Abstract
This study investigated the influence of age on the functional status of mitochondria isolated from skeletal muscle of C57BL/6 mice aged 3 and 18 months. We hypothesized that skeletal muscle mitochondria isolated from aged animals will exhibit a decreased respiratory function. Mitochondrial respiratory functional measures (ie, State 3 and 4 respiration, respiratory control ratio and number of nanomoles of ADP phosphorylated by nanomoles of O2 consumed per mitochondrion) and biochemical markers of oxidative damage (aconitase activity, protein carbonyl derivatives, sulfhydryl groups, and malondialdehyde) were measured in isolated mitochondrial suspensions. Along with traditional tests of mitochondrial function, an in vitro repetitive ADP-stimulation test was used to evaluate the mitochondrial capacity to reestablish the homeostatic balance between successive ADP stimulations. The number of mitochondria per mitochondrial suspension, calculated by transmission electron microscopy, was used to normalize functional and biochemical data. Our results confirm the existence of an age-associated decline in mitochondrial function of mixed skeletal muscle, which is significantly correlated with higher levels of mitochondrial oxidative damage.
Keywords: Aging, Mitochondria, Respiratory function
AGING is characterized by a progressive decline in cellular function and a decrease in the cell's ability to maintain homeostasis. In this regard, it is clear that mitochondria have a critical role in maintaining cellular homeostasis as mitochondria are involved in cellular bioenergetics, production of reactive oxygen species (ROS), the maintenance of calcium homeostasis, and the induction of apoptotic processes (1,2). Several reports suggest that mitochondria might be among the most adversely affected organelles with advanced age (3,4). For example, a decline in mitochondrial bioenergetic potential and an increase in the oxidative damage to mitochondrial biomolecules occur with aging in many tissues (5–8). An age-related decline in mitochondrial bioenergetic function could contribute to a loss of cell viability, leading to an increase in cellular necrosis and/or apoptosis (9–12).
Although it is well established that aging results in loss of skeletal muscle contractile dysfunction and mass, the role that mitochondrial damage has in this phenomenon remains controversial. For example, although several reports suggest that mitochondria isolated from aged skeletal muscle exhibit impaired morphological, biochemical, and functional alterations (13–18), other investigators have failed to demonstrate age-related changes in mitochondrial phenotypes in humans (21,22,25) and other animals (23,24). These conflicting results could be due to several methodological issues associated with the study of isolated mitochondria along with differences in the ages of the animals investigated. A key methodological issue relates to the procedures used to isolate mitochondria (21). For example, protocols designed to isolate intact mitochondria from muscle do not produce a mitochondrial fraction with high purity, leading to contamination with nonmitochondrial proteins. Therefore, normalization of mitochondrial functional measures to the protein content of the mitochondrial suspension could bias these results. In an effort to avoid this pitfall, several authors have measured the level of mitochondrial matrix enzymes, such as citrate synthase (CS), as a marker of mitochondrial mass (21,26). However, even when mitochondrial data are expressed per CS activity, conflicting results still persist (21); this discrepancy could be due, in part, to CS contamination produced by mitochondrial damage associated with the isolation procedures.
Another problem that could contribute to the conflicting results is related to the sensitivity and specificity of the in vitro assays used to evaluate mitochondrial function. Indeed, measurements performed in energized mitochondria in vitro are typically standardized to evaluate the maximal functionality of several components within isolated mitochondria, such as the electron transport chain (ETC) complexes or the ATP synthase and the integrity of the inner membrane. The main objective of these assays is to acutely stimulate the previously energized mitochondria and thereby to assess their maximal functionality during a brief period of time. However, considering the in vivo physiology of muscle contraction, it is predicted that prolonged alterations of the ATP-to-ADP ratio resulting from the continuous actin–myosin interaction (27–29) would impose a continuous overload to the mitochondria with a prolonged State 3. Therefore, the in vivo bioenergetic function of skeletal muscle mitochondria appears to be very different from the metabolic stress transitorily imposed by the traditional in vitro tests. It follows that these routinely used in vitro mitochondrial tests might not be sensitive or specific enough to detect age-related alterations in skeletal muscle mitochondrial function.
A final issue that could contribute to the variance in the literature concerning the impact of age on mitochondrial function is the wide variation in animal ages between studies. That is, age per se might also constitute an additional problem because the occurrence of age-related concomitant disease in very old animals will bias the results (30). Therefore, it is important to study aging itself without the influence of other confounding factors, such as diseases.
Therefore, the objective of the present study was to investigate the influence of age on the functional status of mitochondria isolated from skeletal muscle avoiding the aforementioned confounding factors that may influence the results. To avoid the methodological issues associated with the isolation of mitochondria, we normalized all mitochondrial functional and biochemical measures to the number of mitochondria quantified by transmission electron microscopy in the final mitochondrial suspension. Moreover, to mimic the bioenergetic function of skeletal muscle mitochondria during muscular contractions, we have developed a maximal standardized in vitro protocol that involves successive ADP stimulations of mitochondrial State 3 respiration. Finally, to avoid the pitfalls associated with studying old animals that are diseased, our experimental design incorporated mature animals. We tested the hypothesis that compared with young animals, mitochondria isolated from aged mature animals will exhibit a decreased respiratory function. If our experiments reveal that aging results in a decline in mitochondrial function, we also predict that this age-related decline will be associated with increased mitochondrial oxidative stress.
METHODS
Experimental Design
Sixteen male C57BL/6 mice were divided into two groups according to their ages: young group (3 months old; n = 8) and aged mature group (18 months old; n = 8). Based on the survival curves for these animals (31), 18-month-old animals can be classified as both aged and mature. The option to use aged mature instead of old senescent animals was based on the assumption that the occurrence of any age-related concomitant disease might bias the results. Mice were provided with food and water ad libitum and were sacrificed after 1 week of quarantine. All animals were maintained at a constant temperature (21°C–25°C) on a daily light schedule of 12 hours of light versus dark until sacrifice. Housing and experimental treatment of animals were in accordance with the Guide for the Care and Use of Laboratory Animals from the Institute for Laboratory Animal Research (ILAR 1996). The local ethics committee approved the study, and experiments complied with national laws.
Animal Killing, Skeletal Muscle Extraction and Mitochondria Isolation
Animals were killed by cervical dislocation, and the hindlimb muscles (soleus, gastrocnemius, tibialis anterior, and quadriceps) were excised for preparation of isolated mitochondria by conventional methods of differential centrifugation, as previously described by Tonkonogi and Sahlin (32). Briefly, after weighing, the muscles were immediately minced in ice-cold isolation medium containing 100 mM sucrose, 0.1 mM ethylene glycol tetraacetic acid (EGTA), 50 mM Tris–HCl, 100 mM KCl, 1 mM KH2PO4, and 0.2% bovine serum albumin (BSA), pH 7.4. The blood-free tissue was rinsed and suspended in 10 mL of fresh medium containing 0.2 mg/mL bacterial proteinase (Nagarse E.C.3.4.21.62, Type XXVII; Sigma, St Louis, MO) and stirred for 2 minutes. The sample was then homogenized with a tightly fitted Potter–Elvehjen homogenizer and a Teflon pestle. After homogenization, three volumes (30 mL) of Nagarse-free isolation medium were added to the homogenate followed by centrifugation at 700g for 10 minutes. The resulting pellet was removed, and the supernatant was resuspended and centrifuged at 10,000g for 10 minutes. The supernatant was decanted, and the pellet was gently resuspended in isolation medium (1.3 mL/100 mg initial tissue) and centrifuged at 7,000g for 3 minutes. The supernatant was discarded, and the final pellet, containing the mitochondrial fraction, was gently resuspended (0.4 μL/mg initial tissue) in a medium containing 225 mM mannitol, 75 mM sucrose, 10 mM Tris, and 0.1 mM EDTA, pH 7.4. Total protein concentration in the mitochondrial suspension was estimated spectrophotometrically with the biuret method using BSA as standard. All mitochondrial isolation procedures were performed at 0°C–4°C. The mitochondrial suspensions were studied within 2 hours after the excision of the muscles and were maintained on ice (0°C–4°C) throughout this period.
One aliquot from the final mitochondrial suspension was used for measurement of total protein concentration and CS activity in the mitochondrial suspension. A second aliquot was processed for morphological analysis. Another aliquot was used for measurement of mitochondrial respiratory function using standard in vitro methods. The remaining mitochondrial suspension was used to assess functional and biochemical parameters during an in vitro ADP-consecutive stimulation test that incorporates one, three, and six ADP pulses interspersed by a 90-second recovery between stimulation trying to mimic the prolonged cellular metabolic demands during exercise. The utilization of this in vitro–simulated exercise test was designed to evaluate the capacity of mitochondria to reestablish their homeostatic balance between consecutive ADP stimulations as function of time. All the biochemical parameters were assessed in the whole content of the oxygen chamber following treatment with 0.1% Triton X-100.
Biochemical Analysis in Mitochondrial Fraction
Total protein determination.—
Total protein concentration in the mitochondrial suspension was determined using the biuret method with BSA as the standard.
CS assay.—
CS activity was measured according to Coore and coworkers (33) by spectrophotometric (412 nm) measurement of the amount of 5,5-dithiobis (2-nitrobenzoate) that reacted with acetyl-CoA upon release from the reaction of acetyl-CoA with oxaloacetate. CS activity was assessed in the mitochondrial suspension after treatment with 0.1% Triton X-100.
Mitochondrial Preparation for Transmission Electron Microscopy (TEM)
Mitochondrial preparation for TEM analyses and further morphometric characterization has been previously described (18). Briefly, 100 μL of the mitochondrial suspension was centrifuged at 7,000g for 10 minutes, and the resulting pellet was fixed with 2.5% glutaraldehyde, postfixed with 2% osmiumtetroxide, deyhdrated in graded alcohol, and embedded in LR White. Ultrathin sections mounted on copper grids (300 Mesh) were contrasted with uranyl acetate and lead citrate for transmission electron microscopy (Zeiss EM 10A) analysis. In order to obtain a global characterization of the pellet, several grids were prepared (five to eight grids per animal each containing three to four sections) from different zones ranging through the whole pellet.
Morphometric Analysis of the Mitochondrial Pellet
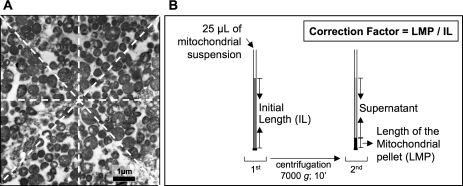
Morphometric analysis was performed as described elsewhere (18) in at least 50 photos per mitochondrial pellet using a morphometric processing program (ImageJ, NIH Image). In each photograph, the number of mitochondria per micrometer and square micrometer were determined. Taking into account the magnification of each micrograph, the number of mitochondria per micrometer was assessed by counting the mitochondria which were situated under the four lines that crossed the center of the micrograph, traced horizontally (n = 1), vertically (n = 1), and obliquely (n = 2) (Figure 1A), resulting in the final number of mitochondria per micrometer as the mean value of the four counts; the number of mitochondria per square micrometer was evaluated by counting the total mitochondria present in each micrograph. For each pellet, the mean number of mitochondria per micrometer and square micrometer was calculated from all analyzed micrographs and their product was used to calculate the mitochondrial concentration in the pellet (number per cubic micrometer with further adjustment to the number per milliliter).
Figure 1.
Methodological procedures to quantify the mitochondrial concentration in each suspension. (A) The number of mitochondria per micrometer was assessed by counting the mitochondria that were situated under the four lines crossing the center of the micrograph; the final number of mitochondria per micrometer was established as the mean value of the four counts. The number of mitochondria per square meter was evaluated by counting the total mitochondria present in each micrograph. (B) Assessment of the correction factor for centrifugation-induced compaction, in order to adjust the data drawn from the microscopic evaluation of the pellet to the real volume of the mitochondrial suspension.
Afterward, in order to estimate the mitochondrial concentration in the mitochondrial suspension from the data obtained in the pellet, we assessed the degree of compression induced by the centrifugation procedure using the following method. In each suspension, a small quantity (25 μL) was introduced in a glass capillary tube followed by closure of one end of the tube. Subsequently, the sample was centrifuged at 7,000g for 10 minutes and the pellet size was measured (mm, with a 0.1-mm graded ruler) and related to the length of the glass capillary tube occupied by the 25 μL of mitochondrial suspension (Figure 1B). This relation, representing the correction factor necessary to expand each pellet to the length occupied by 25 μL of mitochondrial suspension, was multiplied to the respective mitochondria concentration calculated from the TEM analysis, in order to estimate the real number of mitochondria per volume of suspension. The number of total mitochondria per unit of mitochondrial suspension was then used to normalize all functional and biochemical data.
Measurement of Mitochondrial Respiratory Activity
Traditional tests.—
After the determination of the total protein concentration in the mitochondrial suspension (estimated spectrophotometrically with the biuret method using BSA as standard), the mitochondrial respiratory function was polarographically measured using a Clark-type oxygen electrode (DW 1, Hansatech, Norfork, UK). All assays were conducted in a 0.75-mL closed, magnetically stirred, and temperature-controlled (25°C) glass chamber. Mitochondria were added to the chamber (0.5 mg of protein) in a reaction buffer of 225 mM mannitol, 75 mM sucrose, 10 mM Tris, 10 mM KCl, 10 mM K2HPO4, and 0.1 mM EDTA, pH 7.5 (34). After a 1-minute equilibration period, mitochondrial respiration was initiated by adding pyruvate (5 mM) plus malate (2 mM) or succinate (10 mM) plus rotenone (4 μM). State 3 respiration was determined after adding ADP to a final concentration of 200 μM; State 4 respiration was measured as the rate of oxygen consumption in the absence of ADP phosphorylation. The respiratory control ratio (RCR), that is, the ratio between State 3 and State 4 respiration, and number of nanomoles of ADP phosphorylated by nanomoles of O2 consumed per mitochondrion (ADP/O) were calculated according to Estabrook (19), using 235 nmol O2/mL as the value for the solubility of oxygen at 25°C. To quantify mitochondrial inner membrane permeability and the maximal rate of uncoupled oxidative phosphorylation, oligomycin (final concentration of 1.5 μg/mL) and carbonyl cyanide m-chlorophenylhydrazone (CCCP; 2 μM), respectively, were added during State 3 respiration with saturated amounts of ADP (final concentration of 1 mM). This protocol avoids interference of the permeability to protons through the ATP synthase during State 4 respiration (state oligomycin) and ensures that the variations in membrane permeability do not interfere with the inhibition of the respiratory chain, because the permeability in the presence of CCCP is always maximal (20).
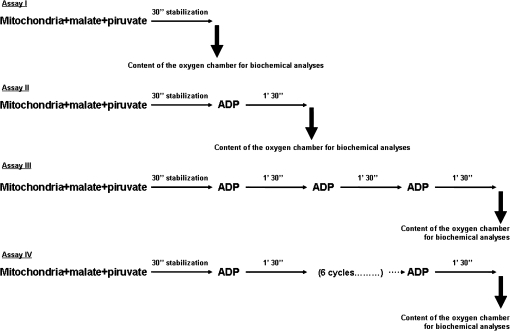
Consecutive ADP-stimulation test.—
In order to mimic the in vivo metabolic stress imposed to mitochondria during muscular exercise, we have performed a consecutive ADP-stimulation test in a Clark-type oxygen electrode (Hansatech DW 1). This in vitro test consisted of four assays (Figure 2)—Assay I: after a 1-minute equilibration period, mitochondrial respiration was initiated by adding pyruvate (5 mM) plus malate (2 mM) followed by 30 seconds of stabilization; Assay II: after a 1-minute equilibration period, mitochondrial respiration was initiated by adding pyruvate (5 mM) plus malate (2 mM) followed by 30 seconds of stabilization and the addition of one pulse of ADP followed by 1 minute 30 seconds; Assay III: after a 1-minute equilibration period, mitochondrial respiration was initiated by adding pyruvate (5 mM) plus malate (2 mM) followed by 30 seconds of stabilization and the addition of three consecutive pulses of ADP interspersed by 1 minute 30 seconds each; Assay IV: after a 1-minute equilibration period, mitochondrial respiration was initiated by adding pyruvate (5 mM) plus malate (2 mM) followed by 30 seconds of stabilization and the addition of six consecutive pulses of ADP interspersed by 1 minute 30 seconds each.
Figure 2.
Repetitive ADP-stimulation test. See text for detailed description.
In Assays II, III, and IV, mitochondrial respiratory function was assessed by analyzing several functional parameters (ie, State 3 and State 4 respiratory rates, the RCR and ADP/O) in each of the ADP cycles. At the end of each of the four assays, the content of the oxygen chamber was collected and used for biochemical analysis (Figure 2). The content of the oxygen chamber at the end of Assay I represents the mitochondrial basal condition (prestimulation); the content of the oxygen chamber after Assays II, III, and IV corresponds to different degrees of ADP stimulation. In all assays a certain quantity of the oxygen chamber (500 μL) was used for the determination of protein carbonyl derivatives, total mitochondrial sulfhydryl (SH) groups, and malondialdehyde (MDA). Furthermore, a portion of the oxygen chamber (250 μL) was centrifuged for 15 minutes at 10,000g in order to determine the content of extramitochondrial cytochrome c. Aconitase (ACON) activity was also quantified in Assay I in order to indirectly assess the age-related superoxide radical generation at basal conditions.
ACON activity.—
The activity of ACON was assayed because ACON activity is redox sensitive and diminished ACON activity has been interpreted as an index of superoxide radical generation and mitochondrial oxidative damage (35). ACON activity was measured spectrophotometrically by monitoring the formation of cis-aconitate at 240 nm after the addition of 20 mM isocitrate at 25°C, according to Krebs and Holzach (36). One unit of activity was defined as the amount of enzyme necessary to produce 1 μM cis-aconitate per minute (molar extinction coefficient [ϵ] at 240 nm (ϵ240) = 3.6 mM−1·cm−1).
Protein carbonyl derivatives.—
For the protein carbonyl derivatives assay, a certain volume (vol) of the oxygen chamber containing 20 μg of protein was derivatized with dinitrophenylhydrazine. Briefly, the sample was mixed with 1 vol of 12% sodium dodecyl sulfate plus 2 vol of 20 mM dinitrophenylhydrazine prepared in 10% trifluoroacetic acid, followed by 30 minutes of dark incubation, after which 1.5 vol of 2 M Tris/18.3% β-mercaptoethanol was added. A negative control was simultaneously prepared for each sample. After the derivatized proteins were diluted in Tris-buffered saline (TBS) to obtain a final concentration of 0.001 μg/μL, a 100-μL volume was slot blotted into a Hybond-polyvinylidene difluoride membrane. Immunodetection of carbonyls was then performed using rabbit polyclonal anti dinitrophenyl: 2000; V0401 DakoCytomation, Freiburg, Germany) as the primary antibody and anti-rabbit IgG peroxidase (Amersham Pharmacia Biotech, Buckinghamshire, UK) as the second antibody (1:2000 dilution). The bands were visualized by treating the immunoblots with enhanced chemiluminescence (ECL) chemiluminescence reagents (Amersham Pharmacia Biotech), according to the supplier's instructions, followed by exposure to X-ray films (Kodak Biomax Light Film, Sigma). The films were analyzed with QuantityOne Software version 4.3.1 (Bio-Rad, Hercules, CA).
Total mitochondrial SH groups.—
The oxidatively modified SH groups, including glutathione (GSH) and other SH-containing proteins, were quantified by spectrophotometric measurement according to the method proposed by Hu (37). Briefly, after 1-minute centrifugation at 1,000g, 50 μL of supernatant was added to a medium containing 150 μL of 0.25 M Tris, 790 μL of methanol, and 10 μL of 10 mM 5,5′-dithio-bis (2-nitrobenzoic acid). The colorimetric assay was performed at 414 nm against a blank test. Total SH content was calculated using the following molar extinction coefficient: ϵ414 = 13.6 mM−1·cm−1.
MDA.—
Nonspecific lipid peroxidation was measured by determining the levels of lipid peroxides as the amount of thiobarbituric acid reactive substances (TBARS) formed according to Rohn and colleagues (38), with some modifications. Mitochondrial protein (0.5 mg) obtained from the oxygen chamber was incubated at 25°C, in 500 μL of a medium consisting of 175 mM KCl and 10 mM Tris, pH 7.4. Samples of 50 μL were taken and mixed with 450 μL of a TBARS reagent (1% thiobarbituric acid, 0.6 N HCl, and 0.0056% butylated hydroxytoluene). The mixture was heated at 80–90°C during 15 minutes and cooled down in ice for 10 minutes before centrifugation at 1,500g for 5 minutes. Lipid peroxidation was estimated by the appearance of TBARS spectrophotometrically quantified at 535 nm. The amount of TBARS formed was calculated using a molar extinction coefficient (ϵ) of 1.56 × 105 M−1·cm−1 (39).
Extramitochondrial cytochrome c.—
After centrifugation, the content of extramitochondrial cytochrome c in the supernatant was determined. Equivalent amounts of proteins were electrophoresed on a 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel, followed by blotting on a nitrocellulose membrane (Hybond-ECL; Amersham Pharmacia Biotech). After blotting, nonspecific binding was blocked with 5% nonfat dry milk in TTBS (TBS with Tween 20) and the membrane was incubated with anti–cytochrome c (1:1,000; Pharmingen) antibody for 2 hours at room temperature, washed, and incubated with secondary horseradish peroxidase–conjugated anti-mouse (Amersham Pharmacia Biotech) for 2 hours. The membrane was then washed and developed with Western blotting chemiluminescence reagents (Amersham Pharmacia Biotech) according to the manufacturer's instructions, followed by exposure to X-ray films (Kodak Biomax Light Film, Sigma). The films were analyzed with QuantityOne Software (Bio-Rad).
Statistical Analysis
Means and standard deviations were calculated for all variables in both groups. An independent t test was used to analyze the differences between groups. One-way analysis of variance was used to analyze the differences between ADP stimulations in each group. If significant differences existed (p < .05), post hoc comparisons were performed using Scheffe test. The Spearman correlation coefficient was used to analyze the correlations between total protein determination, CS activity, and concentration of mitochondria as well as between mitochondrial functional and biochemical data. The Statistical Package for the Social Sciences (SPSS version 10.0) was used for all analyses. Significance was established a priori as p < .05.
RESULTS
Body weights, skeletal muscle wet weights, and CS activity in isolated mitochondria are shown in Table 1. Compared with young animals, a reduction in both skeletal muscle wet weight and the ratio of muscle weight-to-body weight is apparent in the mature animals, suggesting the existence of sarcopenia. When analyzing the mitochondrial suspensions obtained from young and mature animals (Table 2), we observed a significantly higher number of mitochondria in the suspensions of the mature animals; this finding agrees with the CS activity of isolated mitochondria shown in Table 1. In contrast, total protein content in the mitochondrial suspension was not significantly different between the groups (Table 2). Likewise, we did not find significant differences in the mitochondrial areas between the young and the mature animals (data not shown). With regard to the correlation coefficients, strong correlations existed between CS activity and the number of mitochondria (r = .885; p < .05). The correlations between the protein content and CS activity (r = .664; p < .05) and between the protein content and the number of mitochondria (r = .384; p < .05) were also significant.
Table 1.
Body Weights, Skeletal Muscle Wet Weights, and CS Activity in Isolated Mitochondria From Young and Mature Animals
| Young | Mature | |
| Body weight (g) | 25.57 ± 0.92 | 36.64 ± 2.34* |
| Dissected skeletal muscle weight (soleus, gastrocnemius, tibialis anterior, and quadriceps) (g wet weight) | 1.92 ± 0.09 | 1.78 ± 0.11* |
| Ratio dissected muscle weight-to-body weight | 0.075 ± 0.002 | 0.048 ± 0.008* |
| Isolated mitochondria CS activity (nmol/min/mg) | 379.50 ± 34.9 | 524.08 ± 40.5* |
Notes: Data are mean ± standard deviation of eight separate experiments in young and mature animals. CS = citrate synthase.
Significantly different from young animals (p < .05).
Table 2.
Characterization of the Mitochondrial Suspensions Obtained in Young and Mature Animals
| Young | Mature | |
| Protein content (mg/mL) | 15.47 ± 3.04 | 16.94 ± 3.89 |
| CS activity (nmol/min/mL) | 5871.17 ± 489.23 | 8878.67 ± 682.76* |
| Mitochondria (n × 1010/mL) | 2.36 ± 1.10 | 7.87 ± 3.20* |
Notes: Data are mean ± standard deviation. Biochemical data represent eight experiments in young and mature animals. The morphometric data represent the counts in at least 50 micrographs of each mitochondrial pellet. After the counts, the correction factor of each sample was applied in order to estimate the concentration of mitochondria in the suspension (number of mitochondria per milliliter). CS = citrate synthase.
Significantly different from young animals (p < .05).
Age-Related Mitochondrial Functional Alterations
Traditional in vitro tests.—
The data of the age-related changes of respiratory function of skeletal muscle mitochondria are presented in Tables 3 and 4. Our results reveal that older animals exhibit a significant impairment in the mitochondrial respiratory rates when energized with both malate + pyruvate (M + P) and succinate + rotenone (S + R) as substrates.
Table 3.
Functional Data Obtained From Skeletal Muscle Mitochondria Isolated From Young and Mature Animals With Complex I–Linked Substrates, Pyruvate (5 mM) and Malate (2 mM)
| Young | Mature | |
| State 3 (nmol O2/min/mit) | 13.3 × 10−8 ± 7.2 × 10−8 | 2.35 × 10−8 ± 0.79 × 10−8* |
| State 4 (nmol O2/min/mit) | 1.4 × 10−8 ± 0.8 × 10−8 | 0.44 × 10−8 ± 0.15 × 10−8 |
| RCR | 9.4 ± 0.6 | 5.2 ± 0.8* |
| ADP/O per mit | 2.66 × 10−9 ± 0.4 × 10−9 | 0.78 × 10−9 ± 0.1 × 10−9* |
Notes: Data are mean ± standard deviation and represent eight separate experiments in each group. State 3 and State 4 respiratory rates are expressed as nanomoles of O2 consumed per minute per mitochondrion. RCR = respiratory control ratio; ADP/O, number of nanomoles of ADP phosphorylated by nanomoles of O2 consumed per mitochondrion; mit = mitochondria.
Significantly different from young animals (p < .05).
Table 4.
Functional Data Obtained From Skeletal Muscle Mitochondria Isolated From Young and Mature Animals With Complex II–Linked Substrates, Succinate (10 mM) and Rotenone (4 μM)
| Young | Mature | |
| State 3 (nmol O2/min/mit) | 15.9 × 10−8 ± 9.0 × 10−8 | 3.6 × 10−8 ± 1.1 × 10−8* |
| State 4 (nmol O2/min/mit) | 7.2 × 10−8 ± 4.1 × 10−8 | 2.3 × 10−8 ± 0.7 × 10−8* |
| RCR | 2.2 ± 0.1 | 1.5 ± 0.1* |
| ADP/O per mit | 1.15 × 10−9 ± 0.2 × 10−9 | 0.33 × 10−9 ± 0.03 × 10−9* |
Notes: Data are mean ± standard deviation and represent 8 separate experiments in each group. State 3 and State 4 respiratory rates are expressed as nanomoles of O2 consumed per minute per mitochondrion. RCR = respiratory control ratio; ADP/O = number of nanomoles of ADP phosphorylated by nanomoles of O2 consumed per mitochondrion; mit = mitochondria.
Significantly different from young animals (p < .05).
Interestingly, State 4 respiratory rates were unchanged with age. To assess the mitochondrial oxidative phosphorylation efficiency, we calculated the ADP/O ratio normalized to the number of mitochondria. Our results indicate that when expressed per mitochondrion, younger animals reveal higher oxidative phosphorylation efficiency when compared with the aged and mature animals. As for the RCR, our results were comparable with other data reported elsewhere on mitochondria isolated from skeletal muscle of different ages, with older animals presenting lower values when using both M + P and S + R as respiratory substrates (16,40).
The results of our study suggest that the age-related functional impairment of mitochondria is mainly targeted at the State 3 respiratory rates, this assumption being further supported by the results of the oligomycin-inhibited State 3 respiration and CCCP-induced uncoupled respiration (Table 5).
Table 5.
Effect of Age on Olygomycin-Inhibited State 3 Respiration and CCCP-Induced Uncoupled Respiration in Skeletal Muscle Mitochondria Isolated From Young and Mature Animals
| Young | Mature | |
| State Olygomycin (nmol O2/min/mit) | 1.7 × 10−8 ± 0.9 × 10−8 | 0.5 × 10−8 ± 0.1 × 10−8 |
| State CCCP (nmol O2/min/mit) | 9.4 × 10−8 ± 5.2 × 10−8 | 2.14 × 10−8 ± 0.6 × 10−8* |
Notes: Data are mean ± standard deviation and represent 8 separate experiments in each group. Results are expressed as nanomoles of O2 consumed per minute per mitochondrion. Respiration was induced with pyruvate (5 mM) and malate (2 mM) as energizing substrates and saturated ADP concentration (1 mM) to initiate State 3 respiration. State 3 was inhibited after the addition of olygomycin (final concentration 1, 5 μg/mL) and CCCP (final concentration 2 μM) to uncouple mitochondrial respiration. CCCP = m-chlorophenylhydrazone; mit = mitochondria.
Significantly different from young animals (p < .05).
Mitochondria isolated from the older animals, when energized with M + P and stimulated with excess ADP, showed no significant alterations in the respiratory rate in the presence of oligomycin when compared with the younger animals (Table 5). In contrast, uncoupled mitochondrial respiration with CCCP was significantly diminished in the older animals.
Repetitive ADP-stimulation test.—
To mimic the bioenergetic demand that occurs in skeletal muscle mitochondria during repeated bouts of exercise, we designed a repetitive ADP simulation test. This test consisted of exposing separate aliquots of mitochondria that were exposed to one, three, or six repetitive ADP stimulation “cycles”; mitochondrial oxygen consumption was measured during each trial. Following each of these protocols, each sample of mitochondria was removed from the oxygen chamber and biochemically analyzed for biomarkers of oxidative damage.
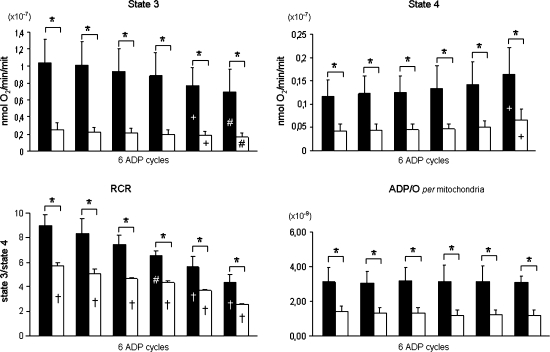
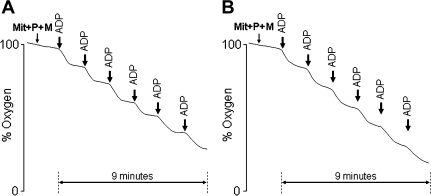
State 3 respiratory rate was acutely diminished with the consecutive ADP stimulations, in both the young and aged animals (Figure 3). In the aged animals, State 3 respiratory rates were significantly lower in all ADP cycles. Figure 4 shows a typical representation of the mitochondrial oxygen consumption in Assay IV for one young (Figure 4A) and one aged (Figure 4B) animal. Notice that isolated mitochondria from aged animals were unable to reestablish State 4 conditions in the fourth, fifth, and sixth ADP cycles.
Figure 3.
Functional data obtained from skeletal muscle mitochondria isolated from young and mature animals with Complex I–linked substrates, pyruvate (5 mM) and malate (2 mM), in the repetitive ADP-stimulation test. Data are mean ± standard deviation. State 3 and State 4 respiratory rates are expressed as nanomoles of O2 consumed per mitochondrion. Filled bars: young animals; open bars: mature animals. *Age differences (p < .05); +significantly different from first cycle; #significantly different from first and second cycle; †significantly different from all ADP cycles (p < .05).
Figure 4.
Schematic representation of the Assay IV (6 ADP cycles) from the repetitive ADP-stimulation test in one young animal (A) and one mature animal (B).
In both experimental groups, the ADP/O ratio remained unaltered between the ADP cycles; nonetheless, compared with aged animals, the ADP/O ratio was significantly higher in the younger animals. In both young and aged animals, the RCR decreased as a function of each successive ADP stimulation trial. Compared with young animals the RCR was significantly lower in the aged animals in all ADP cycles.
Age-Related Mitochondrial Biochemical Alterations
In order to assess the age-related alterations in oxidative stress and damage, we determined the activity and content of several oxidative stress biomarkers in mitochondria obtained from young and mature animals (Table 6) at basal conditions (Assay I).
Table 6.
Biochemical Data Obtained From Skeletal Muscle Mitochondria Isolated From Young and Mature Animals
| Young | Mature | |
| Aconitase (U/mit) | 3.8 × 10−5 ± 2.2 × 10−5 | 0.6 × 10−5 ± 0.2 × 10−5* |
| Carbonyls (OD/mit) | 1.3 × 10−3 ± 0.6 × 10−3 | 6.0 × 10−3 ± 2.0 × 10−3* |
| SH groups (nmol/mit) | 1.5 × 10−9 ± 0.8 × 10−9 | 0.3 × 10−9 ± 0.1 × 10−9* |
| MDA (nmol/mit) | 6.8 × 10−12 ± 4.6 × 10−12 | 3.5 × 10−12 ± 1.4 × 10−12 |
Notes: Data are mean ± standard deviation. Biochemical data represent eight experiments for each group. All data are expressed per mitochondrion. Protein carbonyl derivatives are expressed as optical density (OD) arbitrary units per mitochondrion. mit = mitochondria; SH = sulfhydryl; MDA = malondialdehyde.
Significantly different from young animals (p < .05).
Our results reveal a significantly diminished ACON activity in the mature animals; this observation is consistent with an augmented inactivation probably mediated by an increased superoxide production (41). We further found increased levels of oxidative damage in mitochondria isolated from the mature animals, more specifically at the level of proteins, with increased contents of protein carbonyls and a reduced expression of SH groups. However, our MDA results do not corroborate this assumption because its levels were lower in the mature animals, suggesting that nonspecific lipid peroxidation was not increased in the older animals.
Repetitive ADP-stimulation test.—
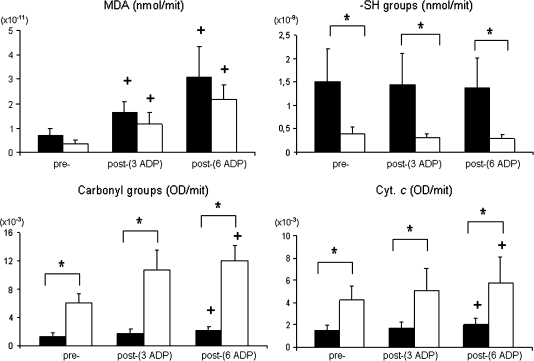
When stimulated with consecutive ADP cycles, mitochondria from both young and aged animals showed a significant increase in the MDA levels and protein carbonyl and extramitochondrial cytochrome c content (Figure 5). In contrast, these changes did not occur in either experimental group following a single ADP stimulation (data not shown).
Figure 5.
Acute biochemical alterations induced by the repetitive ADP-stimulation test in skeletal muscle mitochondria isolated from young and mature animals. Data are mean ± standard deviation. All data are expressed per mitochondrion. Data from protein carbonyl derivatives and cytochrome c are expressed as optical density arbitrary units per mitochondrion. Filled bars: young animals; open bars: mature animals. *Age differences (p < .05); +significantly different from prestimulation (p < .05).
When correlating the data from mitochondrial respiratory activity with oxidative damage biomarkers, strong correlations were found between State 3 respiratory rate and the content of SH and carbonyl groups (r = .967 and r = −.533, respectively; p < .05) and State 4 respiratory rate with MDA levels (r = .827; p < .05).
Discussion
Overview of the Principal Findings
Our results support the hypothesis that aging is associated with diminished respiratory function in skeletal muscle mitochondria. Indeed, when mitochondria were acutely stimulated with ADP, both State 3 respiration and the RCR were significantly lower in skeletal muscle mitochondria from aged animals compared with young animals. Similarly, when mitochondria were repeatedly stimulated with the addition of ADP (ie, simulated exercise), the functional decline induced by the consecutive ADP-stimulation test was significantly greater in mitochondria from aged animals. We also predicted that this age-related decline in mitochondrial respiratory function would be associated with higher levels of mitochondrial oxidative damage. Our findings are consistent with this postulate as aging was associated with a decline in mitochondrial SH groups, diminished ACON activity, and increased mitochondrial protein oxidation. A brief discussion of these and related issues follows.
Aging Impairs Skeletal Muscle Mitochondrial Respiratory Function
The impact of aging on skeletal muscle mitochondrial function remains controversial. Although some studies indicate that mitochondria isolated from aged skeletal muscle exhibit impaired respiratory function (13–17), other investigations report no age-related changes in mitochondrial State 3 respiratory function (21–25). We reasoned that these opposing findings could result from both methodological issues associated with investigating isolated mitochondria along with the wide variation in animal ages across studies. To avoid the methodological problems associated with studying isolated mitochondria (ie, normalization errors resulting from contamination of mitochondrial suspension with nonmitochondrial proteins), we normalized mitochondrial functional and biochemical measures to the number of intact mitochondria present in our suspension. Further, to mimic the bioenergetic function of skeletal muscle mitochondria during repeated muscular contractions (ie, muscular exercise), we employed a maximal in vitro protocol using standardized and successive ADP stimulations of mitochondrial State 3 respiration. Finally, to avoid the potential problems associated with studying very old and possibly diseased animals, our experimental design incorporated aged, mature animals. Our results clearly support the notion that compared with young animals, skeletal muscle mitochondria isolated from aged animals exhibit a decreased respiratory function; these findings agree with other studies demonstrating that mitochondrial function is diminished with age, both in humans (8,16) and in mice (17,18).
Traditional tests of mitochondrial function.—
Our results from traditional in vitro functional tests reveal that mitochondria from aged animals exhibit an impaired State 3 respiratory rate with both Complex I– and Complex II–linked substrates (Tables 3 and 4), indicating that the maximal rate of mitochondrial oxygen consumption was diminished by increased age. Decreased State 3 respiratory rate and RCR was also reported in skeletal muscle of senile mice (40) as well as in liver mitochondria of senescene-accelerated mice (42). These results were further confirmed by the maximal rate of uncoupled respiration that was significantly reduced in the aged animals, supporting the concept of an age-related reduction in the maximal functional capacity of the respiratory chain. The functional impairment observed during State 3 respiration was similar in the Complex I– and Complex II–linked substrate assays (77.11% and 73.94%, respectively), indicating that both Complex I–supported and succinate-supported respiration were similarly affected with increased age. In this context, Desai and colleagues (43) also reported a remarkable decrease (50–75%) in the activity of ETC complexes (ie, Complex I, III, and IV) in the gastrocnemius muscle of 20-month-old mice compared with 10-month-old mice. Despite published data suggesting that age-related decreases in the activities of mitochondrial ETC complexes are mainly targeted at the level of the Complex I, III, and IV (44), our findings indicate that mitochondrial respiration with both Complex I– and Complex II–linked substrates is diminished with increasing age.
Moreover, mitochondria from aged animals exhibited significantly decreased RCR when compared with their younger counterparts; this observation suggests that when analyzed through traditional tests the coupling between the ETC and oxidative phosphorylation is impaired by increased age. Interpreting RCR as a measure of the functional integrity of mitochondria (20), our results reveal that mitochondria from aged animals exhibit impaired function compared with mitochondria from younger animals. This age-related decrease in RCR was a consequence of the decrease in State 3 respiration, as State 4 respiration did not differ between groups. Moreover, our findings disclose that mitochondrial phosphorylation efficiency (ADP/O) was significantly diminished by age, suggesting that ATP synthase complex activity was depressed in mitochondria from the aged animals.
The magnitude of the age-related reduction in skeletal muscle mitochondrial respiratory function in the current experiments is large compared with the literature (17,40). We hypothesize that this is due to the fact that we normalized our respiratory data to the number of mitochondria. Indeed, mitochondrial functional data are frequently normalized to the protein content and this normalization standard may be biased by contamination with nonmitochondrial proteins. In fact, we have previously demonstrated (18) that when normalizing the mitochondrial State 3 respiratory rate of young and mature animals to the protein content or to the number of mitochondria, we observe differences of 27.02% and 79.41%, respectively, which supports our hypothesis that the normalization to the total protein content attenuates the age-related mitochondrial alterations.
Simulated muscular exercise: the repetitive ADP-stimulation test.—
To investigate the capacity of aged skeletal muscle mitochondria to reestablish their homeostatic balance between successive bouts of exercise, we developed a mitochondrial respiration test comprising a series of repetitive ADP challenges. Mitochondrial function declined following each successive ADP stimulation cycle, and the pattern of functional decline was similar across the two age groups (Figure 3). Nonetheless, compared with mitochondria from aged animals, skeletal muscle mitochondria from young animals exhibited superior functional responses during each ADP stimulation cycles. Specifically, mitochondria from young animals exhibited higher State 3 respiratory rates, RCR, and ADP/O ratios compared with aged animals in each repetitive ADP cycle. In addition, State 4 respiration increased and the RCR decreased in both age groups at the completion of the simulated exercise test. Interestingly, this increase in State 4 respiration was greater in young animals compared with aged animals. The mechanism to explain this observation is unclear as State 4 respiration is governed by several factors including the integrity of the inner membrane and the content of uncoupling proteins along with ATP synthase complexes (23).
Note that although the mitochondrial RCR decreased during the simulated exercise test in the young animals, the RCRs remained relatively high and were indicative of fully coupled and functional mitochondria. Indeed, throughout the simulated exercise test, the mitochondria from younger animals were able to phosphorylate the ADP added in each ADP cycle (Figure 4). In contrast, from the fourth cycle onward, mitochondria isolated from aged animals were unable to phosphorylate all the ADP added, suggesting that mitochondria from mature animals were incapable of maintaining ATP homeostasis in response to this energetic challenge. This decreased ADP phosphorylation capacity could be due to impaired ETC function (eg, depressed proton pumping), thereby diminishing the proton electrochemical gradient and compromising the generation of ATP. Finally, the ADP/O, normalized to the number of mitochondria, was not altered between the ADP cycles for both the young and the aged animals, suggesting that the efficiency of ADP phosphorylation was not acutely changed.
Collectively, our data indicate an age-related dysfunction of mitochondria isolated from mixed skeletal muscle; however, it is important to highlight that our final mitochondrial suspensions were obtained from different muscle phenotypes containing specific mitochondrial characteristics (45–48). Moreover, it might also be expected that the relative contribution of each used muscle to the final mitochondrial suspension has been different among hindlimb muscles. Therefore, and bearing in mind the well-known variability of age-related alterations among skeletal muscles and fiber types (49–52), the extrapolation of our results to a specific muscle or fiber must be done with caution.
Age-Related Decline in Mitochondrial Respiratory Function Is Associated With Oxidative Damage
We also predicted that the observed age-related decline in mitochondrial function would be associated with mitochondrial oxidative stress as evidenced by an increase in oxidative biomarkers. Our results support this hypothesis as mitochondria isolated from skeletal muscle of aged animals possessed significantly fewer SH groups, lower ACON activity, and higher levels of protein carbonyls during baseline measurements. Collectively, these results are consistent with the notion that the pro-oxidant/antioxidant balance was shifted in favor of pro-oxidants in mitochondria isolated from old animals. These results agree with data from several studies that have reported age-related increases in oxidative stress and levels of mitochondrial oxidative damage (15,17).
This age-related increase in mitochondrial oxidative damage could have several functional consequences. First, our data reveal a marked age-related decrease in the activity of the tricarboxylic acid cycle (TCA) enzyme, ACON. This enzyme is particularly sensitive to a reaction with superoxide and consequently to oxidative damage, due to the iron–sulfur clusters (4Fe-4S) in its active site (53). Further, it has been suggested that age-related mitochondrial protein oxidation can also occur in the adenine nucleotide translocase (ANT) (41,54). Indeed, oxidative damage to one or both of these proteins could impair mitochondrial function and has been hypothesized to constitute an important mechanism linking aging and oxidative stress with mitochondrial dysfunction (7). For example, impaired ACON activity can diminish TCA cycle flux, leading to decreased electron flow within the respiratory chain and, therefore, decreased oxidative phosphorylation (41). Further, oxidative damage to the ANT could promote a decrease in State 3 respiration and increased mitochondrial H2O2 production (7). Moreover, in agreement with previous reports (41,53–56), our results reveal an age-related increase in mitochondrial oxidative damage (ie, diminished SH groups and increased protein carbonyl levels). This is significant because oxidative damage to mitochondrial complexes could negatively impact electron transport and compromise mitochondrial respiration. Moreover, published reports indicate an age-related increase in mitochondrial membrane lipid peroxidation (40,57), and this oxidative injury could contribute to the decreased mitochondrial membrane function observed with increased age (58). Nonetheless, in the current study, we failed to observe an age-related change in mitochondrial levels of MDA. This result could be due to the lack of specificity of the MDA assay or simply because the levels of mitochondrial lipid peroxidation were not significantly increased at 18 months of age in our animals.
During the repetitive ADP-stimulation test, at the end of three (Assay III) and six ADP stimulations (Assay IV), an increase in the extramitochondrial cytochrome c content was observed, indicating enhanced outer mitochondrial membrane permeability, both for the young and the mature animals. Because the content of extramitochondrial cytochrome c was significantly higher in the older animals, not only at rest but also after stimulation, it is feasible that this enhanced permeabilization of the outer mitochondrial membrane of aged animals after repeated stimulation will favor the occurrence of apoptotic phenomenon in aged animals (12,59). Moreover, an increase in the lipid peroxidation levels at the end of Assay IV was also evident in both young and mature animals, suggesting an enhanced phospholipid membrane dysfunction with alterations in membrane fluidity. This result could have a significant impact on the activity of the respiratory chain as well as in the generation of the electrochemical proton gradient (3), resulting in increased State 4 respiratory rates and decreased RCR. Likewise, an increase in the protein oxidative damage in response to the acute and repeated ADP stimulation was obvious particularly in the mitochondria from the mature animals, which may be explained by a (i) higher production of ROS resulting from the acute stimulation or (ii) an impaired antioxidant capacity. This concept may partly explain the acute functional impairment imposed by the consecutive ADP stimulations, resulting in a progressive inability of mature animals to reestablish their homeostatic status.
Nonetheless, future experiments will be required to determine if oxidative damage to key mitochondrial proteins are the sole explanation for age-related declines in respiratory function; this remains an important area for future research.
SUMMARY AND PERSPECTIVES
The current experiments were performed in an effort to resolve the controversy associated with the question, “does aging impair skeletal muscle mitochondrial function!” Although this query has been addressed in numerous studies, the results of previous investigations have failed to produce a consistent answer. We postulated that these conflicting findings are due to both methodological issues associated with the study of isolated mitochondria and the use of very old animals in several studies. Therefore, our experimental approach was designed to avoid these previous experimental design shortcomings. Specifically, our experiments incorporated a unique “simulated exercise” protocol to investigate mitochondrial function in vitro. Compared with traditional in vitro assays of mitochondrial function, we predict that our repetitive ADP stimulation protocol provides a more physiological assessment of the in vivo bioenergetic demands of skeletal muscle mitochondria during muscular exercise. Further, to avoid problems associated with normalization of mitochondrial functional measures (eg, contamination of sample with nonmitochondrial proteins), we developed a novel method of standardizing mitochondrial function to the number of intact mitochondria in the experimental sample. Finally, to avoid the occurrence of age-related concomitant disease in very old animals, we used aged and mature animals in our experiments as these animals are not likely to possess old age-related diseases that could influence our experimental results. Hence, our study design provides a robust experimental approach to the study of age-related changes in mitochondrial function in skeletal muscle.
Our findings are consistent with the hypothesis that aging is associated with diminished respiratory function in skeletal muscle mitochondria. Indeed, our results indicate that State 3 functional impairments in the mitochondria of aged animals are present with both Complex I and Complex II substrates. Moreover, compared with aged animals, mitochondria isolated from skeletal muscle of young animals exhibited higher functional responses during repetitive ADP stimulation cycles designed to simulate repeated bouts of muscular exercise. Indeed, mitochondria from young animals exhibited higher State 3 respiratory rates, RCR, and ADP/O ratios compared with aged animals during each repetitive ADP cycle. We speculate that this mitochondrial dysfunction could be related with an age-dependent increased oxidative damage to the mitochondrial biomolecules. Our results, however, only provide evidences that a functional limitation exists at the level of isolated mitochondria obtained from mixed skeletal muscle types, giving a merely overall view of age-related skeletal muscle mitochondrial dysfunction.
Additionally, although our results indicate that aging is associated with impaired mitochondrial function in skeletal muscle, our investigation does not identify the mechanisms responsible for this age-related decline in mitochondrial function. Our speculation that oxidation of mitochondrial proteins contributed to the observed respiratory dysfunction evolved from our finding that aging is associated with increased mitochondrial protein damage and the knowledge that oxidative modification of mitochondrial proteins can impair respiratory function (7,13,41,44,54,58). Future cause and effect experiments using mitochondrial targeted antioxidants could prove useful in determining if mitochondrial oxidative stress is a primary cause of age-related impairment in mitochondrial function.
FUNDING
This work was supported by a grant by Fundação para a Ciência e Tecnologia (PTDC/10DES/70757/2006). Pedro A. Figueiredo is supported by a grant of Programa Operacional Ciência e Inovação 2010 and Fundo Social Europeu.
References
- 1.Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516(Pt 1)::1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholls DG. Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol. 2002;34:1372–1381. doi: 10.1016/s1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- 3.Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373::16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- 4.Salvioli S, Bonafe M, Capri M, Monti D, Franceschi C. Mitochondria, aging and longevity—a new perspective. FEBS Lett. 2001;492:9–13. doi: 10.1016/s0014-5793(01)02199-8. [DOI] [PubMed] [Google Scholar]
- 5.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Torres M, Gredilla R, Sanz A, Barja G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radic Biol Med. 2002;32::882–889. doi: 10.1016/s0891-5849(02)00773-6. [DOI] [PubMed] [Google Scholar]
- 7.Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 8.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 10.Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282:R423–R430. doi: 10.1152/ajpregu.00296.2001. [DOI] [PubMed] [Google Scholar]
- 11.Pollack M, Leeuwenburgh C. Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci. 2001;56:B475–B482. doi: 10.1093/gerona/56.11.b475. [DOI] [PubMed] [Google Scholar]
- 12.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 13.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugiyama S, Takasawa M, Hayakawa M, Ozawa T. Changes in skeletal muscle, heart and liver mitochondrial electron transport activities in rats and dogs of various ages. Biochem Mol Biol Int. 1993;30::937–944. [PubMed] [Google Scholar]
- 15.Drew B, Phaneuf S, Dirks A, et al. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003;284:R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- 16.Tonkonogi M, Fernstrom M, Walsh B, et al. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- 17.Mansouri A, Muller FL, Liu Y, et al. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006;127::298–306. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Figueiredo PA, Ferreira RM, Appell HJ, Duarte JA. Age-induced morphological, biochemical, and functional alterations in isolated mitochondria from murine skeletal muscle. J Gerontol A Biol Sci Med Sci. 2008;63:350–359. doi: 10.1093/gerona/63.4.350. [DOI] [PubMed] [Google Scholar]
- 19.Estabrook R. Mitochondrial respiratory control and the polarographic measurement of ADP/O ratios. Meth Enzymol. 1967;10:41–47. [Google Scholar]
- 20.Magalhaes J, Ascensao A, Soares JM, et al. Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J Appl Physiol. 2005;99:1247–1253. doi: 10.1152/japplphysiol.01324.2004. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp Gerontol. 2003;38::877–886. doi: 10.1016/s0531-5565(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Arch. 2003;446:270–278. doi: 10.1007/s00424-003-1022-2. [DOI] [PubMed] [Google Scholar]
- 23.Kerner J, Turkaly PJ, Minkler PE, Hoppel CL. Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am J Physiol Endocrinol Metab. 2001;281:E1054–1062. doi: 10.1152/ajpendo.2001.281.5.E1054. [DOI] [PubMed] [Google Scholar]
- 24.Beyer RE, Starnes JW, Edington DW, Lipton RJ, Compton RT, 3rd, Kwasman MA. Exercise-induced reversal of age-related declines of oxidative reactions, mitochondrial yield, and flavins in skeletal muscle of the rat. Mech Ageing Dev. 1984;24:309–323. doi: 10.1016/0047-6374(84)90116-7. [DOI] [PubMed] [Google Scholar]
- 25.Barrientos A, Casademont J, Rotig A, et al. Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem Biophys Res Commun. 1996;229::536–539. doi: 10.1006/bbrc.1996.1839. [DOI] [PubMed] [Google Scholar]
- 26.Wibom R, Hagenfeldt L, von Dobeln U. Measurement of ATP production and respiratory chain enzyme activities in mitochondria isolated from small muscle biopsy samples. Anal Biochem. 2002;311:139–151. doi: 10.1016/s0003-2697(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 27.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 28.Rall JA. Energetic aspects of skeletal muscle contraction: implications of fiber types. Exerc Sport Sci Rev. 1985;13:33–74. [PubMed] [Google Scholar]
- 29.Eisenberg E, Greene LE. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- 30.Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55:B117–B123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazelton GA, Lang CA. Glutathione contents of tissues in the aging mouse. Biochem J. 1980;188:25–30. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonkonogi M, Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiol Scand. 1997;161:345–353. doi: 10.1046/j.1365-201X.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- 33.Coore HG, Denton RM, Martin BR, Randle PJ. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971;125:115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonkonogi M, Walsh B, Svensson M, Sahlin K. Mitochondrial function and antioxidative defence in human muscle: effects of endurance training and oxidative stress. J Physiol. 2000;528(Pt 2):379–388. doi: 10.1111/j.1469-7793.2000.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li QY, Pedersen C, Day BJ, Patel M. Dependence of excitotoxic neurodegeneration on mitochondrial aconitase inactivation. J Neurochem. 2001;78:746–755. doi: 10.1046/j.1471-4159.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- 36.Krebs HA, Holzach O. The conversion of citrate into cis-aconitate and isocitrate in the presence of aconitase. Biochem J. 1952;52:527–528. doi: 10.1042/bj0520527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Meth Enzymol. 1994;233:380–385. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 38.Rohn TT, Hinds TR, Vincenzi FF. Ion transport ATPases as targets for free radical damage. Protection by an aminosteroid of the Ca2+ pump ATPase and Na+/K+ pump ATPase of human red blood cell membranes. Biochem Pharmacol. 1993;46:525–534. doi: 10.1016/0006-2952(93)90530-a. [DOI] [PubMed] [Google Scholar]
- 39.Buege JA, Aust SD. Microsomal lipid peroxidation. Meth Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 40.Faist V, Koenig J, Hoeger H, Elmadfa I. Mitochondrial oxygen consumption, lipid peroxidation and antioxidant enzyme systems in skeletal muscle of senile dystrophic mice. Pflugers Arch. 1998;437:168–171. doi: 10.1007/s004240050764. [DOI] [PubMed] [Google Scholar]
- 41.Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci USA. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakahara H, Kanno T, Inai Y, et al. Mitochondrial dysfunction in the senescence accelerated mouse (SAM) Free Radic Biol Med. 1998;24:85–92. doi: 10.1016/s0891-5849(97)00164-0. [DOI] [PubMed] [Google Scholar]
- 43.Desai VG, Weindruch R, Hart RW, Feuers RJ. Influences of age and dietary restriction on gastrocnemius electron transport system activities in mice. Arch Biochem Biophys. 1996;333:145–151. doi: 10.1006/abbi.1996.0375. [DOI] [PubMed] [Google Scholar]
- 44.Van Remmen H, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol. 2001;36:957–968. doi: 10.1016/s0531-5565(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 45.Connor MK, Bezborodova O, Escobar CP, Hood DA. Effect of contractile activity on protein turnover in skeletal muscle mitochondrial subfractions. J Appl Physiol. 2000;88:1601–1606. doi: 10.1152/jappl.2000.88.5.1601. [DOI] [PubMed] [Google Scholar]
- 46.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 47.Lyons CN, Mathieu-Costello O, Moyes CD. Regulation of skeletal muscle mitochondrial content during aging. J Gerontol A Biol Sci Med Sci. 2006;61:3–13. doi: 10.1093/gerona/61.1.3. [DOI] [PubMed] [Google Scholar]
- 48.Figueiredo PA, Mota MP, Appell HJ, Duarte JA. The role of mitochondria in aging of skeletal muscle. Biogerontology. 2008;9:67–84. doi: 10.1007/s10522-007-9121-7. [DOI] [PubMed] [Google Scholar]
- 49.Ansved T, Edstrom L. Effects of age on fibre structure, ultrastructure and expression of desmin and spectrin in fast- and slow-twitch rat muscles. J Anat. 1991;174:61–79. [PMC free article] [PubMed] [Google Scholar]
- 50.Figueiredo PA, Mota MP, Appell HJ, Duarte J. Ceasing of muscle function with aging: is it the consequence of intrinsic muscle degeneration or a secondary effect of neuronal impairments? Eur Rev Aging Phys Act. 2006;3:75–83. [Google Scholar]
- 51.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 52.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5::129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 53.Floyd RA, West M, Hensley K. Oxidative biochemical markers; clues to understanding aging in long-lived species. Exp Gerontol. 2001;36:619–640. doi: 10.1016/s0531-5565(00)00231-x. [DOI] [PubMed] [Google Scholar]
- 54.Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci USA. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 56.Stadtman ER. Importance of individuality in oxidative stress and aging. Free Radic Biol Med. 2002;33:597–604. doi: 10.1016/s0891-5849(02)00904-8. [DOI] [PubMed] [Google Scholar]
- 57.Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp Gerontol. 2001;36:1425–1457. doi: 10.1016/s0531-5565(01)00131-0. [DOI] [PubMed] [Google Scholar]
- 58.Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- 59.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]