Abstract
Trichosporon species have been reported as emerging pathogens and usually occur in severely immunocompromised patients. In the present work, 27 clinical isolates of Trichosporon species were recovered from 27 patients. The patients were not immunocompromised, except for one with acute myeloid leukemia. Sequence analysis revealed the isolation of Trichosporon dohaense Taj-Aldeen, Meis & Boekhout sp. nov., with CBS 10761T as the holotype strain, belonging to the Ovoides clade. In the D1-D2 large-subunit rRNA gene analysis, T. dohaense is a sister species to T. coremiiforme, and in the internal transcribed spacer analysis, the species is basal to the other species of this clade. Molecular identification of the strains yielded 17 T. asahii, 3 T. inkin, 2 T. japonicum, 2 T. faecale, and 3 T. dohaense isolates. The former four species exhibited low MICs for five antifungal azoles but showed high MICs for amphotericin B. T. dohaense demonstrated the lowest amphotericin B MIC (1 mg/liter). For the majority of T. asahii isolates, amphotericin B MICs were high (MIC at which 90% of isolates were inhibited [MIC90], ≥16 mg/liter), and except for fluconazole (MIC90, 8 mg/liter), the azole MICs were low: MIC90s were 0.5 mg/liter for itraconazole, 0.25 mg/liter for voriconazole, 0.25 mg/liter for posaconazole, and 0.125 mg/liter for isavuconazole. The echinocandins, caspofungin and anidulafungin, demonstrated no activity against Trichosporon species.
Trichosporon species are yeast-like fungi, widely distributed in nature and commonly isolated from soil and other environmental sources, which have been involved in a variety of opportunistic infections and have been recognized as emerging fungal pathogens in immunocompromised hosts (19, 79, 80). Disseminated Trichosporon infections are potentially life-threatening and are often fatal in neutropenic patients (7, 22). Although uncommon, pathogenic species of this genus have been reported increasingly, mostly in patients with malignant diseases (3, 6, 9, 10, 11, 20, 32, 44, 47, 48, 63, 77), neonates (18, 56, 84), a bone marrow transplant recipient (22), a solid organ transplant recipient (50), and patients with human immunodeficiency virus (34, 35, 46). Trichosporon has also been reported to cause fungemia (5, 9, 25, 29, 30, 33, 53, 62). Members of the genus Trichosporon have occasionally been implicated as nail pathogens (16, 28, 74) and in subcutaneous infections (66). Trichosporon is considered an opportunistic agent, and therefore, recovery of Trichosporon species capable of growing at 37°C, especially from immunocompromised patients, should be regarded as potentially significant. Several reports have addressed the difficulty of identifying Trichosporon to the species level by physiological and biochemical characteristics (2, 64); therefore, molecular methods based on the sequencing of the internal transcribed spacer (ITS) have been developed (15, 69, 71, 72).
In the present paper, we report the isolation of Trichosporon species from clinical specimens over a 4-year period in Qatar, the poor performance of biochemical identification methods, the significance of molecular identification, and the antifungal susceptibility data for the isolates. While investigating the molecular identification of Trichosporon species, we found three strains that do not match any of the published strains in the literature. We describe this organism as Trichosporon dohaense Taj-Aldeen, Meis & Boekhout, sp. nov., the name proposed for this species.
MATERIALS AND METHODS
Patients.
Twenty-seven patients from different regions and with various clinical symptoms presented at Hamad Hospital, Doha, Qatar. The demographic data, clinical specimens, and fungal etiology are reported in Table 1.
TABLE 1.
Clinical data and Trichosporon species recovered from clinical specimens
| Case no. | Clinical specimen | Clinical finding(s)a | Patient age/sexb | Patient origin | Identification of isolate by the Vitek II yeast ID/API ID 32 C system | Closest hit (BLAST)c | No. of identical nucleotides/total nucleotides based on the rDNA sequence
|
Identification | |
|---|---|---|---|---|---|---|---|---|---|
| LSU | ITS | ||||||||
| 1 | Urine | Pyuria, RBC | 26/M | India | T. asahii | T. asahii | 556/556 | 439/440 | T. asahii |
| 2 | Urine | Pyuria | 63/M | Qatar | T. asahii | T. asahii | 555/555 | 458/459 | T. asahii |
| 3 | Urine | Pyuria | 32/M | Egypt | Trichosporon species | T. faecale | 576/577 | 490/490 | T. faecale |
| 4 | Urine | Pyuria, | 23/F | Egypt | T. asahii | T. asahii | 571/571 | 550/550 | T. asahii |
| 5 | Urine | Pyuria | 68/M | Palestine | T. asahii | T. asahii | 555/555 | 469/471 | T. asahii |
| 6 | Urine | Pyuria | 77/F | Qatar | T. asahii | T. asahii | 554/554 | 469/471 | T. asahii |
| 7 | Urine | Pyuria | 41/M | Nepal | T. asahii | T. asahii | 576/577 | 558/558 | T. asahii |
| 8 | Toenail | Distolateral onychomycosis, whitish discoloration | 72/M | United States | T. asahii | T. asahii | 557/557 | 498/499 | T. asahii |
| 9 | Toenail | Onychomycosis | 30/M | Egypt | Trichosporon species | T. asahii | 556/556 | 467/469 | T. asahii |
| 10 | Toenail | Onychomycosis | 50/M | Sudan | T. inkin | T. asahii | 533/533 | 490/490 | T. asahii |
| 11 | Toenail | Onychomycosis | 25/M | Pakistan | Trichosporon species | T. asahii | 515/515 | 335/336 | T. asahii |
| 12 | Fingernail | Onychomycosis, whitish-yellowish, (subungual hyperkeratosis) | 22/M | Nepal | T. asahii | T. asahii | 567/567 | 550/550 | T. asahii |
| 13 | Nail | Onychomycosis | 25/M | Philippines | T. asahii | T. asahii | 587/588 | 565/565 | T. asahii |
| 14 | Toenail | Onychomycosis | 70/F | Qatar | Trichosporon species | T. coremiiforme/T. asahii | 560/561 | 556/566 | T. dohaense |
| 15 | Ear discharge | Pus discharge, ear pain | 15/M | Qatar | T. asahii | T. asahii | 557/557 | 469/470 | T. asahii |
| 16 | Ear discharge | Pus discharge, ear pain | 26/F | India | Trichosporon species | T. asahii | 556/556 | 467/469 | T. asahii |
| 17 | Catheter | Catheter site infection | 41/M | Bangladesh | Trichosporon species | T. coremiiforme/T. asahii | 545/546 | 651/662 | T. dohaense |
| 18 | Skin/intertrigo | Tinea pedis of the diabetic foot | 31/M | Qatar | T. asahii | T. japonicum | 571/571 | 466/466 | T. japonicum |
| 19 | Scalp hair | White piedra | 28/F | Qatar | Trichosporon species | T. inkin | 600/600 | 498/502 | T. inkin |
| 20 | Skin scraping | Tinea pedis | 34/M | India | Trichosporon species | T. coremiiforme/T. asahii | 560/561 | 549/560 | T. dohaense |
| 21 | Tissue | Leg cellulitis | 18/M | Pakistan | Trichosporon species | T. asahii | 581/582 | 541/541 | T. asahii |
| 22 | Skin | Tinea pedis | 33/M | Qatar | Trichosporon species | T. japonicum | 621/621 | 541/541 | T. japonicum |
| 23 | Bone | Osteomyelitis | 65/M | India | Trichosporon species | T. asahii | 585/586 | 557/559 | T. asahii |
| 24 | Blood | Fungemia | 6/F | Qatar | Trichosporon species | T. faecale | 620/621 | 522/522 | T. faecale |
| 25 | Swab | Urethral discharge | 28/M | Kenya | Trichosporon species | T. inkin | 603/603 | 539/539 | T. inkin |
| 26 | Swab | Balanitis | 39/M | India | T. asahii | T. inkin | 526/526 | 508/512 | T. cf inkin |
| 27 | Bronchial lavage | Trauma, cough | 52/M | Unknown | T. asahii | T. asahii | 424/424 | 498/498 | T. asahii |
RBC, red blood cells.
M, male; F, female.
Valid for both the LSU and the ITS, except for cases 14, 17, and 20, where the first name is the hit for the LSU and the second name is the hit for the ITS.
Isolation and identification of Trichosporon species.
A total of 27 clinical specimens positive for Trichosporon species were recorded over a 4-year period. Trichosporon species were isolated and identified according to standard laboratory procedures. The clinical specimens were generally cultured on either Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, MI) plus 40 U/ml streptomycin and 20 U/ml penicillin (SDA+SP), SDA without antibiotics, or brain heart infusion plus 40 U/ml streptomycin and 20 U/ml penicillin. Blood cultures were performed using the Bactec automated culturing system (BD Diagnostic Systems). For culturing of urine, cysteine lactose electrolyte-deficient agar (Mast Diagnostics, United Kingdom) was added for isolation and enumeration of the organism. According to standard laboratory guidelines, total colony counts in the range of 104 to 105 CFU/ml or more were considered significant, and the presence of yeast cells observed by direct microscopy of urine, with associated clinical symptoms such as pyuria, suggested urinary tract infection. Organisms isolated from specimens that did not meet such criteria were excluded from the study. Trichosporon species isolated from nails were considered significant after two successive isolations from a patient, direct microscopy showing compatible fungal cells, and the absence of dermatophytes in culture, according to the diagnostic criteria of Gupta et al. (26). Culture plates were incubated at 28°C and 37°C and were observed daily for growth up to 5 days. Colonies appearing yeast-like in consistency were examined in a lactophenol cotton blue wet mount for microscopic characteristics. Trichosporon showed budding yeast cells, hyphae, and arthroconidia.
Biochemical identification.
A small inoculum from an isolated colony of each isolate was inoculated onto SDA+SP plates, incubated at 37°C for 48 h, and used to prepare inocula for substrate assimilation profiles employing the Vitek II yeast identification (ID) system (Biomerieux, France), as recommended by the manufacturer. The yeast suspension was automatically inoculated into a Vitek II ID yeast card, which was programmed to identify only three Trichosporon species, viz., Trichosporon asahii, T. inkin, and T. mucoides. The isolates were also identified using the API ID 32 C system (Biomérieux).
Morphology, physiology, and rRNA gene sequencing.
The morphology of the isolates was investigated using line inoculations onto the following media: YPGA (1% yeast extract-0.5% bacteriological peptone L37 [Oxoid]-4% glucose agar), yeast malt extract agar (Difco), yeast morphology agar (YMoA; Difco), malt extract agar (Oxoid), SDA (Difco), and potato dextrose agar (PDA; Difco). The formation of blastoconidia was also investigated using these media. Growth at different temperatures (25, 30, 35, 37, 40, and 42°C) was evaluated using inoculated YPGA slants placed in incubators at the appropriate temperatures. The nutritional requirements, fermentative capabilities, reactions to diazonium blue B, and urease activities of the yeast strains were assessed according to the work of Yarrow (83).
Genomic DNA was extracted as described by Bolano et al. (8), with minor adjustments. Molecular identification of the isolate was performed by sequence analysis of the D1-D2 domains and the ITS1 and ITS2 regions of ribosomal DNA according to the method of Okoli et al. (54). The sequences generated were compared to the available data in the NCBI database with the Basic Local Alignment Search Tool (BLASTn) (4). Trees were generated with PAUP (version 4.0b10) using the neighbor-joining algorithm with Kimura 2 as a distance measure and 1,000 bootstrap replicates (75).
Susceptibility testing.
The susceptibilities of all the strains to amphotericin B (Bristol-Myers Squibb, Woerden, The Netherlands), itraconazole (Janssen Research Foundation, Beerse, Belgium), fluconazole, voriconazole, anidulafungin (Pfizer Central Research, Sandwich, United Kingdom), caspofungin (Merck Sharp & Dohme BV, Haarlem, The Netherlands), posaconazole (Schering-Plough, Utrecht, The Netherlands), and isavuconazole (Basilea Pharmaceutica, Basel, Switzerland) were tested using the standard broth microdilution method as described in NCCLS (now CLSI) document M27-A2 (49).
Each isolate was grown on SDA at 35°C for 24 to 48 h, and a stock inoculum suspension was prepared according to the recommendations of the NCCLS (49). This suspension was then adjusted with a spectrophotometer to 75 to 77% transmittance at a wavelength of 530 nm. The working suspension was made by a 1:1,000 dilution of the suspension in RPMI 1640 (GIBCO BRL, Life Technologies, Woerden, The Netherlands) to produce the final test concentration of 1 × 103 to 5 × 103 CFU/ml. Aliquots (100 μl) of the diluted suspension were inoculated into 96-well flat-bottom microtiter plates (Costar; Omnilabo International BV, Breda, The Netherlands) with antifungals by using a multichannel pipette. The concentrations of amphotericin B, itraconazole, voriconazole, and posaconazole ranged from 0.016 to 16 mg/liter, that of fluconazole from 0.063 to 64 mg/liter, those of anidulafungin and caspofungin from 0.008 to 8 mg/liter, and that of isavuconazole from 0.004 to 4 mg/liter.
MIC end points were determined after 48 h of incubation at 35°C with an Anthos HT3 spectrophotometer (Salzburg, Austria) and the MikroWin 2000 program at 450 nm. The MIC of amphotericin B was taken from the well with the lowest concentration with 100% inhibition of growth (compared to the growth control well), while the MICs of fluconazole, itraconazole, voriconazole, posaconazole, isavuconazole, caspofungin, and anidulafungin were taken from the wells with prominent decreases in turbidity (approximately ≤50% growth inhibition) from that of the growth control well. Ranges for MICs and for MICs at which 50 and 90% of the isolates of T. asahii tested were inhibited (MIC50 and MIC90) were calculated. The MICs for the quality control strains Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were all within the reference ranges (data not shown).
Nucleotide sequence accession numbers.
The T. dohaense strains identified in this study were assigned the following GenBank accession numbers: FJ228473 for the large subunit (LSU) and FJ228476 for the ITS of the case 14 isolate; FJ228472 for the LSU and FJ228474 for the ITS of the case 17 isolate; and FJ228471 for the LSU and FJ228475 for the ITS of the case 20 isolate.
RESULTS
Twenty-seven isolates of Trichosporon species originating from 27 patients were obtained. Information pertaining to the source of isolation and the clinical symptoms of the patients yielding these isolates is provided in Table 1. Low discernible differences were observed in the microscopic morphologies of the strains. The assimilation profiles of the strains by the Vitek II and/or the API ID 32 C system were not discriminatory for some strains. When the clinical isolates were subjected to identification by these two biochemical methods, 13 isolates were identified as T. asahii and 1 as T. inkin, while 13 isolates yielded variable results, but the systems were not discriminatory, and hence, these isolates are referred to as “Trichosporon species,” indicating the inability of biochemical methods to discriminate among various Trichosporon species. When conventional identification methods were employed, Trichosporon species such as T. japonicum and T. inkin were misidentified as T. asahii, or T. asahii was misidentified as T. inkin (Table 1).
Molecular identification of the strains yielded 17 T. asahii isolates and 10 isolates of other Trichosporon species: 3 T. inkin, 3 T. dohaense, 2 T. faecale, and 2 T. japonicum isolates. Of the specimens examined, seven were from urine, seven from nails, two from ear discharges, four from superficial sites (three from skin and one from hair), and one each from tissue, bone, urethral discharge, blood, respiratory, balanitis, and a catheter. There were 21 males and 6 females, aged 6 to 77 years (median age, 38.5 years). The apparent bias toward male patients can be explained by the fact that the majority of the immigrant workers in Qatar who form the main patient groups are males. All patients had one or more preexisting clinical manifestations, such as pyuria, onychomycosis, skin infection, fungemia, or a respiratory problem.
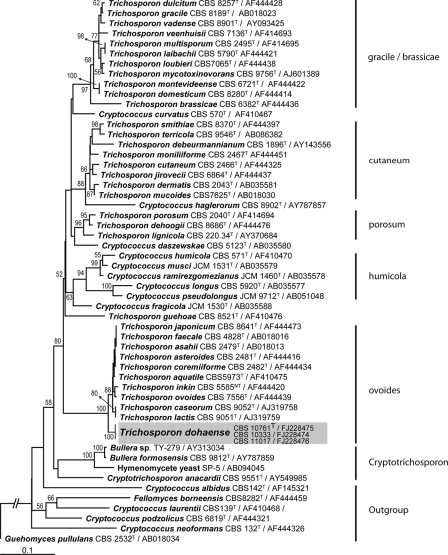
Phylogenetically, three strains (viz., those from case 14 [CBS 11017; also called IHEM 22874], case 17 [CBS 10333; also called IHEM 22872], and case 20 [CBS 10761T; also called IHEM 22873]) of T. dohaense sp. nov. belonged to the Ovoides clade but did not match any known species (Table 1). In the D1-D2 LSU rRNA gene analysis, T. dohaense is a sister species to T. coremiiforme, and in the ITS analysis, T. dohaense is basal to the other species of this clade, viz. T. aquatile, T. asahii, T. asteroides, T. caseorum, T. coremiiforme, T. faecale, T. inkin, T. japonicum, T. lactis, and T. ovoides (Fig. 1) (67). Based on the sequencing analysis, the closest relative to the three strains of T. dohaense is T. coremiiforme, with 97.8% similarity for the ITS and 99.8% similarity for the D1-D2 region. The percentages of similarity and numbers of mismatches with other species in the Ovoides cluster are given in Table 2. T. inkin can be differentiated by its growth at 42°C; T. caseorum utilizes lysine; and the other species, except for T. lactis, do not assimilate glucosamine, which is slowly and weakly assimilated by T. dohaense. T. dohaense differs from T. lactis by growth on melezitose, starch, methanol, glucosamine (N source), and 0.01% cycloheximide; growth at 37°C; and lack of growth on sorbose, mannitol, lactate, and nitrite (67). On the basis of these data, we propose the following description of the new species T. dohaense.
FIG. 1.
Phylogenetic position of Trichosporon dohaense in the Ovoides clade of the Trichosporonales, based on phylogenetic analysis of the ITS regions of the rRNA gene using PAUP, version 4.0b10, for Macintosh (74). Neighbor-joining analysis was performed with the Kimura 2 substitution model. Strain names and GenBank accession numbers are given after each species name. Next to the tree branches, bootstrap support values after 1,000 replications are given. Clade names are given on the right.
TABLE 2.
Percentages of similarity and numbers of mismatches of the closest relatives from the Ovoides clade with the type strain of T. dohaense in the ITS regions and the D1-D2 domains of the LSU of the rRNA gene in GenBank
| Straina | GenBank accession no. | Species | ITS (405b)
|
LSU (517b)
|
||
|---|---|---|---|---|---|---|
| % Similarity | No. of mismatches | % Similarity | No. of mismatches | |||
| CBS 2482 | AF444434 | Trichosporon coremiiforme | 97.8 | 9 | 99.8 | 1 |
| CBS 2481 | AF444416 | Trichosporon asteroides | 97.8 | 9 | 98.6 | 7 |
| CBS 8641 | AF444473 | Trichosporon japonicum | 97.5 | 10 | 98.8 | 6 |
| CBS 4828 | AB018016 | Trichosporon faecale | 97.5 | 10 | 98.8 | 6 |
| CBS 2479 | AB018013 | Trichosporon asahii | 97.5 | 10 | 98.8 | 6 |
| CBS 5973 | AF410475 | Trichosporon aquatile | 97.0 | 12 | 98.3 | 9 |
| CBS 5585 | AF44420 | Trichosporon inkin | 96.3 | 15 | 99.0 | 5 |
| CBS 7556 | AF444439 | Trichosporon ovoides | 96.3 | 15 | 98.8 | 6 |
| CBS 9051 | AJ319759 | Trichosporon lactis | 96.3 | 15 | 98.3 | 9 |
| CBS 9052 | AJ319758 | Trichosporon caseorum | 96.3 | 15 | 97.7 | 12 |
Strains are listed in descending order with regard to the percentage of similarity, based first on the ITS and second on the LSU values.
Query length (base pairs).
Latin description of Trichosporon dohaense Taj-Aldeen, Meis & Boekhout, sp. nov.
In medio liquido cellulae zymoideae globosae vel ellipsoideae, 5.5 ad 9.0 per 3.5 ad 5.0 μm, polariter gemmantes. In agaro YMoA post 10 dies 25°C, coloniae variabiles, ca. 20 mm diametro, leves vel modice irregulares vel verrucosae, marginem versus sulcatae, butyrosae, cremeae vel pallide isabellinae, marginem versus albidae, glabrae vel mycelio aerio albido obtectae. Cellulae zymoideae sicut sopra, nonnumquam etiam e latere, e basi lata gemmantes; cellulae maiores, 5 ad 11 μm diameter et filamenta ad 90 per 2.0 ad 3.0 μm praesentes; hyphae vel pseudohyphae nonnumquam praesentes; arthroconidia cylindrica, magnitudine variabilia, 5 ad 20 per 2 ad 4 μm. Non fermentat. d-Glucosio, d-galactosio, d-glucosamino (d,w), d-ribosio (+,d), d-xylosio, d-arabinosio (d), l-arabinosio, sucrosio, maltosio, trehalosio (+,dw), methyl-α-d-glucosido, cellobiosio, salicino (+,dw), arbutino (+,dw), lactosio (dw), raffinosio (w), melezitosio, amylo solubili, glycerolo, meso-erythritolo, myo-inositolo (w), 2-keto-d-gluconato, d-gluconato, d-glucuronato, succinato, methanolo, ethanolo, propane 1,2 diolo (dw), butane 2,3 diolo (dw), acido galacturonico (+,d) utitur; neque l-sorbosio, melibiosio, galactitolo, d-galacturonato, dl-lactato, citrato, acido quinico, saccharato, vel verosimile l-rhamnosio (−,w), inilino (−,w), ribitolo (−,w), l-arabinitolo (−,w), d-glucitolo (−,w), d-mannitolo (−,w). Ethylamino, l-lysino, cadaverino, et d-tryptophano utitur, neque nitrato et glucosamino. Vitaminis absentibus crescere potest an non. Substantia amyloidea vix formatur. Temperaturis 25 ad 40°C crescere potest, neque 42°C; 0.01% cycloheximido addito haud crescit, neque in medio 50% glucosii addito; reactiones urei et diazonium blue B positivae. Holotypus CBS 10761T (CBS H-20142), isolatus ex cute humana; depositus in collectione herbario CBS Fungal Biodiversity Centre, Utrecht, The Netherlands.
Description of Trichosporon dohaense Taj-Aldeen, Meis & Boekhout sp. nov. (i) Etymology.
The specific epithet dohaense is derived from Doha, the capital of Qatar, where the isolates were recovered.
(ii) Morphological characterization.
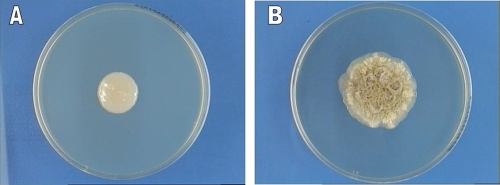
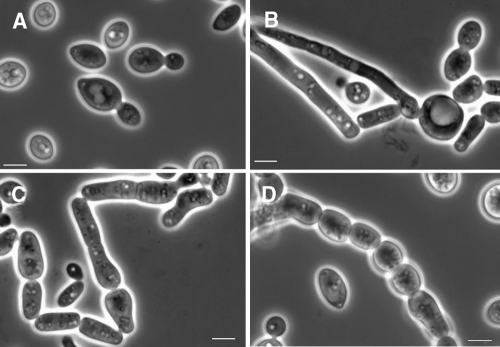
After 2 weeks at 25°C in 2% glucose broth in yeast nitrogen base, a ring, flocks, and sediment are present. Yeast cells are globose or subglobose to ellipsoidal, 5.5 to 9.0 by 3.5 to 5.0 μm in size, and show polar budding. On YMoA after 10 days at 25°C, colonies are somewhat variable, ca. 20 mm in diameter, slightly convex, smooth to somewhat irregular to warty, and transversely ridged toward the margin. They are butyrous, cream to pale café au lait (isabella), but toward the margin they become whitish, dull to shiny, glabrous, or covered with a whitish aerial mycelium. The margin is entirely or locally submerged with hyphal growth. On SDA, colonies are Candida-like, smooth with a mucoid texture (Fig. 2A), and they become irregular to warty in older cultures (Fig. 2B) Yeast cells are globose to ellipsoidal, or somewhat irregularly shaped, 5.5 to 8.0 (or 12.0) by 3.5 to 6.5 μm, with polar or occasionally lateral budding on a rather broad base; somewhat bigger and refractive cells, 5.0 to 11.0 μm in diameter, are present (Fig. 3A and B). Filaments as large as ca. 90 by 2.0 to 3.0 μm are present. Hyphae or pseudohyphae may be present or absent. Arthroconidia are cylindrical and somewhat variable in size, 5.0 to 20.0 by 2.0 to 4.0 μm (Fig. 3C and D). Extensive hyphae are present in Dalmau plate culture on YMoA. On malt extract agar, the surface of the colony may be covered with tapered synnemata.
FIG. 2.
Colony morphology of Trichosporon dohaense strain Myco 194 (CBS 10333), grown on SDA+SP at 37°C. (A) Mucoid appearance at an early stage (96 h) of growth; (B) irregular warty growth after 45 days.
FIG. 3.
T. dohaense. (A) Yeast cells (CBS 10671T). (B) Globose cells with hyphae (CBS 10333) in liquid medium (2% glucose in yeast nitrogen base) after 5 days at 25°C. (C) Arthroconidia and yeast cells (CBS 10333) after 5 days in liquid medium (2% glucose in yeast nitrogen base) at 25°C. (D) Arthroconidia (CBS 10671T) on cornmeal agar after 10 days at 25°C. Phase-contrast optics were used. Bar, 5 μm.
(iii) Assimilation.
Fermentation is absent. Growth is positive on d-glucose, d-galactose, d-glucosamine (d,w), d-ribose (+,d), d-xylose, d-arabinose (d), l-arabinose, sucrose, maltose, trehalose (+,dw), methyl-α-d-glucoside, cellobiose, salicin (+,dw), arbutin (+,dw), lactose (dw), raffinose (w), melezitose, soluble starch, glycerol, meso-erythritol, myo-inositol (w), 2-keto-d-gluconate, d-gluconate, d-glucuronate, succinate, methanol, ethanol, propane-1,2-diol (dw), butane-2,3-diol (dw), and galactonic acid (+,d). Growth is absent in l-sorbose, melibiose, galactitol, d-galacturonate, dl-lactate, citrate, quinic acid, and saccharate. Growth is absent or latent in l-rhamnose (−,w), inulin (−,w), ribitol (−,w), l-arabinitol (−,w), d-glucitol (−,w), d-mannitol (−,w). Ethylamine, l-lysine, cadaverine, and d-tryptophan are assimilated, but nitrate and glucosamine (N source) are not. Growth without vitamins is variable (Myco 194 is negative and Myco 483 is positive). Formation of starch-like compounds is absent or weak (in both regular and acidified glucose fermentation media). There is growth between 25 and 40°C, but no growth at 42°C. There is no growth with 0.01% cycloheximide and no growth on 50% glucose. Results of urea and diazonium blue B tests are positive.
(iv) Type strain.
The type strain is Myco 483 (CBS 10761T).
(v) Origin of strains.
Myco 483 (CBS 10761T; IHEM 22873; MycoBank accession number 513091) was isolated from infected skin (tinea pedis), Myco 194 (CBS 10333; IHEM 22872) from an infected catheter site, and Myco 643 (CBS 11017; IHEM 22874) from a patient with onychomycosis.
(vi) Clinical origin.
T. dohaense was isolated from cutaneous specimens. Strain Myco 483 (CBS 10761T) was isolated from a 34-year-old male patient from India with tinea pedis. The patient had irritated, erythematous scaly lesions on the left lower limb (dorsal and plantar) for 4 years. The patient was successfully treated with oral terbinafine tablets, local econazole cream, and Whitfield's ointment (salicylic acid and benzoic acid).
Antifungal susceptibility testing.
Table 3 demonstrates the MIC ranges of amphotericin B and five azole antifungals for 25 Trichosporon species isolates. For 15 T. asahii isolates, MIC50s and MIC90s are also given. For the majority of isolates, amphotericin B MICs were high and azole MICs were low. The new species T. dohaense demonstrated the highest susceptibility to amphotericin B (MICs, 0.5 to 1 mg/liter) and the azoles posaconazole and isavuconazole. There was one T. asahii isolate for which the fluconazole MIC was 64 mg/liter and the voriconazole MIC was also higher (2 mg/liter). The new azole isavuconazole was the most potent drug, with the lowest MICs for all species. The echinocandins, caspofungin and anidulafungin (both with MICs of >8 mg/liter), demonstrated no activity against Trichosporon species (not shown).
TABLE 3.
Results of antifungal susceptibility testing of clinical isolates of Trichosporon species
| Trichosporon species (no. of isolates) | MIC (mg/liter) of:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B
|
Fluconazole
|
Itraconazole
|
Voriconazole
|
Posaconazole
|
Isavuconazole
|
|||||||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | |
| T. asahii (15)a | 2-≥16 | 8 | ≥16 | 0.25-64 | 4 | 8 | 0.063-1 | 0.25 | 0.5 | 0.016-2 | 0.125 | 0.25 | 0.063-0.25 | 0.25 | 0.25 | 0.008-0.5 | 0.125 | 0.125 |
| T. faecale (2) | 2 | 4-8 | 0.25-0.5 | 0.5 | 0.25 | 0.125 | ||||||||||||
| T. inkin (3) | 1-4 | 0.25-4 | 0.031-0.125 | 0.031-0.063 | 0.063-0.25 | 0.002-0.016 | ||||||||||||
| T. japonicum (2) | 4-16 | 4 | 0.125 | 0.063-0.125 | 0.125-0.25 | 0.031-0.063 | ||||||||||||
| T. dohaense (3) | 1 | 1-4 | 0.031-0.25 | 0.016-0.25 | 0.031-0.125 | 0.063-0.25 | ||||||||||||
Antifungal susceptibility testing was done for 15 of 17 Trichosporon asahii isolates.
DISCUSSION
The reported clinical cases caused by opportunistic fungal infections are constantly rising, and new species within the genus Trichosporon are emerging. Cases of Guehomyces pullulans (T. pullulans) (17) infection of patients with chronic granulomatous disease (45) or the isolation of this species from the oral cavities of AIDS patients (52) have been reported. T. mucoides has been reported to cause infection in a heart and kidney transplant recipient (51). T. dermatis has been reported to cause fungemia in a 13-month-old male with a history of autoimmune enteropathy (25). Five species of Trichosporon were reported during this study. T. asahii is the most common species associated with clinical specimens in Qatar, representing 62.9% of the cases, while T. inkin and T. dohaense together account for 11.1%, and T. faecale and T. japonicum account for 7.4% each. It is worth noting that several species belonging to the Ovoides clade are well-known human pathogens, namely, T. asahii, T. asteroides, T. coremiiforme, T. faecale, T. inkin, T. japonicum, and T. ovoides (67). T. asahii is isolated mainly from the blood, lung tissue, and urine of patients suffering from deep-seated trichosporonosis (23, 67), but it is also isolated from skin (23) or white piedra (14, 78). T. asahii is the most common species, isolated in the present work from a variety of specimens, including urine, nail, skin, tissue, and bone (Table 1). T. asahii is thought to be much more common in cases of systemic infection than other Trichosporon species (81). Our study supports the view that T. asahii is the most common species associated with human clinical specimens and has a wide geographical distribution (2).
Guého et al. (23, 24) significantly revised the taxonomy of the genus Trichosporon on the basis of partial 26S rRNA sequences, combined with a reanalysis of morphological and biochemical properties and an analysis of the coenzyme Q system. The genus Trichosporon was delineated as containing six clearly differentiated opportunistic pathogens of humans (23): T. asahii and T. mucoides are known to cause deep invasive infections; T. asteroides and T. cutaneum cause superficial skin infections; T. ovoides causes white piedra of the scalp; and T. inkin causes white piedra of the pubic hair. Unfortunately, most of the literature on serious opportunistic trichosporonosis refers to the older nomenclature of T. beigelii. Several new taxa have recently been proposed for inclusion in the genus (17, 21, 38, 39, 40-42, 43, 70, 73). The genus Trichosporon now comprises 36 species. The number of Trichosporon species causing disseminated disease is expanding; T. asteroides, T. loubieri, and T. dermatis have recently been shown to cause disseminated trichosporonosis (25, 33, 38, 55). Trichosporon has been reported to be the most common cause of non-Candida yeast infections in patients with hematological malignancies, and the infections were associated with high mortality rates, despite antifungal therapy (59). Accurate identification of Trichosporon species is important, since different species may have different antifungal susceptibilities (57, 59, 64); T. asahii, T. faecale, and T. coremiiforme exhibited high MICs for amphotericin B, while other species showed lower MICs (64, 65). For most of the Trichosporon isolates in our study, high amphotericin B MICs were found, confirming previous results. Although echinocandins are increasingly regarded as the preferred treatment choice for candidemia in patients with severe sepsis and septic shock (58), clinical failure and breakthrough infections with Trichosporon have been reported with the use of caspofungin and micafungin (3, 7). In this study, caspofungin and anidulafungin demonstrated no in vitro activity against Trichosporon species. The general conclusion is that polyenes and echinocandins should not be used to treat Trichosporon infections. The five azoles tested in our series were all active in vitro, confirming previous reports on voriconazole and itraconazole (64). Only one T. asahii isolate exhibited a fluconazole MIC of 64 mg/liter, with a simultaneous increase in the voriconazole MIC (2 mg/liter). In general, itraconazole, voriconazole, posaconazole, and isavuconazole are active against Trichosporon species in vitro, with the most potent agent being the new azole isavuconazole.
The assimilation of a large number of carbon and nitrogen compounds traditionally forms the basis for the species identification of yeasts, although the inconsistency of assimilation may cause misleading identification results. The Vitek II and API ID 32 C systems are programmed to identify only three species of Trichosporon, namely, T. asahii, T. inkin, and T. mucoides. Consequently, strains may be misidentified, and genetically distinct species could be overlooked (Table 1). The application of modern molecular methods, including the sequencing of rRNA genes, offers a reliable means of overcoming this difficulty (17, 43, 64).
T. inkin is frequently isolated from clinical specimens (66), such as white piedra (13, 23, 76), but also from patients with peritonitis (12, 36, 37), endocarditis (61), lung abscesses (60), subcutaneous nodules (rheumatoid arthritis patients receiving corticosteroid therapy) (65), sternal surgical wound infections (13), and invasive infections (31, 82). In the present study, T. inkin was isolated in cases of white piedra, balanitis, and urethral discharge. T. asteroides is known to have been isolated from skin (2, 23) and from a patient with a nosocomial bloodstream infection (33). T. ovoides is not reported to cause systemic infections but is known to cause white piedra (14), and the species is isolated from the homes of patients with summer-type hypersensitivity pneumonitis (67, 68, 72). The isolation of Trichosporon species from various clinical specimens in the present work further suggests that the species-specific patterns of infection previously delineated in Trichosporon infection (23) need reconsideration.
Although T. coremiiforme, T. faecale, and T. japonicum have been isolated from the houses of patients with summer-type hypersensitivity pneumonitis (64, 67, 68), they have occasionally been isolated from clinical specimens. More recently, T. faecale was isolated from the skin of a tinea pedis patient (27), and T. japonicum was isolated from a sputum specimen (1). During this study, T. japonicum was isolated in two cases of tinea pedis. T. faecale was isolated from two patients: a 32-year old male patient with pyuria and a 6-year old female patient with fungemia (Table 1). To our knowledge, this report describes the first case of fungemia caused by T. faecale, which was successfully treated with liposomal amphotericin B (5.8 mg/kg of body weight/day) for 2 weeks. The present study reports the emergence of T. japonicum and T. faecale as potent human pathogens.
The new species, T. dohaense was isolated three times from cutaneous sites (tinea pedis, onychomycosis, and an infected catheter site) during the past 4 years in Doha, Qatar. The emergence of T. dohaense as a human pathogen supports the idea that Trichosporon species are potent opportunistic human pathogens, and therefore, the recovery of a Trichosporon species from a clinical specimen should be regarded as potentially significant. Moreover, the currently available Vitek II yeast identification and API ID 32 C systems are not reliable enough to identify correctly all the clinically relevant Trichosporon species, and molecular analysis is required to achieve an accurate identification of the species.
Acknowledgments
We gratefully thank Walter Gams, CBS Fungal Biodiversity Institute, The Netherlands, for the Latin text.
Footnotes
Published ahead of print on 25 March 2009.
REFERENCES
- 1.Ağirbasli, H., H. Bilgen, S. K. Ozcan, B. Otlu, G. Sinik, and N. Cerikçioğlu. 2008. Two possible cases of Trichosporon infections in bone-marrow-transplanted children: the first case of T. japonicum isolated from clinical specimens. Jpn. J. Infect. Dis. 61130-132. [PubMed] [Google Scholar]
- 2.Ahmad, S., M. Al-Mahmeed, and Z. U. Khan. 2005. Characterization of Trichosporon species isolated from clinical specimens in Kuwait. J. Med. Microbiol. 54639-646. [DOI] [PubMed] [Google Scholar]
- 3.Akagi, T., K. Yamaguti, T. Kawamura, T. Nakumura, K. Kubo, and H. Takemori. 2006. Breakthrough trichosporonosis in patients with acute myeloid leukemia receiving micafungin. Leuk. Lymphoma 471182-1183. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 5.Antachopoulos, C., E. Papakonstantinou, J. Dotis, E. Bibashi, M. Tamiolaki, D. Koliouskas, and E. Roilides. 2005. Fungemia due to Trichosporon asahii in a neutropenic child refractory to amphotericin B: clearance with voriconazole. J. Pediatr. Hematol. Oncol. 27283-285. [DOI] [PubMed] [Google Scholar]
- 6.Asada, N., H. Uryu, M. Koseki, M. Takeuchi, M. Komatsu, and K. Matsue. 2006. Successful treatment of breakthrough Trichosporon asahii fungemia with voriconazole in a patient with acute myeloid leukemia. Clin. Infect. Dis. 43e39-e41. [DOI] [PubMed] [Google Scholar]
- 7.Bayramoglu, G., M. Sonmez, I. Tosun, K. Aydin, and F. Aydin. 2008. Breakthrough Trichosporon asahii fungemia in neutropenic patient with acute leukemia while receiving caspofungin. Infection 3668-70. [DOI] [PubMed] [Google Scholar]
- 8.Bolano, A., S. Stinchi, R. Preziosi, F. Bistoni, M. Allegrucci, F. Baldelli, A. Martini, and G. Cardinali. 2001. Rapid methods to extract DNA and RNA from Cryptococcus neoformans. FEMS Yeast Res. 1221-224. [DOI] [PubMed] [Google Scholar]
- 9.Chagas-Neto, T. C., G. M. Chaves, A. S. A. Melo, and A. L. Colombo. 2009. Bloodstream infections due to Trichosporon spp.: species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing, and antifungal susceptibility testing. J. Clin. Microbiol. doi: 10.1128/JCM.01614-08. [DOI] [PMC free article] [PubMed]
- 10.Chan-Tack, K. M. 2005. Fatal Trichosporon asahii septicemia in a Guatemalan farmer with acute lymphoblastic leukemia. South. Med. J. 98954-955. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhary, A., S. Ahmad, Z. U. Khan, D. C. Doval, and H. S. Randhawa. 2004. Trichosporon asahii as an emerging etiologic agent of disseminated trichosporonosis: a case report and an update. Indian J. Med. Microbiol. 2216-22. [PubMed] [Google Scholar]
- 12.Crowther, K. S., A. T. Webb, and P. H. McWhinney. 2003. Trichosporon inkin peritonitis in a patient on continuous ambulatory peritoneal dialysis returning from the Caribbean. Clin. Nephrol. 5969-70. [DOI] [PubMed] [Google Scholar]
- 13.Davies, F., S. Logan, E. Johnson, and J. L. Klein. 2006. Sternal wound infection by Trichosporon inkin following cardiac surgery. J. Clin. Microbiol. 442657-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Hoog, G. S., and E. Guého. 2005. White piedra, black piedra, and tinea nigra, p. 195-201. In W. G. Merz and R. J. Hay (ed.), Topley & Wilson's microbiology and microbial infections: medical mycology, 10th ed. Hodder Arnold, London, United Kingdom.
- 15.Diaz, M. R., and J. W. Fell. 2004. High-throughput detection of pathogenic yeasts of the genus Trichosporon. J. Clin. Microbiol. 423696-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmer, K. B., D. M. Elston, and L. F. Libow. 2002. Trichosporon beigelii infection presenting as white piedra and onychomycosis in the same patient. Cutis 70209-211. [PubMed] [Google Scholar]
- 17.Fell, J. W., and G. Scorzetti. 2004. Reassignment of the basidiomycetous yeasts Trichosporon pullulans to Guehomyces pullulans gen. nov., comb. nov. and Hyalodendron lignicola to Trichosporon lignicola comb. nov. Int. J. Syst. Evol. Microbiol. 54995-998. [DOI] [PubMed] [Google Scholar]
- 18.Fisher, D. J., C. Christy, P. Spafford, W. M. Maniscalco, D. J. Hardy, and P. S. Graman. 1993. Neonatal Trichosporon beigelii infection: report of a cluster of cases in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 12149-155. [PubMed] [Google Scholar]
- 19.Fleming, R. V., T. J. Walsh, and E. J. Anaissie. 2002. Emerging and less common fungal pathogens. Infect. Dis. Clin. N. Am. 16915-933. [DOI] [PubMed] [Google Scholar]
- 20.Fournier, S., W. Pavageau, M. Feuillhade, S. Deplus, A. M. Zagdanski, O. Verola, H. Dombret, and J. M. Molina. 2002. Use of voriconazole to successfully treat disseminated Trichosporon asahii infection in a patient with acute myeloid leukaemia. Eur. J. Clin. Microbiol. Infect. Dis. 21892-896. [DOI] [PubMed] [Google Scholar]
- 21.Fuentefria, A. M., S. O. Suh, M. F. Landell, J. Faganello, A. Schrank, M. H. Vainstein, M. Blackwell, and P. Valente. 2008. Trichosporon insectorum sp. nov., a new anamorphic basidiomycetous killer yeast. Mycol. Res. 11293-99. [DOI] [PubMed] [Google Scholar]
- 22.Ghiasian, S. A., A. M. Maghsood, and S. H. Mirhendi. 2006. Disseminated, fatal Trichosporon asahii infection in a bone marrow transplant recipient. J. Microbiol. Immunol. Infect. 39426-429. [PubMed] [Google Scholar]
- 23.Guého, E., L. Improvisi, G. S. de Hoog, and B. Dupont. 1994. Trichosporon on humans: a practical account. Mycoses 373-10. [DOI] [PubMed] [Google Scholar]
- 24.Guého, E., M. T. Smith, G. S. de Hoog, G. Billo-Grand, R. Christen, and W. H. Batenburg-Van der Vegte. 1992. Contributions to a revision of the genus Trichosporon. Antonie van Leeuwenhoek 61289-316. [DOI] [PubMed] [Google Scholar]
- 25.Gunn, S. R., X. T. Reveles, J. D. Hamlington, L. C. Sadkowski, T. L. Johnson-Pais, and J. H. Jorgensen. 2006. Use of DNA sequencing analysis to confirm fungemia due to Trichosporon dermatis in a pediatric patient. J. Clin. Microbiol. 441175-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta, A. K., E. A. Cooper, P. MacDonald, and R. C. Summerbell. 2001. Utility of inoculum counting (Walshe and English criteria) in clinical diagnosis of onychomycosis caused by nondermatophytic filamentous fungi. J. Clin. Microbiol. 392115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahner, D., R. Kirschner, M. Piepenbring, and H. Schöfer. 2008. First isolation of the anamorphic basidiomycetous yeast Trichosporon faecale in Germany, from the skin of a patient with tinea pedis. Mycopathologia 165149-153. [DOI] [PubMed] [Google Scholar]
- 28.Han, M. H., J. H. Choi, K. J. Sung, K. C. Moon, and J. K. Koh. 2000. Onychomycosis and Trichosporon beigelii in Korea. Int. J. Dermatol. 39266-269. [DOI] [PubMed] [Google Scholar]
- 29.Itoh, T., H. Hosokawa, U. Kohdera, N. Toyazaki, and Y. Asada. 1996. Disseminated infection with Trichosporon asahii. Mycoses 39195-199. [DOI] [PubMed] [Google Scholar]
- 30.Karabay, O., M. G. Madariaga, E. Kocoglu, N. Ince, and E. Kandirali. 2006. Trichosporon asahii fungemia in a patient with non-hematological malignancy. Jpn. J. Infect. Dis. 59129-131. [PubMed] [Google Scholar]
- 31.Koyanagi, T., N. Nishida, S. Osabe, Y. Imamura, S. Yamamoto, A. Shichiji, and Y. Nakamura. 2006. Autopsy case of disseminated Trichosporon inkin infection identified with molecular biological and biochemical methods. Pathol. Int. 56738-743. [DOI] [PubMed] [Google Scholar]
- 32.Krcmery, V., Jr., F. Mateicka, A. Kunová, S. Spánik, J. Gyarfás, Z. Sycová, and J. Trupl. 1999. Hematogenous trichosporonosis in cancer patients: report of 12 cases including 5 during prophylaxis with itraconazole. Support. Care Cancer 739-43. [DOI] [PubMed] [Google Scholar]
- 33.Kustimur, S., A. Kalkanci, K. Caglar, M. Dizbay, F. Aktas, and T. Sugita. 2002. Nosocomial fungemia due to Trichosporon asteroides: firstly described bloodstream infection. Diagn. Microbiol. Infect. Dis. 43167-170. [DOI] [PubMed] [Google Scholar]
- 34.Lascaux, A., F. Bouscarat, V. Descamps, E. Casalino, C. Picard-Dahan, B. Crickx, and S. Belaich. 1998. Cutaneous manifestations during disseminated trichosporonosis in an AIDS patient. Ann. Dermatol. Venereol. 125111-113. (In French.) [PubMed] [Google Scholar]
- 35.Leaf, H. L., and M. S. Simberkoff. 1989. Invasive trichosporonosis in a patient with the acquired immunodeficiency syndrome. J. Infect. Dis. 160356-357. [DOI] [PubMed] [Google Scholar]
- 36.Lopes, J. O., S. H. Alves, C. Klock, L. T. Oliveira, and N. R. Dal Forno. 1997. Trichosporon inkin peritonitis during continuous ambulatory peritoneal dialysis with bibliography review. Mycopathologia 13915-18. [DOI] [PubMed] [Google Scholar]
- 37.Madariaga, M. G., A. Tenorio, and L. Proia. 2003. Trichosporon inkin peritonitis treated with caspofungin. J. Clin. Microbiol. 415827-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marty, F. M., D. H. Barouch, E. P. Coakley, and L. R. Baden. 2003. Disseminated trichosporonosis caused by Trichosporon loubieri. J. Clin. Microbiol. 415317-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Middelhoven, W. J. 2004. Trichosporon wieringae sp. nov., an anamorphic basidiomycetous yeast from soil, and assimilation of some phenolic compounds, polysaccharides and other non-conventional carbon sources by saprophytic Trichosporon species. Antonie van Leeuwenhoek 86329-337. [DOI] [PubMed] [Google Scholar]
- 40.Middelhoven, W. J., G. Scorzetti, and J. W. Fell. 1999. Trichosporon guehoae sp. nov., an anamorphic basidiomycetous yeast. Can. J. Microbiol. 45686-690. [DOI] [PubMed] [Google Scholar]
- 41.Middelhoven, W. J., G. Scorzetti, and J. W. Fell. 2000. Trichosporon veenhuisii sp. nov., an alkane-assimilating anamorphic basidiomycetous yeast. Int. J. Syst. Evol. Microbiol. 50381-387. [DOI] [PubMed] [Google Scholar]
- 42.Middelhoven, W. J., G. Scorzetti, and J. W. Fell. 2001. Trichosporon porosum comb. nov., an anamorphic basidiomycetous yeast inhabiting soil, related to the loubieri/laibachii group of species that assimilate hemicelluloses and phenolic compounds. FEMS Yeast Res. 115-22. [DOI] [PubMed] [Google Scholar]
- 43.Middelhoven, W. J., G. Scorzetti, and J. W. Fell. 2004. Systematics of the anamorphic basidiomycetous yeast genus Trichosporon Behrend with the description of five novel species: Trichosporon vadense, T. smithiae, T. dehoogii, T. scarabaeorum and T. gamsii. Int. J. Syst. Evol. Microbiol. 54975-986. [DOI] [PubMed] [Google Scholar]
- 44.Miura, Y., M. Kaneko, M. Nishizawa, K. Okamoto, M. Hirai, H. Kaneko. M. Watanabe, and M. Tsudo. 2007. Breakthrough infection of Trichosporon asahii in a patient with chronic lymphocytic leukemia. Int. J. Hematol. 85177-178. [DOI] [PubMed] [Google Scholar]
- 45.Moylett, E. H., J. Chinen, and W. T. Shearer. 2003. Trichosporon pullulans infection in 2 patients with chronic granulomatous disease: an emerging pathogen and review of the literature. J. Allergy Clin. Immunol. 1111370-1374. [DOI] [PubMed] [Google Scholar]
- 46.Nahass, G. T., S. P. Rosenberg, C. L. Leonardi, and N. S. Penneys. 1993. Disseminated infection with Trichosporon beigelii. Report of a case and review of the cutaneous and histologic manifestations. Arch. Dermatol. 1291020-1023. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa, T., K. Nakashima, T. Takaiwa, and K. Negayama. 2000. Trichosporon cutaneum (Trichosporon asahii) infection mimicking hand eczema in a patient with leukemia. J. Am. Acad. Dermatol. 42929-931. [DOI] [PubMed] [Google Scholar]
- 48.Nakajima, M., T. Sugita, and Y. Mikami. 2007. Granuloma associated with Trichosporon asahii infection in the lung: unusual pathological findings and PCR detection of Trichosporon DNA. Med. Mycol. 45641-644. [DOI] [PubMed] [Google Scholar]
- 49.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 50.Netsvyetayeva, I., E. Swoboda-Kopec, M. Sikora, M. Jaworska-Zaremba, S. Blachnio, and M. Luczak. 2008. Trichosporon asahii as a prospective pathogen in solid organ transplant recipients. Mycoses. doi: 10.1111/j.1439-0507.2008.01590.x. [DOI] [PubMed]
- 51.Nettles, R. E., L. S. Nichols, K. Bell-McGuinn, M. R. Pipeling, P. J. Scheel, Jr., and W. G. Merz. 2003. Successful treatment of Trichosporon mucoides infection with fluconazole in a heart and kidney transplant recipient. Clin. Infect. Dis. 36E63-E66. [DOI] [PubMed] [Google Scholar]
- 52.Neves, R. P., M. A. Cavalcanti, G. M. Chaves, and O. M. Magalhaes. 2002. Trichosporon pullulans (Lidner) Diddens & Lodder isolated from the oral cavity of AIDS patient. Braz. J. Microbiol. 33241-242. [Google Scholar]
- 53.O'Gorman, C., R. McMullan, C. H. Webb, and A. Bedi. 2006. Trichosporon asahii. Blood-stream infection in a non-cancer patient receiving combination antifungal therapy. Ulster Med. J. 75226-227. [PMC free article] [PubMed] [Google Scholar]
- 54.Okoli, I., C. A. Oyeka, K. J. Kwon-Chung, B. Theelen, V. Robert, J. Z. Groenewald, D. C. McFadden, A. Casadevall, and T. Boekhout. 2007. Cryptotrichosporon anacardii gen. nov., sp. nov., a new trichosporonoid capsulate basidiomycetous yeast from Nigeria that is able to form melanin on niger seed agar. FEMS Yeast Res. 7339-350. [DOI] [PubMed] [Google Scholar]
- 55.Padhye, A. A., S. Verghese, P. Ravichandran, G. Balamurugan, L. Hall, P. Padmaja, and M. C. Fernandez. 2003. Trichosporon loubieri infection in a patient with adult polycystic kidney disease. J. Clin. Microbiol. 41479-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panagopoulou, P., J. Evdoridou, E. Bibashi, J. Filioti, D. Sofianou, G. Kremenopoulos, and E. Roilides. 2002. Trichosporon asahii: an unusual cause of invasive infection in neonates. Pediatr. Infect. Dis. J. 21169-170. [DOI] [PubMed] [Google Scholar]
- 57.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2002. In vitro antifungal susceptibilities of Trichosporon species. Antimicrob. Agents Chemother. 461144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pappas, P. G., C. A. Kauffman, D. Andes, D. K. Benjamin, Jr., T. F. Calandra, J. E. Edwards, Jr., S. G. Filler, J. F. Fisher, B. J. Kullberg, L. Ostrosky-Zeichner, A. C. Reboli, J. H. Rex, T. J. Walsh, and J. D. Sobel. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48503-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfaller, M. A., and D. J. Diekema. 2004. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 424419-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piwoz, J. A., G. J. Stadtmauer, E. J. Bottone, I. Weitzman, E. Shlasko, and C. Cummingham-Rundles. 2000. Trichosporon inkin lung abscesses presenting as a penetrating chest wall mass. Pediatr. Infect. Dis. J. 191025-1027. [DOI] [PubMed] [Google Scholar]
- 61.Ramos, J. M., M. Cuenca-Estrella, F. Gutierrez, M. Elia, and J. L. Rodriguez-Tudela. 2004. Clinical case of endocarditis due to Trichosporon inkin and antifungal susceptibility profile of the organism. J. Clin. Microbiol. 422341-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rastogi, V. L., and P. S. Nirwan. 2007. Invasive trichosporonosis due to Trichosporon asahii in a non-immunocompromised host: a rare case report. Indian J. Med. Microbiol. 2559-61. [DOI] [PubMed] [Google Scholar]
- 63.Rieger, C., S. Geiger, T. Herold, C. Nickenig, and H. Ostermann. 2007. Breakthrough infection of Trichosporon asahii during posaconazole treatment in a patient with acute myeloid leukaemia. Eur. J. Clin. Microbiol. Infect. Dis. 26843-845. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Tudela, J. L., T. M. Diaz-Guerra, E. Melado, V. Cano, C. Tapia, A. Perkins, A. Gomez-Lopez, L. Rodero, and M. Cuenca-Estrella. 2005. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob. Agents Chemother. 494026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Tudela, J. L., A. Gomez-Lopez, A. Alastruey-Izquierdo, E. Melado, L. Bernal-Martinez, and M. Cuenca-Estrella. 2007. Genotype distribution of clinical isolates of Trichosporon asahii based on sequencing of intergenic spacer 1. Diagn. Microbiol. Infect. Dis. 58435-440. [DOI] [PubMed] [Google Scholar]
- 66.Song, H. J., S. L. Chung, and K. S. Lee. 2007. Trichosporon inkin subcutaneous infection in rheumatoid arthritis patient. Int. J. Dermatol. 46282-283. [DOI] [PubMed] [Google Scholar]
- 67.Sugita, T. Trichosporon Behrend (1890). In C. P. Kurtzman, J. W. Fell, and T. Boekhout (ed.), The yeasts: a taxonomic study, 5th ed., in press. Elsevier, Amsterdam, The Netherlands.
- 68.Sugita, T., R. Ikeda, and A. Nishikawa. 2004. Analysis of Trichosporon isolates obtained from the houses of patients with summer-type hypersensitivity pneumonitis. J. Clin. Microbiol. 425467-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sugita, T., M. Nakajima, R. Ikeda, T. Matsushima, and T. Shinoda. 2002. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J. Clin. Microbiol. 401826-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugita, T., and T. Nakase. 1998. Trichosporon japonicum sp. nov. isolated from the air. Int. J. Syst. Bacteriol. 481425-1429. [DOI] [PubMed] [Google Scholar]
- 71.Sugita, T., A. Nishikawa, R. Ikeda, and T. Shinoda. 1999. Identification of medically relevant Trichosporon species based on sequences of internal transcribed spacer regions and construction of a database for Trichosporon identification. J. Clin. Microbiol. 371985-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugita, T., A. Nishikawa, and T. Shinoda. 1998. Identification of Trichosporon asahii by PCR based on sequences of the internal transcribed spacer regions. J. Clin. Microbiol. 362742-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugita, T., M. Takashima, T. Nakase, T. Ichikawa, T. Shinoda, and A. A. Nishikawa. 2002. A basidiomycetous anamorphic yeast, Trichosporon terricola sp. nov., isolated from soil. J. Gen. Appl. Microbiol. 48293-297. [DOI] [PubMed] [Google Scholar]
- 74.Svejgaard, E. L., and J. Nilsson. 2004. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses 47131-135. [DOI] [PubMed] [Google Scholar]
- 75.Swofford, D. L. 2002. PAUP, version 4.0b10. Sinauer Associates, Sunderland, MA.
- 76.Taj-Aldeen, S. J., H. I. Al-Ansari, T. Boekhout, and B. Theelen. 2004. Co-isolation of Trichosporon inkin and Candida parapsilosis from a scalp white piedra case. Med. Mycol. 4287-92. [DOI] [PubMed] [Google Scholar]
- 77.Tashiro, T., H. Nagai, P. Kamberi, Y. Goto, H. Kikuchi, M. Nasu, and S. Akizuki. 1994. Disseminated Trichosporon beigelii infection in patients with malignant diseases: immunochemical study and review. Eur. J. Clin. Microbiol. Infect. Dis. 13218-224. [DOI] [PubMed] [Google Scholar]
- 78.Thérizol-Ferly, M., M. Kombila, M. Gomez de Diaz, T. H. Duong, and D. Richard-Lenoble. 1994. White piedra and Trichosporon species in equatorial Africa. I. History and clinical aspects: an analysis of 449 superficial inguinal specimens. Mycoses 37249-253. [DOI] [PubMed] [Google Scholar]
- 79.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: challenges to immunocompromised patients for the twenty-first century. Transpl. Infect. Dis. 1247-261. [DOI] [PubMed] [Google Scholar]
- 80.Walsh, T. J., A. Groll, J. Hiemenz, R. Fleming, E. Roilides, and E. Anaissie. 2004. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 10(Suppl. 1)48-66. [DOI] [PubMed] [Google Scholar]
- 81.Wolf, D. G., R. Falk, M. Hacham, B. Theelen, T. Boekhout, and G. Scorzetti. 2001. Multidrug-resistant Trichosporon asahii infection of nongranulocytopenic patients in three intensive care units. J. Clin. Microbiol. 394420-4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wynne, S. M., K. J. Kwon-Chung, Y. R. Shea, A. C. Filie, A. Varma, P. Lupo, and S. M. Holland. 2004. Invasive infection with Trichosporon inkin in 2 siblings with chronic granulomatous disease. J. Allergy Clin. Immunol. 1141418-1424. [DOI] [PubMed] [Google Scholar]
- 83.Yarrow, D. 1998. Methods for the isolation, maintenance and identification of yeasts, p. 77-100. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts: a taxonomic study, 4th ed. Elsevier, Amsterdam, The Netherlands.
- 84.Yoss, B. S., L. Sautter, and H. J. Brenker. 1997. Trichosporon beigelii, a new neonatal pathogen. Am. J. Perinatol. 14113-117. [DOI] [PubMed] [Google Scholar]