared to those receiving Fluzone® alone showed a significant enhancement in survival following viral challenge with (A) influenza A H1N1 (PR/8/34) ***P<0.0005; (B) influenza A H3N2 (HKx31) ***P<0.0005; or (C) influenza B (B/Lee/40) *P<0.05. P values were calculated using a log-rank (Mantel-Cox) test. Similarly, mice vaccinated with JVRS-100-Fluzone® compared to those receiving Fluzone® alone showed less weight loss following viral challenge with (D) influenza A H1N1 (PR/8/34) and (E) influenza A H3N2 (HKx31). There was not an improvement in weight loss following challenge with influenza B (B/Lee/40) (F), although the surviving mice (80%) recovered.
Immunized mice lethally challenged with drifted influenza strains showed considerable benefit from the JVRS-100 adjuvanted vaccine. Mice vaccinated with JVRS-100-Fluzone® and challenged with 2xLD50 of PR/8/34 (H1N1) or HKx31 (H3N2) had minimal weight loss (~10–15%) and 100% survival compared to >30% weight loss and 0% survival for mice vaccinated with unadjuvanted Fluzone®. Mice vaccinated with JVRS-100-Fluzone® and challenged with 2xLD50 of B/Lee/40 showed a similar weight loss profile compared to unadjuvanted Fluzone® until day 6, after which the infection was lethal for the control and unadjuvanted groups. In contrast, the JVRS-100-adjuvanted group recovered with 80% survival [p<0.05, log-rank (Mantel-Cox) test].
3.4 Non-human primate antibody response
To determine the effectiveness of the JVRS-100 adjuvant in non-human primates, rhesus macaques were vaccinated on days 0 and 14 with the Fluzone® vaccine (22.5 μg) adjuvanted with varying doses (2.5 μg, 7.5 μg, or 22.5 μg) of JVRS-100.
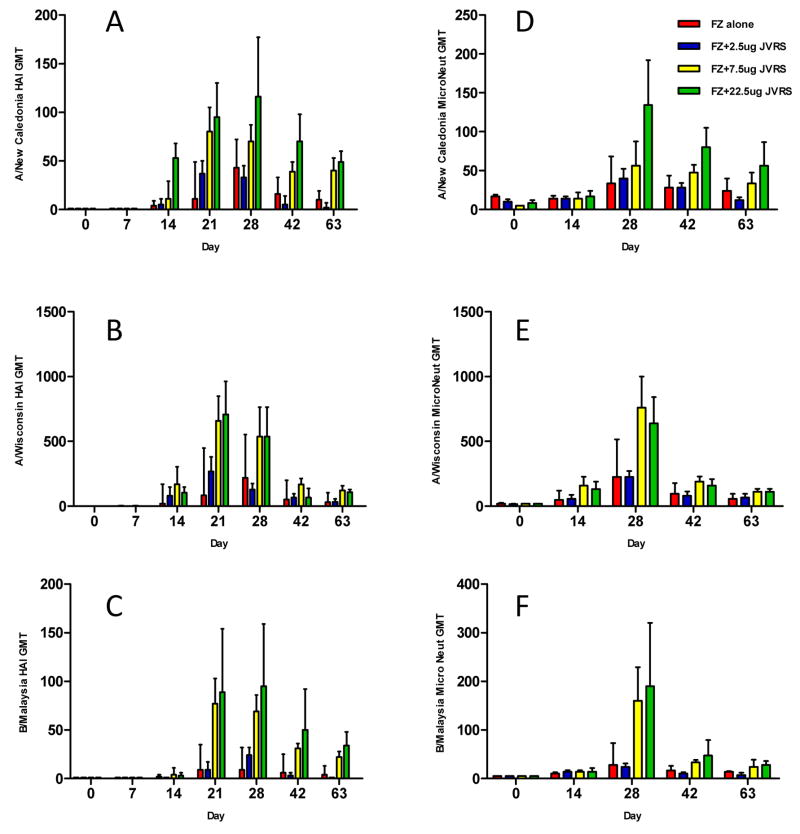
The administration of JVRS-100/Fluzone® vaccine resulted in an enhancement of geometric mean HAI antibody titers (Fluzone® antigen) compared to animals receiving Fluzone® vaccine alone (Figure 5A, B, and C). In these Fluzone® naïve monkeys, the JVRS-100 adjuvant appeared most effective following the booster immunization and continued for at least two months duration thereafter, whereas the HAI titers in the Fluzone® only group had a more pronounced decline in HAI response. Furthermore, after a single-dose priming immunization, there was an increase in HAI titer in the 7.5 μg and 22.5 μg JVRS-100/Fluzone® dose groups, but not in the Fluzone® only group on day 14.
Fig. 5.
Rhesus macaques were vaccinated intramuscularly with 22.5 μg of Fluzone® and increasing amounts of JVRS-100 (2.5, 7.5 22.5 μg) at Day 0 and 14. Viral strain-specific HAI antibody and microneutralization titers were determined, and demonstrated significantly higher antibody titers after vaccination with Fluzone® plus JVRS-100 compared to Fluzone® alone. (A) influenza A H1N1 (A/New Caledonia/20/99)-specific HAI-titers ***P<0.0005. (B) influenza A H3N2 (A/Wisconsin/67/2005)-specific HAI-titers *P<0.05. (C) influenza B (B/Malaysia/2506/2004)-specific HAI titers *P<0.05). (D) influenza A H1N1-specific microneutralization titers ***P<0.0005. (E) influenza A H3N2-specific microneutalization titer *P<0.05. (F) influenza B-specific microneutalization titers **P<0.005). P values were calculated using a two-way ANOVA test.
Antibody responses were also measured by microneutralization (MN) tests using the individual viruses included in the Fluzone® vaccine (Figure 5D, E, and F). Two-way ANOVA analysis of the dose response demonstrated statistical significance between the dose of JVRS-100 and the enhanced MN response tested at 0, 2, 4, 6 and 9 weeks for H1N1 – A/New Caledonia/20/99 (P<0.0005), H3N2 – A/Wisconsin/67/2005 (P<0.05), and influenza B –B/Malaysia/2506/2004 (P<0.005) viral isolates. These results indicated that the JVRS-100-Fluzone® virus neutralization responses were directed against all three viral serotypes contained in the vaccine.
The treated and control monkeys were monitored for clinical and hematological parameters including white and red blood cell counts, hemoglobin, hematocrit, red cell indices (MCV, MCH, MCHC), platelet counts, and plasma total protein levels. There were no clinical signs of illness and no significant toxicologically meaningful excursions in these parameters, outside the normal limits, during the course of immunization. Furthermore, there were no injection site reactions following the immunization schedule (data not shown).
3.5 Non-human primate CD4+ and CD8+ T-cell responses
Cryopreserved PBMC obtained from immunized macaques were thawed, restimulated in vitro with Fluzone® vaccine, and evaluated by intracellular cytokine-staining (IFN-γ, IL-2, and TNF-α) and surface staining for the presence of activated CD4+ and CD8+ T cells. The results from the high-dose immunization group [JVRS-100 (22.5 μg) + Fluzone® (22.5 μg)], indicated that the JVRS-100-adjuvanted Fluzone® vaccine, when compared to Fluzone® vaccine alone, resulted in higher levels of antigen-specific CD4+ and CD8+ T cell responses (Figure 6), which increased up to 9 weeks post-vaccination. The charts (Figure 7) summarize the mean T cell responses and the extent to which the CD4+ and CD8+ T cell responses were polyfunctional within the immunized monkeys. The results revealed that the JVRS-100-adjuvanted Fluzone® vaccine, when compared to Fluzone® vaccine alone, resulted in enhanced responses that exhibited multi-functional T cell characteristics.
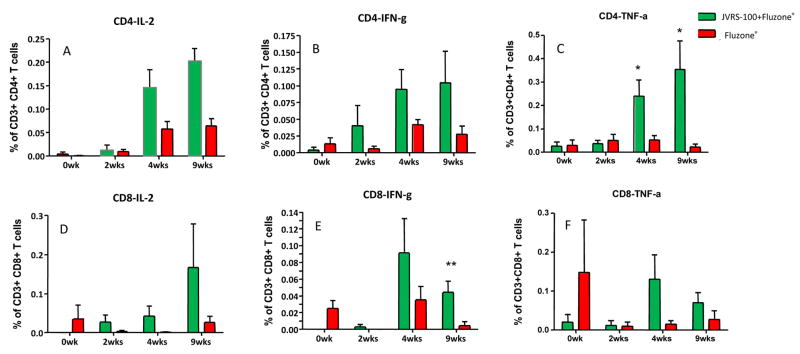
Fig. 6.
Fluzone®-specific cytokine production by rhesus monkey T cells is increased after 22.5μg (high dose) JVRS-100-adjuvanted Fluzone® vaccination compared to Fluzone® alone. PBMCs were restimulated in vitro and intracellularly stained for IL-2, IFN-γ and TNF-α producing CD4+ and CD8+ T cells. The T cell populations were evaluated by flow cytometric analysis. *P<0.05; **P<0.01.
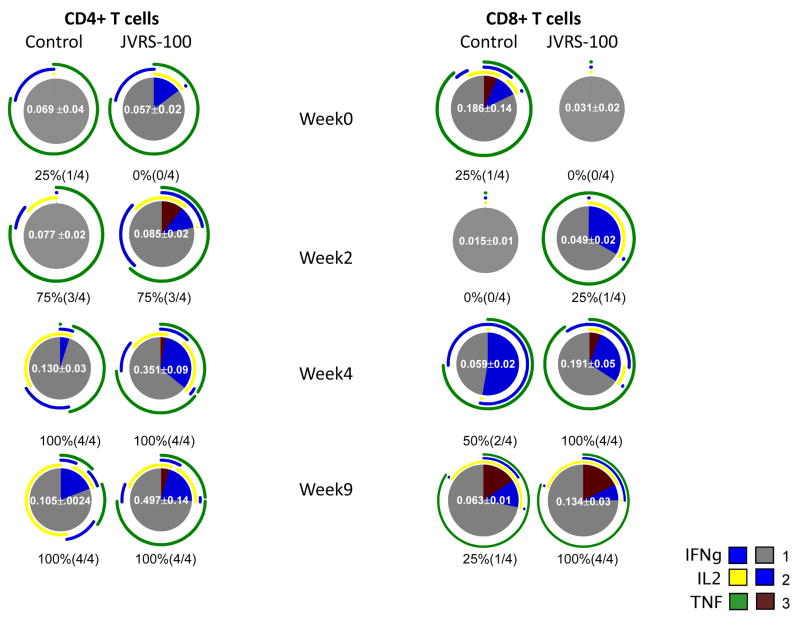
Fig. 7.
Fluzone®-specific multifunctional cytokine production by rhesus monkey T cells is increased after 22.5μg (high dose) JVRS-100-adjuvanted Fluzone® vaccination compared to Fluzone® alone. The response to Fluzone® stimulation is shown. The pie charts summarize the mean T cell responses of all immunized animals and the extent to which the CD4+ and CD8+ T cell responses were polyfunctional. For each pie chart the percentage (and proportion) of responding animals is indicated below the chart. The mean frequency (± s.e.m.) of each response is shown as a white number in the middle of the pie chart; only animals with a positive response are included. Each portion of a pie chart indicates the percentage of Fluzone®-specific T cells that responded with one, two, or three functions; and the arcs around the pie show the function or combination of functions to which the specific response corresponds (see color legend). Where arcs overlap this indicates that cells were secreting two or three cytokines in response to stimulation with Fluzone®. All differences are below statistical significance in this pilot study (n=4/group) with the exception of CD4+/IL-2+/TNF-α+ cells at 4 weeks (P=0.002) and 9 weeks (P=0.01).
The responding animals and percentages are indicated below each pie chart. The mean frequency of each group (± s.e.m.) is shown in the center of the pie. Only positive responses were considered in the mean and s.e.m. calculations. Sections of the pie chart indicate the proportion of T-cells which responded with one, two, or three cytokines (represented by gray, blue, or maroon colors, respectively) in response to restimulation with Fluzone®. The arcs around the pie charts indicate the cytokine(s) elicited by restimulation. Points at which the arcs overlap are representative of cell populations which are secreting two or three cytokines.
The differences noted between adjuvanted and unadjuvanted vaccine in this pilot study (n=4/group) were statistically significant for CD4+/TNF-α+ cells at 4 weeks and 9 weeks (P<0.05), CD8+/IFN-γ+ cells at 9 weeks (P<0.01), and CD4+/IL-2+/TNF-α+ cells at 4 weeks (P=0.002) and 9 weeks (P=0.01). All p values were calculated using a two-tailed student’s t-test.
4. Discussion
In this study, we have demonstrated that a cationic liposome-DNA complex-based adjuvant, JVRS-100, enhanced the quantity and the quality of the adaptive immune response to a commercially available split influenza vaccine in both mice and non-human primates. In addition to inducing protective HAI titers and neutralizing antibody against homotypic virus, JVRS-100 enhanced both the CD4+ and CD8+ T-cell response to the split influenza vaccine.
The antibody response induced by JVRS-100-Fluzone® vaccine is of particular interest both in the magnitude of the HAI titer as well as the antibody isotype profile. An HAI titer of ≤1:40 is considered to represent a protective titer against homotypic virus in humans [37]. The addition of the JVRS-100 adjuvant induced a 2.4–17.7 fold higher titer than this minimal protective level in non-human primates and 2.7–10.5 fold higher than Fluzone® alone. This enhanced immune activity may allow for the induction of protective antibody titers using a fraction of vaccine antigen, which would be important in a setting of limited antigen, e.g. contamination of a production facility or in the event of a pandemic. The humoral response was biased toward antibody isotypes that have a higher affinity for Fc-receptors (IgG2a) [38]. These antibody isotypes may enhance effector functions such as complement activation or antibody-dependent cell-mediated cytotoxicity (ADCC) in vivo as compared with other isotypes [37]. The enhanced antibody responses may account for the ability of JVRS-100-Fluzone® immunization to provide protection from a lethal heterosubtypic viral challenge, although we cannot exclude a role for Fluzone®-specific T-cell immunity in this protection. The bias toward an IgG2a response also suggests a Th1 orientation of the immune response; expected based on prior experience with CLDC [39], and associated with a cytotoxic T cell response.
One observation in the mouse and non-human primate antibody responses to vaccination is the variable immunogenicity and efficacy data for the different strains of TIV. The immunodominant antigenic structure of the response of H3 > H1 > B has been noted in previous preclinical studies with unadjuvanted and adjuvanted TIV [40]. We have extended these observations by showing that this immunodominant structure is reflected in weight loss from drifted influenza challenge in mice, although eventual survival was 100%. In clinical studies, the cold-adapted influenza vaccine (Flumist) shows exactly the same phenomenon even after multiple doses, eliminating interference as the cause [41]. In addition, the recombinant HA (FluBlok) shows H3 responses dominant over H1 [42].
JVRS-100 induced T-cell-mediated immunity to influenza split vaccine, with an increase in both CD4+ and CD8+ Fluzone®-specific T cells. The importance of the CD4+ T-cell response to influenza has been demonstrated in the murine model; adoptive transfer of primed CD4+ effector T cells conferred protection against lethal influenza challenge in naïve recipients [43]. In addition, influenza-specific CD4+ T cells play an important role in enhancing B-cell mediated anti-influenza effects [44].
The role of CD8+ T cells in influenza virus clearance has been well-established. Mice deficient in CD8+ T cells have delayed clearance of influenza virus after infection [45]. Adoptive transfer of influenza-specific CD8+ T cells can confer protection in B-cell-deficient mice [46]. Here, we found that the JVRS-100 adjuvanted influenza vaccine enhanced both the CD4+ and CD8+ T-cell response in non-human primates.
In addition to an increase in the magnitude of the influenza-specific CD4+ and CD8+ T cells, we found that non-human primates given JVRS-100 adjuvanted influenza vaccine also had an increase in the number of multi-functional T cells. Recently, it has been shown in responses to both Leishmania and the tuberculosis vaccine Bacillus Calmette-Guerin (BCG), that Th1 CD4+ cells producing multiple cytokines including IFN-γ, TNF-α, and IL-2, have greater effector function and may be a better correlate of protection after vaccination [47]. CD8+ T cells may also be further delineated into different effector populations by their ability to secrete multiple cytokines, which has been associated with high level anti-influenza responses. [9]. Overall, this multi-functional T cell response measurement may be an important way to assess the quality of the T cell-mediated response after vaccination [48]. The presence of large numbers of multi-functional CD4+ and CD8+ T cells suggests that the JVRS-100 adjuvant induces a CD4+ and CD8+ T-cell response with optimal effector function and, thus, may be more effective at clearing influenza virus after infection than unadjuvanted vaccine. Interestingly, the non-human primate T cell responses appeared to increase up to 9 weeks following JVRS-100-Fluzone® vaccination. This result may suggest a role of the adjuvant in expanding these multi-functional T cell populations.
There has been considerable interest in developing a vaccine that induces heterosubtypic protection against drifted or pandemic (shifted) strains of influenza. Heterosubtypic protection has been shown to be mediated by cross-reactive antibodies [14] as well as by CD8+ T cells [49]. Thus, an influenza vaccine that induces a robust antibody and cell-mediated response would likely induce the greatest amount of heterosubtypic protection.
There are certain limitations when comparing this work with previous studies examining adjuvanted TIV. These parameters include the use of different seasonal vaccines, vaccination regimen, strains of mice, and the use of lethal or non-lethal murine challenge. Furthermore, there are several novel aspects of these studies including drifted murine challenge of vaccinated mice and the examination of multi-functional T-cells in vaccinated non-human primates that have not previously been reported. However, previous investigators have shown the increased immunogenicity of CLDC adjuvanted vaccines compared with several immunoadjuvants combined with model and disease antigens [32].
In the present study employing a murine heterosubtypic lethal challenge model, the addition of JVRS-100 to split influenza vaccine conferred drifted-protection as demonstrated by statistically significant differences in survival when compared with Fluzone® alone. It is unclear whether this protection is mediated by CD4+ or CD8+ T cells or by cross-reactive antibodies. Since split influenza vaccines lack significant amounts of influenza internal proteins, which are the usual targets for CD8+ T-cell immunity to influenza [9], antibody-mediated heterosubtypic protection may play a larger role. Alternatively, CD8+ T cells that recognize influenza peptides outside of the usual immunodominant hierarchies, may be mediating this protection. Further studies employing adoptive transfer (serum or cells) or using mice that lack CD8+ T cells or B cells may help to delineate the relative contributions to cross-protection in this system.
In summary, we describe the immunogenicity and protection afforded by a trivalent split influenza vaccine adjuvanted with a cationic liposome-DNA-complex adjuvant, JVRS-100. In both mice and non-human primates, JVRS-100 induced a robust quantitative and qualitative antibody response as well as an increase in CD4+ and CD8+ T-cell responses. In addition, a statistically significant difference in protection was seen in JVRS-100-Fluzone® vaccinated mice after lethal challenge with drifted influenza strains compared with Fluzone® alone. Finally, non-human primate studies revealed that JVRS-100-Fluzone® vaccination resulted in a strikingly increased number of influenza-specific CD4+ and CD8+ T cells with a poly-functional phenotype (i.e., multiple cytokines expressed per cell). Taken together, these data suggest that JVRS-100 may be a safe and appropriate adjuvant for improving the effectiveness of a currently available seasonal influenza vaccine.
Acknowledgments
The authors would like to thank the Influenza Division of the Centers for Disease Control and Prevention for kindly supplying the human reassortant influenza viruses used in these studies. This work was supported by NIAID grant 1U01AI074512-1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy BR, Kasel JA, Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972 Jun 22;286(25):1329–32. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 2.Virelizier JL. Host defenses against influenza virus: the role of anti-hemagglutinin antibody. J Immunol. 1975 Aug;115(2):434–9. [PubMed] [Google Scholar]
- 3.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004 Jan;59(1):1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 4.Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002 Aug 19;20(25–26):3068–87. doi: 10.1016/s0264-410x(02)00254-2. [DOI] [PubMed] [Google Scholar]; Clements ML, Betts RF, Tierney EL, Murphy BR. Resistance of adults to challenge with influenza A wild-type virus after receiving live or inactivated virus vaccine. J Clin Microbiol. Jan 19;23(1):73–6. doi: 10.1128/jcm.23.1.73-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986 Jul;24(1):157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986 Jul;24(1):157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, Treanor J, Zangwill K, Hayden FG, Bernstein DI, Kotloff K, King J, Piedra PA, Block SL, Yan L, Wolff M. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000 Mar;181(3):1133–7. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 8.Smith H, Sweet C. Lessons for human influenza from pathogenicity studies with ferrets. Rev Infect Dis. 1988 Jan-Feb;10(1):56–75. doi: 10.1093/clinids/10.1.56. [DOI] [PubMed] [Google Scholar]
- 9.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006 May;7(5):449–55. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 10.Kilbourne ED. Influenza immunity: new insights from old studies. J Infect Dis. 2006 Jan 1;193(1):7–8. doi: 10.1086/498984. [DOI] [PubMed] [Google Scholar]
- 11.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–7. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 12.Lee LY, Do LH, Simmons C, deJong MD, Nguyen VC, Schumacher R, Yan CP, McMicheal AJ, Farrar JJ, Smith GL, Townsend A, Askonas BA, Rowland-Jones S, Dong T. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008 Oct;118(10):3478–90. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benton KA, Misplon JA, Lo CY, Brutkiewicz RR, Prasad SA, Epstein SL. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NK T cells, or gamma delta T cells. J Immunol. 2001 Jun 15;166(12):7437–45. doi: 10.4049/jimmunol.166.12.7437. [DOI] [PubMed] [Google Scholar]
- 14.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001 Jun;75(11):5141–50. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein SL. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J Infect Dis. 2006 Jan1;193(1):49–53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 16.Steinhoff MC, Fries LF, Karron RA, Clements ML, Murphy BR. Effect of heterosubtypic immunity on infection with attenuated influenza A virus vaccines in young children. J Clin Microbiol. 1993 Apr;31(4):836–8. doi: 10.1128/jcm.31.4.836-838.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemble G, Greenberg H. Novel generations of influenza vaccines. Vaccine. 2003 May 1;21(16):1789–95. doi: 10.1016/s0264-410x(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 18.Beyer WE, Palache AM, deJong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002 Oct 4;20(9–10):1340–53. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson I, Zambon MC, Rudin A, Colegate A, Podda A, Bugarini R, Del Giudice G, Minutello A, Bonnington S, Holmgren J, Mills KH, Nicholson KG. Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers. J Virol. 2006 May;80(10):4962–70. doi: 10.1128/JVI.80.10.4962-4970.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006 Jan;24(1):5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999 Oct;5(10):1157–63. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 22.Kodihalli S, Kobasa DL, Webster RG. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine. 2000 May 22;18(23):2592–9. doi: 10.1016/s0264-410x(99)00485-5. [DOI] [PubMed] [Google Scholar]
- 23.Baird MR, Wilson L, Young L, Williman J, Young S, Wilson M, Slobbe E, Lockhart E, Buchan G. Bystander help within a polyepitope DNA vaccine improves immune responses to influenza antigens. Scand J Immunol. 2004 Oct;60(4):363–71. doi: 10.1111/j.0300-9475.2004.01487.x. [DOI] [PubMed] [Google Scholar]
- 24.Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, Nabel GJ. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005 Nov 16;23(46–47):5404–10. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 25.Palese P, Garcia-Sastre A. Influenza vaccines: present and future. J Clin Invest. 2002 Jul;110(1):9–13. doi: 10.1172/JCI15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rimmelzwaan GF, Claas EC, vanAmerongen G, deJong JC, Osterhaus AD. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine. 1999 Mar 17;17(11–12):1355–8. doi: 10.1016/s0264-410x(98)00390-9. [DOI] [PubMed] [Google Scholar]
- 27.Gluck R, Mischler R, Durrer P, Furer E, Lang AB, Herzog C, Cryz SJ. Safety and immunogenicity of intranasally administered inactivated trivalent virosome-formulated influenza vaccine containing Escherichia coli heat-labile toxin as a mucosal adjuvant. J Infect Dis. 2000 Mar;181(3):1129–32. doi: 10.1086/315337. [DOI] [PubMed] [Google Scholar]
- 28.Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19(17–19):2673–80. doi: 10.1016/s0264-410x(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 29.Benne CA, Harmsen M, van der Graaff W, Verheul AF, Snippe H, Kraaijeveld CA. Influenza virus neutralizing antibodies and IgG isotype profiles after immunization of mice with influenza A subunit vaccine using various adjuvants. Vaccine. 1997;15(9):1039–44. doi: 10.1016/s0264-410x(96)00287-3. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi M, Satou E, Ueki R, Yano M, Miyano-Kurosaki N, Fujii M, Takaku H. Resistance to influenza A virus infection by antigen-conjugated CpG oligonucleotides, a novel antigen-specific immunomodulator. Biochem Biophys Res Commun. 2005 Apr 1;329(1):230–6. doi: 10.1016/j.bbrc.2005.01.116. [DOI] [PubMed] [Google Scholar]
- 31.Cooper CL, Davis HL, Morris ML, Efler SM, Krieg AM, Li Y, Laframboise C, Al Adhami MJ, Khaliq Y, Seguin I, Cameron DW. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluvarix influenza vaccine. Vaccine. 2004 Aug 13;22(23–24):3136–43. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 32.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006 Jun 15;176(12):7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 33.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NC, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999 Apr;37(4):937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genesca M, Rourke T, Li J, Bost K, Chohan B, McChesney MB, Miller CJ. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J Immunol. 2007 Oct 1;179(7):4732–40. doi: 10.4049/jimmunol.179.7.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, MacDonald GH, McCullers JA. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vac Imm. 2006 Sept;13(9):981–990. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beebe DP, Schreiber RD, Cooper NR. Neutralization of influenza virus by normal human sera: mechanisms involving antibody and complement. J Immunol. 1983 Mar;130(3):1317–22. [PubMed] [Google Scholar]
- 37.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of derum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg. 1972 Dec;70(4):767–77. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jegerlehner A, Schmitz N, Storni T, Bachmann ME. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004 May 1;172(9):5598–605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- 39.Dow SW, Fradkin LG, Liggitt DH, Willson AP, Heath TD, Potter TA. Lipid-DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol. 1999 Aug 1;163(3):1552–61. [PubMed] [Google Scholar]
- 40.Wack A, Baudner BC, Hilbert AK, Manini I, Nuti S, Tavarini S, Scheffczik H, Ugozzoli M, Singh M, Kazzaz J, Montomoli E, Del Giudice G, Rappuoli R, O’Hagan DT. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008 Jan 24;26(4):552–61. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 41.Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K, Iacuzio D, Wolff M. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998 May 14;338(20):1405–12. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 42.Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, Holden-Wiltse J, Liang H, Gilbert A, Cox M. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007 Apr 11;297(14):1577–82. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- 43.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006 Sep 1;177(5):2888–98. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 44.Mozdzanowska K, Furchner M, Zharikova D, Feng J, Gerhard W. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J Virol. 2005 May;79(10):5943–51. doi: 10.1128/JVI.79.10.5943-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bender BS, Croghan T, Zhang L, Small PA. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992 Apr1;175(4):1143–5. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997 Dec 15;186(12):2063–8. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darrah PA, Patel DT, DeLuca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Anderson P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 Jul;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 48.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008 Apr;8(4):247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 49.Thomas PG, Brown S, Wen Y, So J, Webby R, Doherty P. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2764–9. doi: 10.1073/pnas.0511185103. [DOI] [PMC free article] [PubMed] [Google Scholar]