Abstract
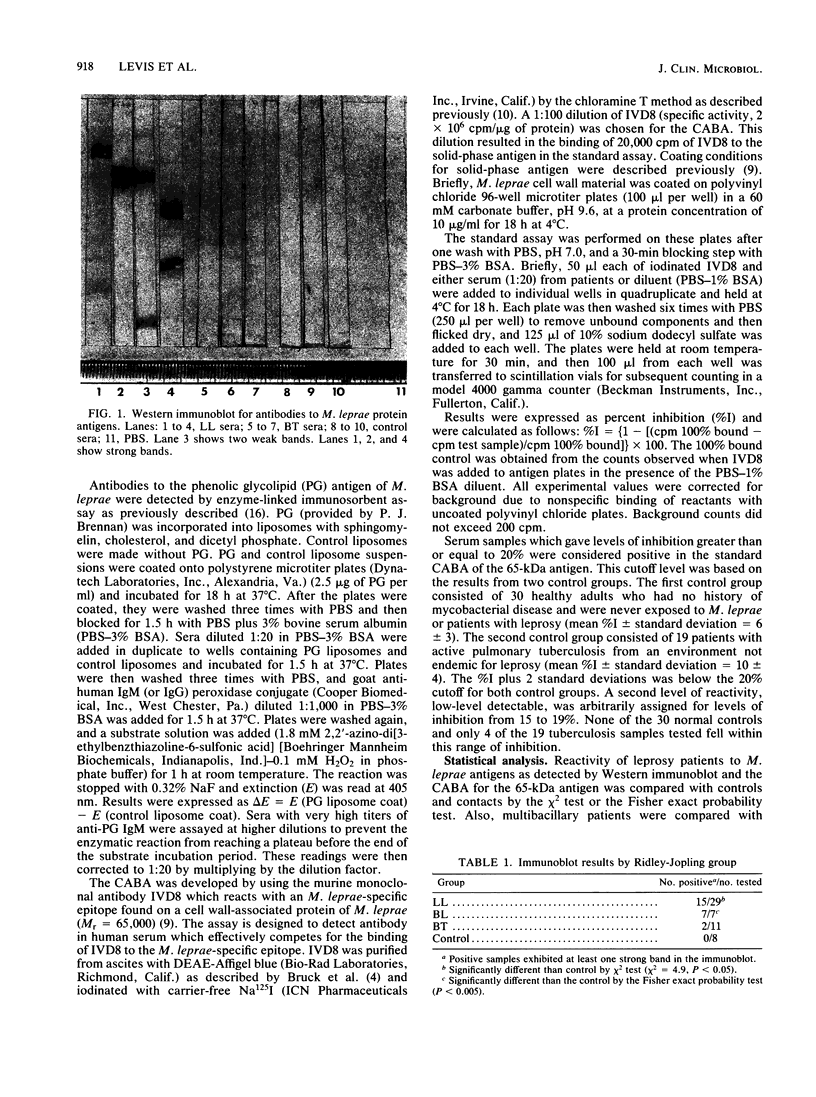
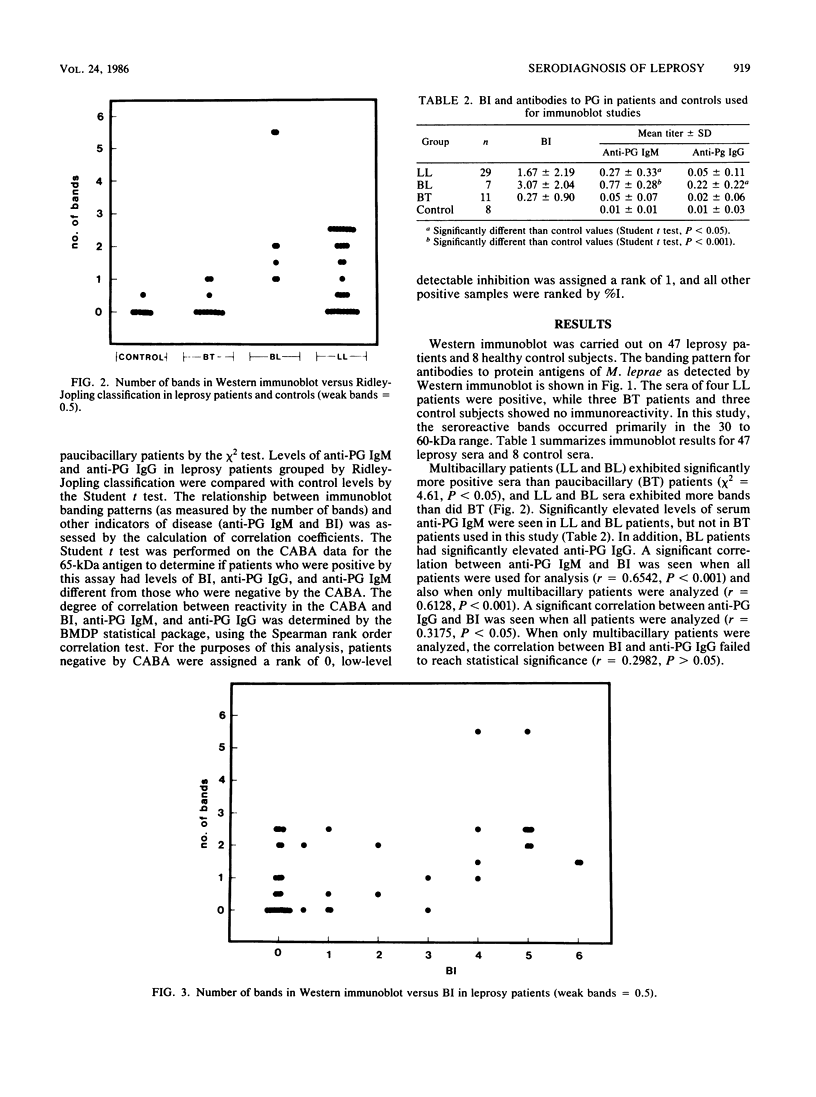
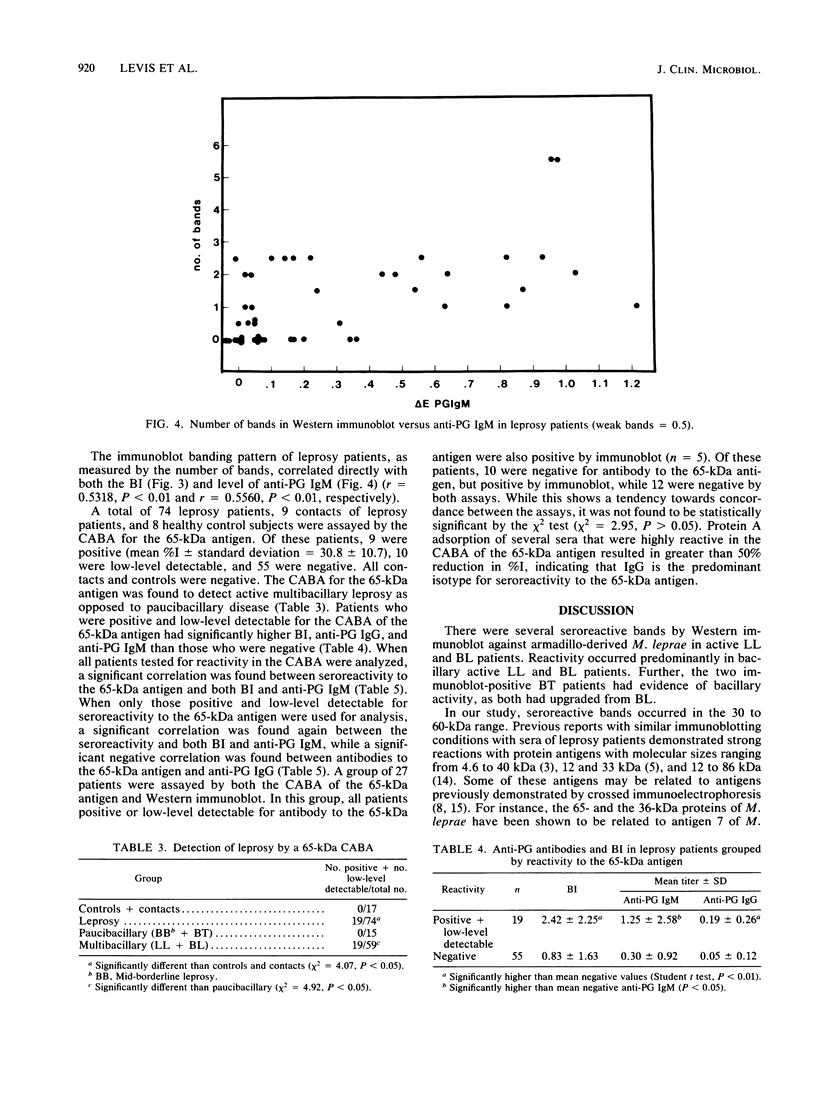
Sera from leprosy patients and controls were assayed for immunoglobulin M (IgM) and IgG antibodies to the Mycobacterium leprae-specific phenolic glycolipid I antigen (PG) by enzyme-linked immunosorbent assay, for IgG antibodies to M. leprae protein antigens by Western immunoblot, and for antibodies to a 65-kilodalton (kDa) protein antigen of M. leprae by a competition antibody binding assay. Elevated levels of anti-PG IgM were seen in lepromatous and borderline lepromatous patients, and elevated levels of anti-PG IgG were seen in borderline lepromatous patients. There was a significant correlation between the bacillary index (BI) and anti-PG IgM whether all leprosy patients or only multibacillary patients were analyzed. A significant correlation was seen between anti-PG IgG and BI when all leprosy patients were used for analysis, but not when only multibacillary patients were used. IgG antibodies to protein antigens of M. leprae, as detected by Western immunoblot, were more prevalent in lepromatous and borderline lepromatous patients than in borderline tuberculoid patients, while one of eight controls showed one weak band. There were significant correlations between the number of M. leprae protein antigens detected by the sera of patients and both BI and the level of anti-PG IgM. The 65-kDa competition antibody binding assay detected active multibacillary leprosy. Patients positive for antibody to the 65-kDa antigen had a significantly higher BI and levels of anti-PG IgM and anti-PG IgG than did patients that were negative. In addition, the level of antibody to the 65-kDa antigen correlated with both the BI and anti-PG IgM. We conclude that testing for antibodies to protein antigens of M. leprae may provide a useful adjunct to testing for antibodies to PG.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Draper P., Payne S. N., Rees R. J. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin Exp Immunol. 1983 May;52(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Raison R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J Immunol. 1985 Dec;135(6):4171–4177. [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. K., Maire M. A., Lambert P. H. SDS-PAGE analysis of M. leprae protein antigens reacting with antibodies from sera from lepromatous patients and infected armadillos. Clin Exp Immunol. 1982 Sep;49(3):523–531. [PMC free article] [PubMed] [Google Scholar]
- Cho S. N., Fujiwara T., Hunter S. W., Rea T. H., Gelber R. H., Brennan P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-beta-D-glucopyranosyl epitope for the serodiagnosis of leprosy. J Infect Dis. 1984 Sep;150(3):311–322. doi: 10.1093/infdis/150.3.311. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Yanagihara D. L., Hunter S. W., Gelber R. H., Brennan P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983 Sep;41(3):1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Mshana R. N., Harboe M. Antigenic analysis of Mycobacterium leprae. Scand J Immunol. 1979 Mar;9(3):297–302. doi: 10.1111/j.1365-3083.1979.tb02735.x. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatser P. R., van Rens M. M., Eggelte T. A. Immunochemical characterization of Mycobacterium leprae antigens by the SDS-polyacrylamide gel electrophoresis immunoperoxidase technique (SGIP) using patients' sera. Clin Exp Immunol. 1984 Jun;56(3):537–544. [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Stanford J. L., Walsh G. P. Studies of mycobacterial antigens, with special reference to Mycobacterium leprae. Infect Immun. 1976 Apr;13(4):1132–1138. doi: 10.1128/iai.13.4.1132-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis W. R., Meeker H. C., Schuller-Levis G., Sersen E., Schwerer B. IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium leprae in leprosy: insight into patient monitoring, erythema nodosum leprosum, and bacillary persistence. J Invest Dermatol. 1986 May;86(5):529–534. doi: 10.1111/1523-1747.ep12354963. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Schwerer B., Meeker H. C., Sersen G., Levis W. R. IgM antibodies against phenolic glycolipid I from Mycobacterium leprae in leprosy sera: relationship to bacterial index and erythema nodosum leprosum. Acta Leprol. 1984 Oct-Dec;2(2-4):394–402. [PubMed] [Google Scholar]
- Young D. B., Buchanan T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science. 1983 Sep 9;221(4615):1057–1059. doi: 10.1126/science.6348948. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]



