Abstract
Magnetoencephalographic responses recorded from auditory cortex evoked by brief and rapidly successive stimuli differed between adults with poor vs. good reading abilities in four important ways. First, the response amplitude evoked by short-duration acoustic stimuli was stronger in the post-stimulus time range of 150–200 ms in poor readers than in normal readers. Second, response amplitude to rapidly successive and brief stimuli that were identical or that differed significantly in frequency were substantially weaker in poor readers compared with controls, for interstimulus intervals of 100 or 200 ms, but not for an interstimulus interval of 500 ms. Third, this neurological deficit closely paralleled subjects’ ability to distinguish between and to reconstruct the order of presentation of those stimulus sequences. Fourth, the average distributed response coherence evoked by rapidly successive stimuli was significantly weaker in the β- and γ-band frequency ranges (20–60 Hz) in poor readers, compared with controls. These results provide direct electrophysiological evidence supporting the hypothesis that reading disabilities are correlated with the abnormal neural representation of brief and rapidly successive sensory inputs, manifested in this study at the entry level of the cortical auditory/aural speech representational system(s).
Keywords: dyslexia, specific language impairment, auditory cortex, magnetoencephalography, electroencephalography
The specific nature of the neurological deficits that underlie language learning impairments and reading impairment has been a subject of intense debate (for review, see refs. 1–5). On the one hand, it has been argued that at least the substantial majority of individuals with language and reading impairments have measurable deficits in their accurate reception of rapidly changing and rapidly successive acoustic inputs (4–13), manifested by abnormal detection, discrimination, or recognition of stimuli occurring in rapid succession (11, 14, 15). Many young children with such early specific language impairments have difficulties in reading initiation (16–18); it has been argued that these children are largely synonymous with the population later identified as reading-impaired. By this controversial view, failure to successfully initiate reading has been described as a “bottom-up” problem. Because of the poor resolution of the fine structure in speech, a relatively weak, unreliable, and abnormally context-dependent processing of sounds emerges through learning—with later consequent difficulties in mastering the sound-to-letter correspondences that underlie facile reading.
On the other hand, it has been argued that because there is no simple relationship between the structure of acoustic inputs and the ability to process phonological inputs, and because most dyslexic individuals ultimately develop facile speech production and aural speech reception abilities, the relationship of non-speech acoustic deficits to language impairments and to reading impairments are at best uncertain (refs. 19–21; for review, see also refs. 2–5 and 16). Failure to successfully initiate and master reading, by this perspective, is more likely attributable to a specific cognitive or higher level performance deficit, for example, to a weakness in a top-down ability to parse words into their sound parts (“phonological awareness”), or to deficits in attention, memory, grammar, or other higher-level aspects of language processing (22–24).
The present study was designed to further investigate the acoustic processing abilities of adults who are poor readers, by using psychophysical and physiological methods. Specifically, it was designed to determine whether there are differences in poor-reading vs. normal individuals in their fundamental cortical processing of brief and rapidly successive acoustic inputs when compared with normal reading adults. Magnetoencephalographic (MEG) recordings were employed to assay cortical responses because they enabled us to track, with fine temporal resolution, evoked neuronal responses that could be localized to the primary auditory cortex and its immediate environs, i.e., to the entry level of cortical auditory/aural speech processing system(s).
METHODS
Subjects.
Informed consent was obtained from all subjects in the study. In an experimental group of poor readers, seven adult subjects (two males, five females, ages 18–42 years) (i) performed poorly on standardized reading tests (25) of words (mean ± SEM = 83 ± 4) or non-words (86 ± 7) and (ii) had poorer than normal performances [<90% accuracy at short interstimulus intervals (ISIs)] on a variety of psychophysical tasks measuring perceptual interference between rapidly successive stimuli including a temporal ordering task that was performed while MEG responses were being recorded (see Fig. 1A). Seven subjects in a control group (two males, five females, ages 23–41 years) (i) had normal reading scores on standardized reading tests of words (107 ± 2) and non-words (110 ± 2) with no overlap in reading scores between control and reading impaired populations and (ii) performed the task of temporal ordering of tone pairs with few errors (>95% accuracy at 100- or 200-ms ISIs).
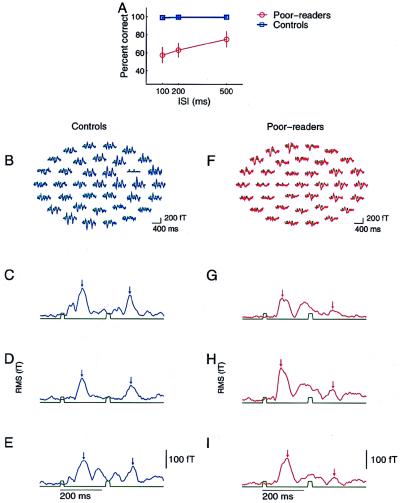
Figure 1.
(A) Behavioral performance at a task in which subjects had to identify and temporally order rapidly successive brief (20-ms duration) tonal stimuli that in some trials did, and in other trials did not, differ in frequency. Performance was defined during MEG recording sessions conducted for poor readers and controls (10–12). The normal readers in the control group performed this task with almost no errors. Poor readers (see Methods for subject selection) made many errors, even at long (500-ms) ISIs. All poor readers performed better at longer than at shorter ISIs. (B) Examples of evoked magnetic responses recorded in the 37 channels centered on left hemisphere auditory cortex, averaged from ≈100 artifact-free presentation of stimulus-pairs at 200-ms ISI in a control subject. The timing of stimulus events is indicated in green. (C–E) Examples of rms waveforms computed across sensors at each time sample for three different control subjects, for tonal stimulus pairs presented with 200-ms ISIs. Stimulus events are indicated in green. Small vertical arrows mark the 100-ms poststimulus time (the time of occurrence of the expected evoked M100 response) for the initial and the second stimulus of the pair. (F) Example of evoked magnetic field response for a 200-ms ISI condition for a poor-reading subject. Whereas a normal-strength response was evoked by the first stimulus, the second rapidly successive stimulus evoked only a weak response. (G–I) Example rms waveforms from three poor-reading subjects.
Three additional selection criteria were applied to all experimental and control subjects. (i) All had normal detection and discrimination thresholds for 250- to 400-ms tones at frequencies of 800, 1,000, and 1,200 Hz, i.e., across the frequency range relevant to the behavioral task; (ii) all subjects included in these MEG comparison populations had evoked magnetic field responses both to long and brief tonal stimuli that had a signal-to-noise-ratio >5 dB (here, noise refers to the average prestimulus sensor mean data); and (iii) the evoked magnetic field responses to both long and brief isolated tones could be accurately spatially localized (model vs. data correlation > 0.95) in all subjects by a single-equivalent current dipole model to within the known variability of the location of the primary auditory cortex. Three poor readers and two control subjects were excluded from experimental and control population samples on the basis of these latter two criteria. Their exclusion eliminated the possibility that population differences could be affected by significant differences in cortical anatomy.
Subjects from experimental and control groups were matched for age and gender and exhibited normal hearing and normal responses recorded in the MEG as described in the text. Although all of our subjects were in the range of normal intelligence, we chose not to match experimental and control populations with respect to nonverbal IQ because IQ–reading discrepancies increase with age into adulthood and because there is a growing consensus that an IQ–reading discrepancy is an invalid basis for defining reading impairment (26–28). At the same time, extensive parallel measures of auditory signal reception, IQ, and reading abilities in a population of 106 adult subjects that included these poor and normal readers have shown that reading ability is strongly correlated with auditory reception abilities. This correlation is not accounted for by variation in IQ (M. Ahissar, A. Protopapas, and M.M.M., unpublished data).
Recordings, Stimuli, and Task.
During performance on the temporal ordering task, subjects lay on their right side with their eyes closed, while concurrent MEG recording was achieved. All MEG responses reported here were recorded by using a 37-channel biomagnetometer device (Biomagnetic Technology, San Diego) centered over the lateral fissure at the level of primary auditory cortex of the left hemisphere. The sensor array was positioned over the left temporal area contralateral to the stimulus presentation; to optimally record a response elicited by a 400-ms, 1-kHz reference tone presented at approximately 90 dB sound pressure levels. After optimal placement of the sensors, epochs of 1.5 s (500-ms pretrigger) were recorded for each trial of a temporal recognition/ordering task. Trials were triggered on the first stimulus of each pair, and 100 trials at each ISI were averaged to obtain an evoked response. Trials at each of the three ISIs were randomly interleaved, and averaging of artifact-free trials was performed post hoc. The duration between trials was approximately 2 s and was randomly jittered by 400 ms. Sequential trial presentations were triggered with time reference to the response of the subject to a preceding trial. The sampling rate was 297.8 Hz per channel.
Subjects were presented with pairs of stimuli, each 20 ms in duration including 5 ms on/off ramps, at each of three different ISIs (100, 200, and 500 ms). Each stimulus of a pair was at 68 dB SPL and either 800 Hz (low tone) or 1,200 Hz (high tone) and presented to both ears via headphones. Subjects signaled which of four possible tone pair sequences (high–high, high–low, low–high, or low–low) was presented by pressing buttons strapped to their thighs. Behavioral responses were stored in a computer. Feedback was provided at the end of each trial by a light tap on the index finger of the right hand signaling incorrect responses. The percentages of correct trials were measured at each ISI to obtain the psychometric functions shown in Fig. 1A. Stimulus-pair combinations were randomized across trials for each ISI condition.
Data Analysis.
Because the magnetic field sensors could be located at different positions in different subjects across different sessions, three spatially invariant response measures were computed to facilitate comparisons across subjects.
First, we obtained the rms waveform for each stimulus condition computed across all sensors at each time point. Jackknife procedures were used to estimate the errors of the mean across subjects at each time point (29). To examine differences in evoked responses between reading impaired adults and controls as a function of time, two-way ANOVA with time and group as factors was performed on 90-ms sliding and nonoverlapping epochs of the rms response.
Second, to examine the average cross-sensor coherence in the evoked response, spatial-frequency singular-value decomposition analysis methods were used. This analysis allows characterization of the average coherence across all channels, rather than examining pairwise coherence (30). In this method, the time-domain magnetic field data across all sensors was first projected into a local time-frequency domain by filtering through a bank of ortho-normal discrete prolate spheroidal sequence functions that were parameterized by a time-bandwidth factor. Performing a singular-value decomposition on this projection matrix at each frequency, a measure of coherence was then given by the ratio of the power in the leading eigenmode to the total power. A complete coherent response at each frequency, C(f), is equal to unity at all frequencies and for a uniform random process, C(f) ≈ 1/K, where K is the number of degrees of freedom in the spectral estimation procedure (29). For statistical purposes, magnitude coherence was transformed by using a hyperbolic tangent operator, Q(f) =  tanh−1 (C(F)), where NW is the time-bandwidth factor, which was chosen to be seven for all calculations. This transformed average cross-sensor coherence was computed over a 750-ms time window following the first stimulus of pair. Jackknife procedures were used to estimate the variance of the mean coherence. Differences in this average coherence as a function of frequency between reading-impaired adults and controls were analyzed by using a two-way ANOVA with frequency and group as factors. Analysis was performed over 5 Hz sliding and nonoverlapping frequency ranges.
tanh−1 (C(F)), where NW is the time-bandwidth factor, which was chosen to be seven for all calculations. This transformed average cross-sensor coherence was computed over a 750-ms time window following the first stimulus of pair. Jackknife procedures were used to estimate the variance of the mean coherence. Differences in this average coherence as a function of frequency between reading-impaired adults and controls were analyzed by using a two-way ANOVA with frequency and group as factors. Analysis was performed over 5 Hz sliding and nonoverlapping frequency ranges.
Finally, for each subject, a spherical model was fit to their digitized head shape, and the location, orientation, and amplitude of a single equivalent current dipole tangential to the surface of the model sphere were estimated for each point in time from the average evoked response filtered between 1 and 20 Hz. The origin of the coordinate system used was the midpoint between the pre-auricular points; the x axis (antero-posterior) joined the origin to the nasion. The y axis (latero-medial) passed between the pre-auricular points; the z axis (infero-superior) was perpendicular to the x–y plane. The M100 response latency was defined as the time of occurrence of the maximum rms magnetic field amplitude in the 90–130 ms poststimulus time window after each stimulus, subject to a model data correlation greater than 0.95. Locations of estimated dipoles of the M100 response were verified, for each subject, to lie within bounds of normal variability in the locations of auditory cortex (31–33). Unpaired t tests were performed to compare the M100 response locations, latencies, M100 rms and M100-∥Q∥ (see Table 1).
Table 1.
M100 response latencies, amplitudes, and dipole strengths
| Tone | ISI, ms | M100 latency, ms
|
M100 rms, fT
|
M100 ∥Q∥, nA-m
|
|||
|---|---|---|---|---|---|---|---|
| Poor readers | Good readers | Poor readers | Good readers | Poor readers | Good readers | ||
| First 20-ms | 500 | 106 ± 8 | 96 ± 5 | 116 ± 8 | 130 ± 16 | 35 ± 7 | 47 ± 9 |
| Second 20-ms | 500 | 89 ± 9 | 109 ± 10 | 59 ± 9 | 69 ± 7 | 31 ± 6 | 21 ± 13 |
| Second 20-ms | 200 | 107 ± 7 | 115 ± 12 | 79 ± 9* | 110 ± 13 | 20 ± 3* | 34 ± 6 |
Model-independent (rms) and model-dependent (∥Q∥) amplitudes are shown for the different conditions. These data were obtained from the averaged evoked response in each sensor filtered between 0 and 20 Hz, for each ISI condition. In spite of the interference between the responses to each stimulus in the 200-ms ISI condition, significant differences were observed between the M100 responses after the second stimuli. All of these single-equivalent current dipoles were localized to within primary auditory cortex and its immediate environs, and the locations were neither significantly different across these different conditions nor between poor and good readers 4.0 ± 0.3 cm antero-posterior, 5.0 ± 0.1 cm latero-medial, 5.8 ± 0.2 cm inferio-superior, see Methods for coordinate system). Note that there were also no significant differences in M100 latencies. Values shown are mean ± SEM. ∗, P < 0.05.
RESULTS
Behavioral data for the temporal ordering task performed during MEG recordings are shown in Fig. 1A for both experimental and control subjects. Subjects in the control group performed this task with almost no errors. Subjects in our experimental group performed poorly at shorter ISIs and above threshold at a longer ISI of 500 ms, although even at the longest ISI, they did not perform as well as control subjects. These data demonstrate the behavioral auditory processing deficits observed in these experimental subjects.
Representative evoked magnetic field responses to brief (20-ms) and rapidly successive stimuli recorded in all 37 channels of the sensor array are shown for a poor reader and a control subject in Fig. 1 B and F. In this control subject (Fig. 1B), many channels with a strong evoked response to a first stimulus, peaking ≈100 ms after the stimulus onset, also responded strongly to a second short-duration stimulus occurring 200 ms later. However, in this poor reader (Fig. 1F), although a clear response was elicited by the first stimulus in many channels, only a weak response was elicited by a second stimulus occurring 200 ms later.
To visualize and facilitate the comparison of responses independent of the position of individual sensors in the MEG recording array, rms waveforms were computed across the 37 magnetic recording channels at each time point for each ISI condition. Examples of rms waveforms elicited by successive and brief stimuli separated by 200 ms in three representative subjects from experimental and control groups are illustrated in Fig. 1 C–E and G–I, respectively. Two differences between experimental and control subjects were immediately evident in these representative examples. First, the amplitudes of responses in the 150- to 200-ms period after a stimulus were generally stronger in experimental subjects than in controls. Second, the responses evoked by a second brief stimulus (presented in these examples with an ISI of 200 ms) were lower in experimental subjects than in controls.
Averaged rms and rms-difference responses to successive brief stimuli expressed across the population in the two groups are shown in Fig. 2. rms responses for stimuli separated by 500 ms are shown in Fig. 2A, where the first 500 ms of poststimulus time represents the responses evoked by the application of a single (the initial) stimulus. Response peaks at ≈100 ms after a stimulus are commonly referred to as the “M100 response,” which in MEG recordings could be localized to the primary auditory cortex and its environs (32) (see Table 1).
Figure 2.
Mean rms and rms-difference waveforms recorded from good-reader (thick blue lines) and poor-reader (thin red lines) subject groups. Error bars indicate jackknife error estimates of the mean across subjects at each time sample. For clarity, error bars (one-sided) are shown at every fifth time point. Stimulus events are indicated in green. Stars indicate epochs, computed over 90-ms nonoverlapping sliding time windows, that were statistically significant at P < 0.0001. (A) rms waveforms for stimulus pair with 500-ms ISIs. (B and C) rms difference waveforms for stimulus pairs with 100 (B) and 200 (C) ms ISIs. These rms differences were computed for each subject by subtracting the rms for the 500-ms ISI condition from the rms waveforms in the 100- and 200-ms ISI conditions over the first 500-ms poststimulus period. These waveforms reflect the sole contribution of the second stimulus to the response. Stars indicate the center of 90-ms-epoch windows in which differences in the responses to the second stimulus were statistically significant between poor readers vs. good readers at P < 0.0001.
M100 responses for each stimulus of a pair were not significantly different between controls and experimental subjects at an ISI of 500 ms (P > 0.05; Fig. 2A and Table 1). However, as indicated by the representative examples shown earlier, significant differences were observed over a time window of 125–250 ms in the evoked response to isolated tonal stimuli between experimental and control populations (P < 0.0001, see Fig. 2A). These later MEG responses could also be localized to auditory cortex, but as in earlier studies, with progressively weaker reliability (33, 34).
In contrast to the 500-ms ISI condition, significant differences in the M100 response to a second brief stimulus were found for the 200-ms ISI condition (P < 0.05; see Table 1). However, responses evoked by a second stimulus occurring at 200 ms ISI and at lower ISIs were complexly summed with the ongoing responses to the first stimulus. To overcome that problem, rms difference functions were created for each experimental and control subject by subtracting rms responses evoked by a single stimulus from the response evoked by a stimulus pair. This analysis for 200-ms and 100-ms ISI conditions revealed highly significant differences between the experimental and control groups, in the M100 response to the second stimulus, contributed solely by the second stimulus (P < 0.0001, Fig. 2 B and C).
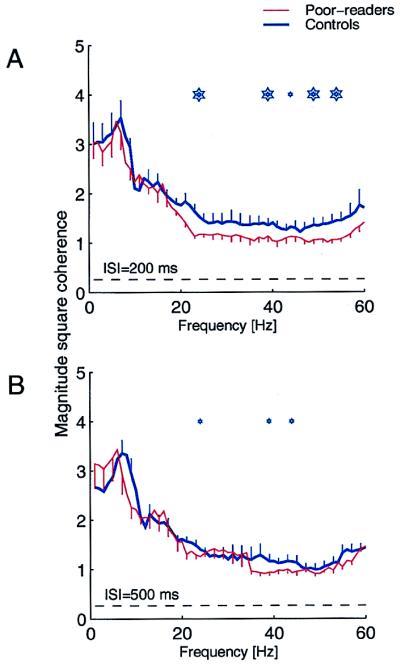
In addition to the differences in the time-domain response dynamics and M100 responses to a second stimulus delivered at ISI of 100 and 200 ms, significantly weaker cross-sensor response coherence also was recorded in the experimental group when compared with controls (Fig. 3A, P < 0.001). Distributed response coherence population differences were much lower when successive stimulus events were presented at a longer ISI of 500 ms (Fig. 3B).
Figure 3.
Average transformed magnitude coherence across sensors expressed over a 750-ms period after the first stimulus of a pair in good readers (thick blue) and poor readers (thin red), at 200 ms (A) and 500 ms (B) ISIs. Mean ± one-sided jackknife errors are shown. The dashed line indicates the coherence obtained by random chance. Stars indicate the center of 5-Hz nonoverlapping sliding frequency windows in which coherence is statistically significant (for small stars P < 0.01, and large stars, P < 0.001). Significant differences were observed in β- and γ-band ranges (20–60 Hz).
DISCUSSION
This study in adults who are poor readers directly demonstrates differences in evoked responses originating from the primary auditory cortex and its immediate environs that correlate with concurrently measured behavioral deficits in the individuation and discrimination of successively occurring stimuli. It demonstrates that in these individuals, there are fundamentally different cortical response dynamics generated by brief stimuli, along with substantially weaker cortical responses to rapidly successive stimuli across the same time-scale over which these individuals exhibited degradation in detection, recognition, and discrimination of rapidly successive simple and complex acoustic stimuli.
How do the three aspects of neurological response difference recorded in this study relate to one another? An initial stimulus event appears to generate stronger than normal poststimulus inhibition. Hypothetically, with deeper, prolonged suppression or inhibition, the recovery time governing the capacity of the cortex to respond to a rapidly following input event is lengthened. Along with smaller distributed neural activation evoked by any brief event within a >200-ms time window after the first event, there is a large difference in the distributed response coherence marking the salient features, (e.g., the intrasyllabic sound parts of words) of a multicomponent stimulus in this time domain. Clearly, these findings directly relate to experiments on temporal integration of brief and successive stimuli and to “sensory memory trace” experiments which, to date, have been conducted only in normal subjects (35–38).
The average sensor array coherence reported in this study is a sensor-position independent measure of distributed response coherence(30) that reflects the degree of response synchrony in the underlying neuronal generators of A-1 and its environs. In studies conducted in animal models, it has been shown that complex acoustic events are represented in primary auditory cortex by distributed, strongly temporally coordinated (“coherent”) populations of excited neurons (39). Our observation of weaker β- and γ-band coherence for shorter (but not for longer) ISIs in experimental subjects is consistent with the hypothesis that coherence in this frequency range contributes to stimulus discrimination and recognition, and to figure–ground (signal–noise) stimulus distinctions (40), which appear to be degraded in this subject population (41). Interestingly, in contrast to earlier studies that have reported clear peaks within the γ-band in the spectra of individual channels in the range of 20–40 Hz (42), the coherence recorded in this study did not exhibit a peak in the β/γ-range frequency, in either poor readers or normal controls.
Several behavioral studies on reading-impaired adults and children have revealed a significant correlation between reading ability and performance in successive auditory signal reception. Indeed, in the population of poor readers drawn from the general population in the present study, the great majority had abnormal perceptual processing of brief, rapidly successive stimuli. Subjects identified as reading impaired in these studies presumably fall on the tail of a continuous distribution of reading-ability and correlated auditory signal processing abilities for processing successive signals (43). Additional studies with larger populations of subjects are required to determine whether the physiological responses to successive events also are correlated with reading and auditory processing abilities across the wider human population distribution, as indicated by the earlier psychophysical studies and these initial MEG imaging studies. It should be noted that we excluded a very small minority of poor readers whose performance on a battery of auditory psychophysical tests assaying abilities to distinguish between, recognize, and sequence rapidly successive acoustic inputs appeared to be normal. We believe that the neurology of such a class of subjects, which appears to represent <5–10% of poor-reading adults, is very different than that of the larger, main population of reading-impaired individuals.
Although reported differences in successive signal processing are presumably not unequivocally limited to our experimental and control subject population comparisons, several electroencephalography, brain imaging, and behavioral studies have described phenomenology that relate to the current observations. McAnally and Stein (44) have shown that frequency-following far-field potential responses evoked by modulated acoustic stimuli are weaker in dyslexic than in normal adults. Witton and colleagues (13) have shown that there is a specific deficit in acoustic frequency-modulation rate discrimination at low modulation frequencies that may well reflect a weakness in response to rapidly successive acoustic stimulus events relevant for speech feature representation. Neville and colleagues (45) have shown that there are differences in the early and late responses to brief auditory stimuli presented at short ISIs in language-learning impaired children who perform poorly in an acoustic temporal ordering task. Several investigators have shown differences in spontaneous β- and γ-band power in the electroencephalography signals between adults and children with reading-impairment compared with normals (46, 47). Kraus and colleagues (48) have shown that there are differences in “mismatch negativity” responses 300–600 ms poststimulus, in language-impaired children. Mismatch negativity responses correlated with the ability of those children to differentiate phonetic stimuli, and mismatch negativity was weak or absent in subjects in which phonetic distinctions were not accurately made. Although the experimental paradigms of the present study differ significantly from these mismatch negativity studies, we have also observed differences in the responses evoked ≈400–700 ms after the first stimulus for short ISIs of 100 and 200 ms (P < 0.0001, data not shown), consistent with the findings of Kraus and colleagues as well as those of other investigators who have shown abnormal late response effects that relate directly to the accuracy of aural speech reception in reading-impaired adults (49–53). It should be noted that although earlier events in the evoked responses recorded in our study could be localized to auditory cortex, these later poststimulus time responses could not be reliably localized to a specific source modeled as a single-equivalent current dipole.
Two other MEG studies and an electroencephalography study of visual processing in reading-impaired adults have also provided evidence that responses evoked in both the visual and “speech” cortical domains have longer latencies than in normal individuals (54–56). These large differences in signal timing may relate to the prolonged response recovery times recorded in successive signal presentation in the current study. A number of other studies have argued that reading-impaired adults commonly also have deficits in visual high-speed signal discrimination and recognition (57–60). It remains to be determined whether these visual deficits ontogenetically precede, parallel, or follow closely related auditory perceptual deficits now known to emerge in infants in the first year of life (61). It also remains to be determined how deficits associated with early reading failure relate to persistent expressions of deficits recorded in adults. It should be noted that acoustic-processing deficits and the neurological “deficits” recorded here comprise only a part of the complex abnormal neurology of reading-impaired individuals, and that a direct linkage of the neurological deficit described here to the origins of or to the expressions of poor reading ability remains to be established.
CONCLUSION
This study provides further evidence that most reading-impaired individuals have an enduring “deficit” in their cortical processing of brief and rapidly successive inputs, paralleled by a fundamental difference in the fidelity of the processing of detailed features of rapidly successive and rapidly changing acoustic inputs. When coupled with related psychophysical and electrophysiological studies, these data clearly suggest that this abnormal signal-processing “problem,” in at least most reading-impaired adults, is lifelong. We emphasize that this demonstration of a cortical entry-level impairment does not rule out the contribution of “top-down” effects on receptive deficits. Differences could potentially arise via top-down effects either from multiple cognitive consequences derived from abnormal language learning and usage or from fundamental deficiencies in attentional or memory resources critical for language development and processing. Nevertheless, because the auditory cortex represents a main gateway for acoustic information entry into the aural speech representational system, these findings strongly suggest that acoustic reception in reading-impaired adults develops with fundamental processing and learning-derived representational forms of complex acoustic inputs like speech that differ substantially from normals. Such representational differences could have widespread consequences for speech and language learning, representation, and usage and for subsequent phonological-to-orthographic symbol representation in reading.
Acknowledgments
Susanne Honma and Mary Mantle provided excellent technical assistance for these experiments. Drs. Athanassios Protopapas, Merav Ahissar, and Miriam Reid assisted with subject screening. We thank Drs. Bret Petersen and Srividya Sundaresan for reading previous versions of this manuscript. This research was supported by National Institutes of Health Grant NS-10414, Office of Naval Research Grant N00014-96-10206, Hearing Research Inc., and the Coleman fund. S.N. was supported by a National Research Service Award Fellowship F32-DC00285. H.M. was supported by a Howard Hughes Medical Institute predoctoral fellowship.
ABBREVIATIONS
- ISI
interstimulus interval
- MEG
magnetoencephalography
References
- 1.Bishop D V M. J Child Psychol Psychiatry. 1992;33:2–66. doi: 10.1111/j.1469-7610.1992.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 2.Leonard L B. Children with Specific Language Impairment. Cambridge, Mass.: MIT Press; 1998. [Google Scholar]
- 3.Reid L G. Ann Dyslexia. 1995;45:3–27. [Google Scholar]
- 4.Farmer M E, Klein R M. Psychonom Bull Rev. 1995;2:460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- 5.Tallal P, Galaburda A M, Llinas R R, von Euler C, editors. Temporal Information Processing in the Nervous System: Special Reference to Dyslexia and Dysplasia. New York: N.Y. Acad. Sci.; 1993. [PubMed] [Google Scholar]
- 6.Tallal P, Piercy M. Neuropsychologia. 1973;11:389–398. doi: 10.1016/0028-3932(73)90025-0. [DOI] [PubMed] [Google Scholar]
- 7.Tallal P. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- 8.Tallal P, Stark R E, Mellits D. Neuropsychologica. 1985;23:527–534. doi: 10.1016/0028-3932(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 9.Hari R, Kiesila P. Neurosci Lett. 1996;205:138–140. doi: 10.1016/0304-3940(96)12393-4. [DOI] [PubMed] [Google Scholar]
- 10.Harel S, Nachson I. Percept Mot Skills. 1997;84:467–473. doi: 10.2466/pms.1997.84.2.467. [DOI] [PubMed] [Google Scholar]
- 11.Protopapas A, Ahissar M, Merzenich M M. J Acoust Soc Am. 1997;102:3188. [Google Scholar]
- 12.Reed M A. J Exp Child Psychol. 1989;48:270–292. doi: 10.1016/0022-0965(89)90006-4. [DOI] [PubMed] [Google Scholar]
- 13.Witton C, Talcott J B, Hansen P C, Richardson A J, Griffiths T D, Rees A, Stein J F, Green G G R. Curr Biol. 1998;8:791–797. doi: 10.1016/s0960-9822(98)70320-3. [DOI] [PubMed] [Google Scholar]
- 14.Wright B A, Lombardino L J, King W M, Puranik C S, Leonard C M, Merzenich M M. Nature (London) 1997;387:176–178. doi: 10.1038/387176a0. [DOI] [PubMed] [Google Scholar]
- 15.Tallal P, Piercy M. Nature (London) 1973;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- 16.Bishop D V M, Adams C. J Child Psychol Psychiatry. 1990;31:1027–1050. doi: 10.1111/j.1469-7610.1990.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 17.Aram D M, Ekelman B L, Nation J E. J Speech Hearing Res. 1984;27:232–244. doi: 10.1044/jshr.2702.244. [DOI] [PubMed] [Google Scholar]
- 18.Silva P A, Williams S, McGee R. Dev Med Child Neurol. 1987;29:630–640. doi: 10.1111/j.1469-8749.1987.tb08505.x. [DOI] [PubMed] [Google Scholar]
- 19.Studdert-Kennedy M, Mody M. Psychonom Bull Rev. 1995;2:508–514. doi: 10.3758/BF03210986. [DOI] [PubMed] [Google Scholar]
- 20.Watson B U, Miller T K. J Speech Hearing Res. 1993;36:850–863. doi: 10.1044/jshr.3604.850. [DOI] [PubMed] [Google Scholar]
- 21.Mody M, Studdert-Kennedy M, Brady S. J Exp Child Psychol. 1997;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- 22.Snowling M. Dyslexia: A Cognitive Development Perspective. Oxford: Blackwell; 1990. [Google Scholar]
- 23.Swan D, Goswami U. J Exp Child Psychol. 1997;66:18–41. doi: 10.1006/jecp.1997.2375. [DOI] [PubMed] [Google Scholar]
- 24.Wagner R K, Torgesen J K. Psychol Bull. 1987;101:192–212. [Google Scholar]
- 25.Woodcock R. Woodcock Reading Mastery Tests-Revised. Circle Pines, MN: American Guidance Service; 1987. [Google Scholar]
- 26.Stanovich K E. Reading Res Q. 1991;26:7–29. [Google Scholar]
- 27.Siegel L S. J Learn Disabil. 1992;25:618–629. doi: 10.1177/002221949202501001. [DOI] [PubMed] [Google Scholar]
- 28.Shaywitz S E, Shaywitz B A, Pugh K R, Fulbright R K, Constable R T, Mencl W E, Shankweiler D P, Liberman A M, Skudlarski P, Fletcher J M, et al. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson D J, Chave A D. Advances in Spectrum Analysis and Array Processing. Vol. 1. Englewood Cliffs, NJ: Prentice–Hall; 1991. pp. 58–113. [Google Scholar]
- 30.Prechtl J C, Cohen L B, Pesaran B, Mitra P P, Kleinfeld D. Proc Natl Acad Sci USA. 1997;94:7621–7626. doi: 10.1073/pnas.94.14.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantev C, Hoke M, Lehnertz K, Lutkenhoner B, Anogianakis G, Wittkowski W. Electroencephalogr Clin Neurophysiol. 1988;69:160–170. doi: 10.1016/0013-4694(88)90211-8. [DOI] [PubMed] [Google Scholar]
- 32.Reite M, Adams M, Simon J, Teale P, Sheeder J, Richardson D, Grabbe R. Cogn Brain Res. 1994;2:13–20. doi: 10.1016/0926-6410(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 33.Lutkenhoner B, Steinstrater O. Audiol Neurootol. 1998;3:191–213. doi: 10.1159/000013790. [DOI] [PubMed] [Google Scholar]
- 34.Alho K, Winkler I, Escera C, Huotilainen M, Virtanen J, Jaaskelainen I P, Pekkonen E, Ilmoniemi R J. Psychophysiology. 1998;35:211–224. [PubMed] [Google Scholar]
- 35.Loveless N, Hari R, Hamalainen M, Tiihonen J. Electroencephalogr Clin Neurophysiol. 1989;74:217–227. doi: 10.1016/0013-4694(89)90008-4. [DOI] [PubMed] [Google Scholar]
- 36.Loveless N, Levänen S, Jousmäki V, Sams M, Hari R. Electroencephalogr Clin Neurophysiol. 1996;100:220–228. doi: 10.1016/0168-5597(95)00271-5. [DOI] [PubMed] [Google Scholar]
- 37.Lu Z L, Williamson S J, Kaufman L. Science. 1992;258:1668–1670. doi: 10.1126/science.1455246. [DOI] [PubMed] [Google Scholar]
- 38.Sams M, Hari R, Rif J, Knuutila J. J Cogn Neurosci. 1993;5:363–370. doi: 10.1162/jocn.1993.5.3.363. [DOI] [PubMed] [Google Scholar]
- 39.Wang X Q, Merzenich M M, Beitel R, Schreiner C E. J Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- 40.Joliot M, Ribary U, Llinas R. Proc Natl Acad Sci USA. 1994;91:11748–11751. doi: 10.1073/pnas.91.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llinas R. Ann NY Acad Sci. 1993;682:48–56. doi: 10.1111/j.1749-6632.1993.tb22958.x. [DOI] [PubMed] [Google Scholar]
- 42.Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C. Proc Natl Acad Sci USA. 1991;88:8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaywitz S E, Escobar M D, Shaywitz B A, Fletcher J M, Makuch R. N Engl J Med. 1992;326:145–150. doi: 10.1056/NEJM199201163260301. [DOI] [PubMed] [Google Scholar]
- 44.McAnally K I, Stein J F. J Speech Lang Hearing Res. 1997;40:939–945. doi: 10.1044/jslhr.4004.939. [DOI] [PubMed] [Google Scholar]
- 45.Neville H J, Coffey S A, Holcomb P J, Tallal P. J Cogn Neurosci. 1993;5:235–253. doi: 10.1162/jocn.1993.5.2.235. [DOI] [PubMed] [Google Scholar]
- 46.Flynn J M, Deering W, Goldstein M, Rahbar M H. J Learn Disabil. 1992;25:133–141. doi: 10.1177/002221949202500207. [DOI] [PubMed] [Google Scholar]
- 47.Ackerman P T, Dykman R A, Oglesby D M, Newton J E O. J Learn Disabil. 1994;27:619–630. doi: 10.1177/002221949402701002. [DOI] [PubMed] [Google Scholar]
- 48.Kraus N, McGee T J, Carrell T D, Zecker S G, Nicol T G, Koch D B. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- 49.Erez A, Pratt H. Int J Neurosci. 1992;63:247–264. doi: 10.3109/00207459208987200. [DOI] [PubMed] [Google Scholar]
- 50.Johannes S, Mangun G R, Kussmaul C L, Munte T F. Neurosci Lett. 1995;195:183–186. doi: 10.1016/0304-3940(95)11814-d. [DOI] [PubMed] [Google Scholar]
- 51.Ortiz Alonso T, Navarro M, Vila Abad E. Funct Neurol. 1990;5:333–338. [PubMed] [Google Scholar]
- 52.SchulteKorne G, Deimel W, Bartling J, Remschmidt H. NeuroReport. 1998;9:337–340. doi: 10.1097/00001756-199801260-00029. [DOI] [PubMed] [Google Scholar]
- 53.Taylor M J, Keenan N K. Psychophysiology. 1990;27:318–327. doi: 10.1111/j.1469-8986.1990.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 54.Livingstone M S, Rosen G D, Drislane F W, Galaburda A M. Proc Natl Acad Sci USA. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salmelin R, Service E, Kiesila P, Uutela K, Salonen O. Ann Neurol. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- 56.Vanni S, Uusitalo M A, Kiesila P, Hari R. NeuroReport. 1997;8:1939–1942. doi: 10.1097/00001756-199705260-00029. [DOI] [PubMed] [Google Scholar]
- 57.Demb J B, Boynton G M, Heeger D J. J Neurosci. 1998;18:6939–6951. doi: 10.1523/JNEUROSCI.18-17-06939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demb J B, Boynton G M, Best M, Heeger D J. Vision Res. 1998;38:1555–1559. doi: 10.1016/s0042-6989(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 59.Demb J B, Boynton G M, Heeger D J. Proc Natl Acad Sci USA. 1997;94:13363–13366. doi: 10.1073/pnas.94.24.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eden G F, Vanmeter J W, Rumsey J M, Maisog J M, Woods R P, Zeffiro T A. Nature (London) 1996;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- 61.Benasich A A, Curtiss S, Tallal P. J Am Acad Child Adolesc Psychiatry. 1993;32:585–594. doi: 10.1097/00004583-199305000-00015. [DOI] [PubMed] [Google Scholar]