Abstract
Background:
Several recent guidelines recommend assessment of patients with TIA within 24 hours, but it is uncertain how many recurrent strokes occur within 24 hours. It is also unclear whether the ABCD2 risk score reliably identifies recurrences in the first few hours.
Methods:
In a prospective, population-based incidence study of TIA and stroke with complete follow-up (Oxford Vascular Study), we determined the 6-, 12-, and 24-hour risks of recurrent stroke, defined as new neurologic symptoms of sudden onset after initial recovery.
Results:
Of 1,247 first TIA or strokes, 35 had recurrent strokes within 24 hours, all in the same arterial territory. The initial event had recovered prior to the recurrent stroke (i.e., was a TIA) in 25 cases. The 6-, 12-, and 24-hour stroke risks after 488 first TIAs were 1.2% (95% confidence interval [CI]: 0.2–2.2), 2.1% (0.8–3.2), and 5.1% (3.1–7.1), with 42% of all strokes during the 30 days after a first TIA occurring within the first 24 hours. The 12- and 24-hour risks were strongly related to ABCD2 score (p = 0.02 and p = 0.0003). Sixteen (64%) of the 25 cases sought urgent medical attention prior to the recurrent stroke, but none received antiplatelet treatment acutely.
Conclusion:
That about half of all recurrent strokes during the 7 days after a TIA occur in the first 24 hours highlights the need for emergency assessment. That the ABCD2 score is reliable in the hyperacute phase shows that appropriately triaged emergency assessment and treatment are feasible.
GLOSSARY
- A&E
= accident and emergency department;
- CI
= confidence interval;
- FASTER
= Fast Assessment of Stroke and Transient Ischemic Attack to prevent Early Recurrence;
- OXVASC
= Oxford Vascular Study.
The risk of recurrent stroke in the week after a TIA or minor stroke is up to 10%.1-4 About 150,000 suspected TIAs and minor strokes are referred to secondary care for assessment and investigation in England alone each year,5 and rates are similar in the United States.6 These “warning” events provide a short window of opportunity for prevention,7 and in recognition of this, the most recent clinical guidelines all now recommend that high-risk patients with TIA should be assessed within 24 hours of the event.8-12 However, the appropriateness of the new 24-hour recommendation depends on three key issues. First, what is the risk of recurrent stroke within the first few hours, which might be prevented by even more urgent assessment? Second, what proportion of patients who have a stroke during the first 24 hours after a TIA seek medical attention prior to the recurrent stroke? Third, do existing risk scores reliably predict the risk of recurrent stroke in the hyperacute phase after TIA such that very high risk individuals might be triaged for emergency care? The ABCD system, which the guidelines recommend be used to identify high-risk cases,3,13 was derived for prediction of the risk of recurrent stroke at 7 days, and there are no published data on its ability to predict recurrence within the first few hours.
In a previous study of the timing of TIAs in patients presenting with stroke, we found that 44% of TIAs during the previous 14 days occurred on same day as the stroke or on the previous day.7 However, this study was retrospective, with many patients assessed several weeks after the stroke, and was therefore potentially prone to recall bias, no data were available on whether patients had sought medical attention prior to the stroke, and the clinical characteristics necessary to calculate the ABCD2 score were not collected. In the absence of reliable prospectively collected data, we performed the first population-based study of risk of stroke during the 24 hours after TIA, with stratification by the ABCD2 score and by whether patients sought medical attention prior to the stroke.
METHODS
The Oxford Vascular Study (OXVASC) is a population-based study of all stroke and all TIA in 91,105 individuals of all ages registered with 63 general practitioners in Oxfordshire, UK. OXVASC is approved by our local ethics review committee. The study methods have been described elsewhere.14,15 Briefly, multiple overlapping methods of “hot pursuit” were used to achieve near complete ascertainment of all individuals presenting to medical attention with TIA or stroke,14-16 including the following:
A daily (weekdays only), urgent open-access TIA clinic to which participating general practitioners and the local accident and emergency department (A&E) send all individuals with suspected TIA or stroke who they would not normally admit to hospital
Daily assessment of admissions to medical, stroke, neurology, and other relevant wards
Daily searches of the local A&E attendance register
In order to identify any patients missed by hot pursuit, such as patients who presented late, patients who were referred to other services, or patients who were not referred to secondary care, we also performed several types of “cold pursuit”: monthly computerized searches of family doctor diagnostic coding, hospital discharge codes, and all cranial and carotid imaging studies performed in local hospitals, and all death certifications in our study population.
All patients were consented and seen by study physicians as soon as possible after their initial presentation. All patients presenting with stroke were asked about symptoms of TIA during the previous 24 hours. Event characteristics and risk factors were recorded and all patients underwent brain imaging, with CT or MRI as appropriate. All cases were subsequently reviewed by the study senior neurologist (P.M.R.) and classified as probable or definite TIA or stroke or other condition using standard definitions.14,15 We excluded patients in whom it was impossible to obtain a definite history of TIAs because they were aphasic, confused, or unconscious. This article includes all cases with events from April 2002 to March 2007.
All patients were followed up at 30 days by a study nurse or physician. Recurrent symptoms, medications, and disability scores were recorded. All recurrent strokes that presented to medical attention would also be identified acutely by ongoing daily case ascertainment within OXVASC. All patients with recurrent events were reassessed by a study physician and reviewed by PMR. Etiologic classification of the early recurrent strokes was performed according to the TOAST classification.17
Analysis.
We restricted analysis to the risk of stroke after the first probable or definite TIA in the study period. We analyzed the risk of stroke in the first 24 hours after TIA both including and excluding patients who did not seek medical attention for their initial symptoms and only presented after the recurrent stroke. A recurrent stroke was defined as a new or persistent neurologic symptom in a patient in whom the initial symptoms had already substantially or fully recovered. In patients who had recurrent stroke within 24 hours of an initial event, the following two types of preceding TIA were defined:
Cases in whom the initial symptoms had definitely fully resolved prior to the stroke (definite TIA)
Cases in whom the initial symptoms were rapidly resolving and had almost completely recovered prior to the stroke, such that complete resolution within 24 hours would have been highly likely (probable TIA)
In cases where there was no, or only partial, resolution of symptoms prior to recurrent stroke, the initial event was classified as a minor stroke.
Analyses of time to event were performed from two separate time points:
Time of onset of the TIA
Time that the patient first called for medical attention (i.e., time that he or she called the family doctor, or presented to the emergency department, or called for an ambulance)
The risks of stroke during the 6, 12, and 24 hours after TIA were determined in relation to the ABCD2 score, a seven-point validated clinical risk prediction tool for triage assessment of TIA.3
RESULTS
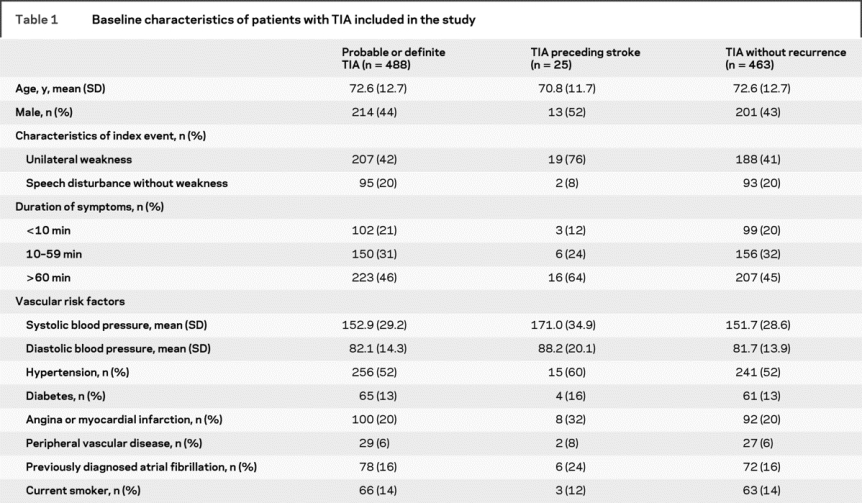
Complete baseline data and complete follow-up to 30 days were available on all 1,247 first TIA or strokes. Table 1 shows the clinical characteristics of all 488 first TIAs.
Table 1 Baseline characteristics of patients with TIA included in the study
Of the 488 first TIAs, 463 had no stroke within 24 hours. In the remaining 25 cases, 23 of whom had incident TIAs, a recurrent stroke occurred within 24 hours of onset of the TIA. The symptoms of the TIA had completely resolved prior to the recurrence in 22 cases (definite TIA), lasting less than 60 minutes in 9 cases. In three cases (probable TIA), the symptoms had almost completely recovered at the time of onset of the stroke, which occurred at 4, 12, and 13 hours after onset of the TIA. A further 10 patients with a recurrent stroke within 24 hours were excluded because the symptoms of the initial event had no more than partially resolved prior to the recurrent event.
Of the 488 first TIAs, 17 had a recurrent stroke within 24 hours of first calling for medical attention. The symptoms of the TIA had completely resolved prior to the recurrence in 14 cases (definite TIA) and had almost completely recovered in three cases (probable TIA; same three cases as above).
Figure 1 shows the 24-hour risk of stroke after TIA. The risks of stroke within 6, 12, and 24 hours of a TIA were 1.2% (95% confidence interval [CI] 0.2–2.2, 6 cases), 2.0% (95% CI 0.8–3.2, 10 cases), and 5.1% (95% CI 3.1–7.1, 25 cases). The equivalent risks measured from the time of first calling for medical attention after the TIA were 1.2% (95% CI 0.2–2.2, 6 cases), 2.0% (95% CI 0.8–3.2, 10 cases), and 3.5% (95% CI 1.9–5.1, 17 cases) (figure 1). Of the 25 cases with recurrent stroke within 24 hours, 14 (56%) patients were already on aspirin at the time of the initial TIA, 5 (20%) were on a statin, and 12 (48%) were on one or more antihypertensive drugs.
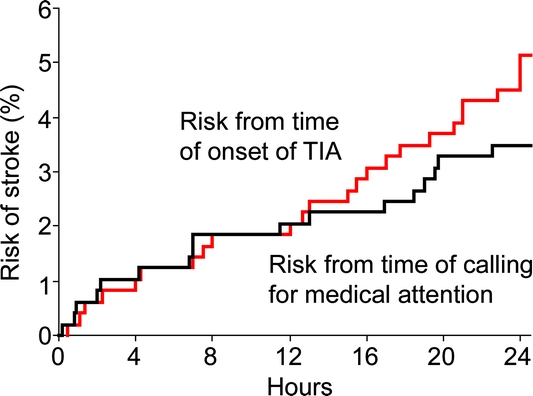
Figure 1 Risk of recurrent stroke within 24 hours of onset of a probable or definite TIA and within 24 hours of first calling for medical attention after a probable or definite TIA
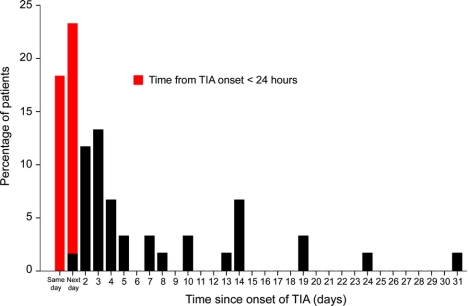
Figure 2 shows the distribution of time from first TIA to stroke. The 25 cases with recurrence within 24 hours accounted for 52% of the 48 strokes during the 7 days and 42% of the 59 strokes during the 30 days after first TIA. Of the recurrences within 24 hours, 14 (56%) had an NIHSS ≥3 at initial assessment after the recurrent stroke, 12 (48%) were associated with a Rankin score ≥3 at 1 month follow-up, and 3 were fatal.
Figure 2 Time from onset of TIA to onset of stroke in all patients who had a stroke within 1 month of a TIA
Sixteen (64%) of the 25 patients with TIA with recurrent stroke within 24 hours of onset of the TIA sought medical attention prior to the stroke, of whom 7 (44%) did not know the cause of their symptoms, 7 (44%) thought it was a stroke or mini-stroke, and 2 were uncertain. Seven (44%) contacted their family practitioner, five called for an emergency ambulance (one was seen by the paramedics and left at home and four were taken to hospital, one of whom had a stroke on arrival at the emergency department), two attended the emergency room directly, and two were inpatients at the time of their event and notified medical staff. At the time of the initial TIA, 10 (56%) of these 16 patients were already on aspirin, 4 (25%) were on a statin, and 8 (50%) were on one or more antihypertensive drugs. None received additional antiplatelet treatment prior to the recurrence. However, the median (interquartile range) time from seeking medical attention to recurrence in these 16 patients was only 6.9 (2.0–18.0) hours. Patients recruited to the EXPRESS study18 were nested within this cohort and of the 337 patients with TIA who were referred to the EXPRESS Study clinic, five cases had a recurrent stroke within 24 hours and sought medical attention for their initial TIA. All five had their recurrence before being seen in the clinic (three in phase 1 of the study and two in phase 2).
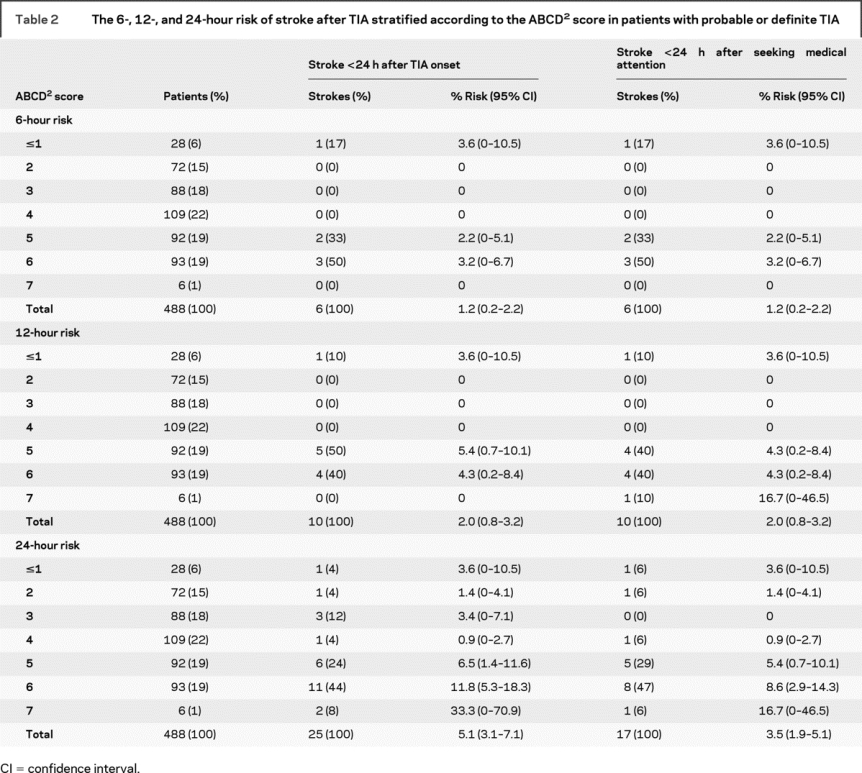
Table 2 shows the 6-, 12-, and 24-hour risks of stroke following TIA stratified by the ABCD2 score, along with the parallel analysis based on time from first calling for medical attention after the TIA. The score was highly predictive (p = 0.00025) of recurrent stroke within 24 hours. Risk of stroke was 2.0% at a score ≤4, 6.5% at 5 (6/92), 11.8% at 6 (11/93), and 33% at 7 (2/6). Nineteen of 25 recurrences had an ABCD2 score of five or more. A similar pattern was seen at 6 and 12 hours, with patients with scores ≥5 having greater risks of stroke than those with lower scores: 6-hour risk, 5/191 (2.6%) vs 1/297 (0.3%); 12-hour risk, 9/191 (4.7%) vs 1/297 (0.3%). An ABCD2 score of five or more also identified the majority of patients who had a stroke within 24 hours of seeking medical attention (14/17, 82%): 6-hour risk, 5/191 (2.6%) vs 1/297 (0.3%); 12-hour risk, 9/191 (4.7%) vs 1/297 (0.3%); 24-hour risk, 14/191 (7.3%) vs 3/297 (1%).
Table 2 The 6-, 12-, and 24-hour risk of stroke after TIA stratified according to the ABCD2 score in patients with probable or definite TIA
No particular etiologic subtype (TOAST classification) was predominant among the 25 TIAs with recurrent stroke within 24 hours of onset: large artery atherosclerosis in 3, cardioembolic in 4, small vessel disease in 9, mixed large artery and cardioembolic in 1, mixed large artery and small vessel disease in 1, undetermined in 5, unknown in 1, and other cause in 1.
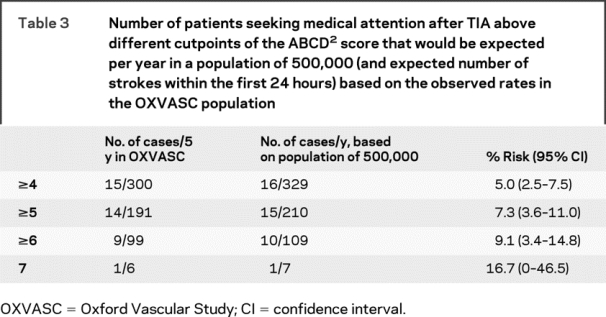
To understand the health economic burden represented by seeing as an emergency those patients with an ABCD2 above a particular cutpoint, we determined the number of cases that would need to be seen per year in a population of 500,000 and the expected number of strokes within the first 24 hours (table 3). For example, based on the rates observed in OXVASC, the expected number of cases with an ABCD2 score ≥6 seeking medical attention prior to a recurrent stroke would be 109/500,000/year, 10 of whom would be expected to have a stroke within the first 24 hours without emergency assessment and treatment.
Table 3 Number of patients seeking medical attention after TIA above different cutpoints of the ABCD2 score that would be expected per year in a population of 500,000 (and expected number of strokes within the first 24 hours) based on the observed rates in the OXVASC population
DISCUSSION
Determining the risk of stroke within 24 after a TIA is methodologically difficult as differentiating between a stroke in evolution and a very early recurrence can be problematic, particularly if only routinely collected data are available. By ascertaining patients as soon as possible after the event and by assessing them ourselves in the acute phase, including detailed recording of the exact timing and evolution of the clinical syndrome, we have been able to gather as accurate an estimate as possible of the risk of early recurrent stroke. In this first rigorous prospective population-based study of the risk of stroke within 24 hours of a TIA, we found a stroke rate of about 5%, with half of all recurrent strokes within 7 days occurring in the first 24 hours, and half of these very early recurrent strokes being disabling or fatal. Analyses of risk of stroke from time of first seeking medical attention, which is perhaps more clinically relevant, were similar.
Previous studies looking at risk of stroke following TIA have used various methods to estimate risk.19 In a recent systematic review including 18 independent cohorts, which reported stroke risk in 10,126 patients with TIA, the pooled stroke risk was 5.2% (95% CI 3.9–6.5) at 7 days, but ranged from 0% to 12.8%.4 Most of the variation in reported risk between studies could be accounted for by differences in methods, with population-based studies that had face-to-face follow-up reporting the highest risks of stroke.2
Previous standard definitions of recurrent stroke have tended to exclude all new symptoms or deteriorations within 24 hours of the initial event,19 and it has often been unclear whether such early recurrences were included in previous studies of the early risk of stroke after TIA.20-27 Our data show that exclusion of such cases would significantly underestimate the true early risk of stroke.
In our previous retrospective study of the timing of TIAs in patients presenting with stroke, we found that 44% of TIAs during the previous 14 days occurred on the same day as the stroke or on the previous day,7 but many patients were assessed several weeks after the stroke and might therefore have either forgotten about any preceding TIA or have been prone to recall bias. It was also unknown whether patients had sought medical attention for these preceding TIAs. The current prospective study has, however, confirmed the very high rate of TIA shortly before stroke.
The ABCD2 score was highly predictive of early recurrent stroke. The score also performed well in the analysis based on risk from time of first calling for medical attention and is therefore likely to be clinically useful for triage of patients with TIA in the hyperacute phase, as well as in public education.
Our study had some potential limitations. First, we cannot be completely precise about the risk of stroke within 24 hours of a TIA because not all patients with TIA seek medical attention. However, our analysis of risk from the time of first seeking medical attention revealed very similar risks. Secondly, we might have underestimated the early risk of stroke slightly because some patients with major stroke with a preceding TIA may not have been identified because we excluded those in whom it was impossible to obtain a definite history of TIAs because they were aphasic, confused, or unconscious. Third, our validation of the ABCD2 score for recurrences in the first few hours after TIA was based on relatively small numbers of outcomes and so further studies would help to confirm or refute our findings. Fourth, our study included patients recruited into the EXPRESS study,18 phase 2 of which involved more urgent investigation and treatment. However, the two patients who sought medical attention and were referred to the phase 2 EXPRESS clinic prior to their recurrent stroke had the recurrence before being seen in the clinic.
The EXPRESS study showed that urgent intervention after TIA was highly effective in preventing recurrent stroke after patients sought medical attention,18 and this observation is supported by other studies in which patients have been treated urgently and intensively.24,28 However, relatively few patients in EXPRESS were treated within a few hours of onset of their TIA. Our current study has shown that the majority of patients who had a recurrent stroke within 24 hours of a TIA did seek medical attention, usually from their family doctor, prior to their recurrence but they were not treated or sent to the EXPRESS clinic as an emergency. However, the fact that the majority of patients sought medical attention prior to their recurrence indicates that emergency triage and treatment are feasible, if front-line services recognize the need.
Our results also have implications for trials of treatment in the acute phase after TIA, such as trials of combination antiplatelet treatment. Although the MATCH trial of aspirin plus clopidogrel vs clopidogrel alone showed no benefit of longer-term combination treatment, there was a beneficial trend in patients randomized within 7 days of a TIA or stroke,29 which was supported by the findings of the CARESS trial,30,31 and more recently by the results of the Fast Assessment of Stroke and Transient Ischemic Attack to prevent Early Recurrence (FASTER) Trial, in which aspirin plus clopidogrel vs aspirin alone tended to reduce the risk of recurrent stroke in patients who had had a TIA or minor ischemic stroke within the previous 24 hours.32 However, there was a clinically significant risk of major bleeding in FASTER, as in similar trials of longer-term treatment with aspirin plus clopidogrel.30,33,34 Further larger trials of aspirin plus clopidogrel are planned in the hyperacute phase after TIA (<12 hours after onset), including FASTER 2 and POINT. Our results suggest that large phase 3 trials in the hyperacute phase are feasible. Based on the planned inclusion and exclusion criteria for these two trials (unpublished protocols), the proportion of potentially eligible patients calculated from our population-based cohort would be 39% (189/488) for FASTER and 40% (196/488) for POINT. The expected 24-hour risk of stroke after seeking medical attention using these criteria would be 4.8% (95% CI 1.9–7.7) and 3.6% (95% CI 1.1–6.1).
ACKNOWLEDGMENT
The authors thank all primary care practices and physicians who collaborated with the Oxford Vascular Study, details of which have been published previously.12,13
Supplementary Material
Address correspondence and reprint requests to Prof. Peter M. Rothwell, Stroke Prevention Research Unit, Oxford University Department of Clinical Neurology, Level 6, West Wing, John Radcliffe Hospital, OX3 9DU, UK peter.rothwell@clneuro.ox.ac.uk.
Supported by the UK Medical Research Council, the National Institute of Health Research, the Stroke Association, the Dunhill Medical Trust, and the Oxford Partnership Comprehensive Biomedical Research Centre.
Disclosure: The authors report no disclosures.
Received December 15, 2008. Accepted in final form March 2, 2009.
REFERENCES
- 1.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901–2906. [DOI] [PubMed] [Google Scholar]
- 2.Coull AJ, Lovett JK, Rothwell PM. Population based study of early risk of stroke after transient ischemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischemic attack. Lancet 2007;369:283–292. [DOI] [PubMed] [Google Scholar]
- 4.Giles MF, Rothwell PM. Risk of stroke early after transient ischemic attack: a systematic review and meta-analysis. Lancet Neurol 2007;6:1063–1072. [DOI] [PubMed] [Google Scholar]
- 5.Giles MF, Rothwell PM. Substantial underestimation of the need for outpatient services for TIA and minor stroke. Age Ageing 2007;36:676–680. [DOI] [PubMed] [Google Scholar]
- 6.Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 2005;36:720–723. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology 2005;64:817–820. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health. National Stroke Strategy (2007). Available at: dh.gov.uk/stroke. Accessed 2008 Dec. 10.
- 9.Guidelines for management of ischemic stroke and transient ischemic attack 2008. Cerebrovasc Dis 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence TIA and Stroke Guidelines (2008). Available at: www.nice.org.uk. Accessed 2008 Dec. 10.
- 11.Royal College of Physicians Intercollegiate Stroke Working Party. Clinical Effectiveness and Evaluation Unit. National Clinical Guideline for Stroke, 3rd ed; London (UK): The College; 2008. [Google Scholar]
- 12.Johnston SC, Nguyen-Huynh MN, Schwarz ME, et al. National Stroke Association guidelines for the management of transient ischemic attacks. Ann Neurol 2006;60:301–313. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischemic attack. Lancet 2005;366:29–36. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Coull AJ, Giles MF, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925–1933. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773–1783. [DOI] [PubMed] [Google Scholar]
- 16.Coull AJ, Silver LE, Bull LM, Giles MF, Rothwell PM. Direct assessment of completeness of ascertainment in a stroke incidence study. Stroke 2004;35:2041–2045. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007;370:1432–1442. [DOI] [PubMed] [Google Scholar]
- 19.Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke: evidence of the need for a standard definition. Stroke 2004;35:1925–1929. [DOI] [PubMed] [Google Scholar]
- 20.Bray JE, Coughlan K, Bladin C. Can the ABCD Score be dichotomised to identify high-risk patients with transient ischemic attack in the emergency department? Emerg Med J 2007;24:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correia M, Silva MR, Magalhaes R, Guimaraes L, Silva MC. Transient ischemic attacks in rural and urban northern Portugal: incidence and short-term prognosis. Stroke 2006;37:50–55. [DOI] [PubMed] [Google Scholar]
- 22.Cucchiara BL, Messe SR, Taylor RA, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke 2006;37:1710–1714. [DOI] [PubMed] [Google Scholar]
- 23.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology 2004;62:2015–2020. [DOI] [PubMed] [Google Scholar]
- 24.Lavallee PC, Meseguer E, Abboud H, et al. A transient ischemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol 2007;6:953–960. [DOI] [PubMed] [Google Scholar]
- 25.Lisabeth LD, Ireland JK, Risser JM, et al. Stroke risk after transient ischemic attack in a population-based setting. Stroke 2004;35:1842–1846. [DOI] [PubMed] [Google Scholar]
- 26.Purroy F, Montaner J, Rovira A, Delgado P, Quintana M, Alvarez-Sabin J. Higher risk of further vascular events among transient ischemic attack patients with diffusion-weighted imaging acute ischemic lesions. Stroke 2004;35:2313–2319. [DOI] [PubMed] [Google Scholar]
- 27.Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke 2006;37:2892–2897. [DOI] [PubMed] [Google Scholar]
- 28.Calvet D, Lamy C, Touze E, Oppenheim C, Meder JF, Mas JL. Management and outcome of patients with transient ischemic attack admitted to a stroke unit. Cerebrovasc Dis 2007;24:80–85. [DOI] [PubMed] [Google Scholar]
- 29.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331–337. [DOI] [PubMed] [Google Scholar]
- 30.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 2005;111:2233–2240. [DOI] [PubMed] [Google Scholar]
- 31.Payne DA, Jones CI, Hayes PD, et al. Beneficial effects of clopidogrel combined with aspirin in reducing cerebral emboli in patients undergoing carotid endarterectomy. Circulation 2004;109:1476–1481. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuck AM, Buchan AM. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomized controlled pilot trial. Lancet Neurol 2007;6(11):961–969. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–1717. [DOI] [PubMed] [Google Scholar]
- 34.Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol 2008;101:960–966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.