Abstract
Global food demand is growing rapidly. Livestock grazing can provide a valuable source of protein, but conventional grazing is often unsustainable. We studied an 800,000-ha section of a threatened ecoregion in southeastern Australia. Conventional management in the region involves continuous livestock grazing with few rest periods and regular fertilizer application. By using remotely sensed data on tree cover and extensive field data on livestock grazing regimes, soil chemistry, tree diameters, and tree regeneration, we show that the region is facing a tree regeneration crisis. Under conventional management, across the region, millions of hectares of land currently supporting tens of millions of trees will be treeless within decades from now. This would have severe negative ramifications for biodiversity and key ecosystem services, including water infiltration and shade provision for livestock. However, we identified an unexpected win–win solution for tree regeneration and commercial grazing. A relatively new practice in the region is fast-rotational grazing, characterized by prolonged rest periods in between short, intensive grazing events. The probability of regeneration under fast-rotational grazing was up to 4-fold higher than under conventional grazing, and it did not differ significantly from the probability of regeneration in ungrazed areas. In addition, trees were more likely to regenerate where soil nutrient levels were low. These findings suggest that the tree regeneration crisis can be reversed by applying low-input, fast-rotational grazing. New policy settings supporting these practices could signal a turning point for the region, from ecological decline to ecological recovery.
Keywords: countryside biogeography, grassy box woodlands, holistic management, rotational grazing, scattered trees
With increases in human population and affluence, demand for agricultural goods is projected to double from the levels of 2000 by 2050 (1). Because conventional agricultural production has come at great ecological costs (2), new trajectories for sustainable agriculture are urgently needed (3). Livestock grazing has a larger geographic extent than any other form of land use (4). Although livestock grazing systems offer a potentially sustainable source of high-quality protein (1), poorly managed livestock grazing poses a severe threat to biodiversity (5).
Australia's temperate grazing region is the heartland of the nation's beef production (Fig. S1), but its southern section coincides with an internationally recognized endangered ecoregion (6). Before European settlement, the region was dominated by grassy Eucalyptus woodlands and dry forests. Approximately 80–95% of tree cover has since been cleared, and especially in foothill areas, most remaining cover occurs as small patches and scattered trees (7). Scattered trees are prominent in livestock grazing landscapes around the world (8–12). They provide important ecosystem services, including enhanced water infiltration and local biodiversity, shade for livestock (11), and improved pasture growth (13, 14).
Although large-scale clearing in Australia has stopped, tree cover continues to be threatened by the ongoing decline of mature trees due to death (10, 15) and their lack of regeneration (16, 17). Like in some tropical landscapes, many remnant trees, therefore, may be the “living dead” (18). They represent relicts of the original vegetation cover, but in the absence of natural regeneration, their disappearance is only a matter of time. Unless tree cover can be maintained, massive biodiversity declines have been predicted within decades from now (19).
Although consensus is growing among ecologists that the ongoing decline of tree cover deserves urgent attention (10, 11, 16, 19), the tree regeneration crisis has received little attention in conservation policy. To this end, 2 knowledge gaps must be closed. First, the extent of tree regeneration failure must be systematically assessed. Second, management practices associated with natural tree regeneration must be identified (20). We addressed these 2 objectives for an 800,000-ha area in the Upper Lachlan Catchment of New South Wales (Fig. S1). The area is dominated by livestock grazing, although some mixed farms practice cropping as well.
Based on a framework of key drivers of tree regeneration, we tested the importance of 3 sets of variables amenable to regional-scale management action (Fig. S2): (i) tree density (which constrains seed supply; ref. 8), (ii) livestock grazing regimes (which can affect tree regeneration by trampling, browsing, and alteration of soil properties; ref. 21), and (iii) soil chemistry (nutrient enrichment in naturally low-nutrient soils negatively affects Australian plants; ref. 22).
We created a high-resolution map of tree cover for our study area (Fig. S1; see SI Methods). In addition, on 33 selected farms, we quantified livestock grazing regimes applied for at least 6 consecutive years. Grazing regimes included conventional practices as well as more recently adopted variations of rotational grazing. We differentiated between “continuous grazing” (>275 days/year), “slow rotation” (from 91 to 275 days/year), “fast rotation” (≤90 days/year; equivalent to high-intensity, short-duration grazing, or cell grazing), and “ungrazed” locations (Fig. S3). Across the 33 farms, at 126 survey sites of 2 ha each, we measured tree diameters, tree regeneration, and soil chemistry (methods for soil chemistry analyses are outlined in SI Methods). Sites spanned the full range of available grazing regimes and tree densities, including paddock sites (median count of trees, 2), scattered tree sites (median count, 16), grazed woodlands (median count, 198), and ungrazed woodlands (median count, 427). Sites comprised 106 “primary survey sites” and 20 additional “validation sites,” which were used specifically to assess the robustness of our results.
There were 3 parts to our analyses. First, based on remotely sensed data, we analyzed across our study area how much remnant tree cover and how many remnant trees occurred at different densities. This large-scale analysis provided the context for more detailed site-level analyses. Second, we summarized the tree diameter distributions at our 126 survey sites characterized by different tree densities. This provided an indication of the age profile of trees in stands of different densities. Third, we used regression models to formally quantify how tree cover, grazing regimes, and soil chemistry were related to (i) the minimum diameter of trees at a site (a proxy for time since last regeneration) and (ii) the presence of seedlings at a site (a proxy for recent regeneration).
Results
The State of Regional Tree Cover.
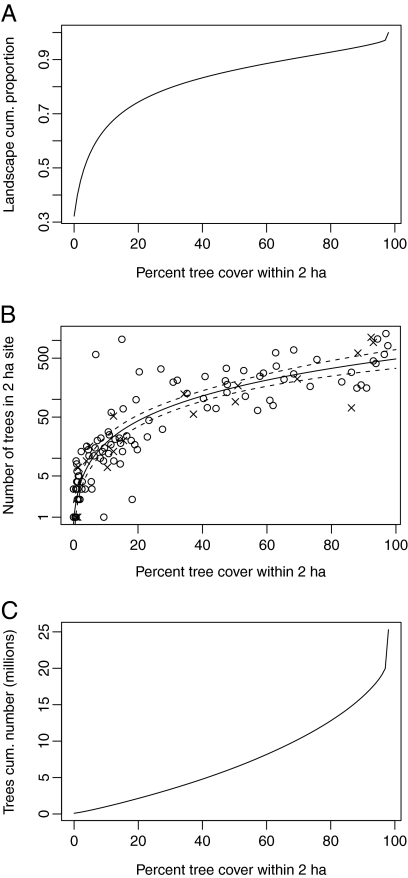
Tree cover was 18% across the study area, and it ranged from 49% to 3% on our farms (median, 12%; Fig. S1). Approximately three-quarters of the study area had ≤30% tree cover, and two-thirds had ≤10% tree cover (calculated within a circular 2-ha moving window; Fig. 1A). Remotely sensed percent tree cover within a 2-ha circle around each of the 126 survey sites was significantly related to the number of trees within the site (P < 0.001; R2 = 0.81; Fig. 1B and Table S1). This relationship was significant whether or not validation sites were included, suggesting it was robust. Based on the relationship between percent tree cover and number of trees within a given 2-ha area, we estimated across the study area how many trees occurred at different levels of tree cover (Fig. 1C). Approximately 3 million trees occurred at densities ≤30%, and 1.5 million trees occurred at densities ≤10% per 2 ha (Fig. 1C).
Fig. 1.
Landscape-level tree cover for the study area, based on a 2-ha moving window to calculate percent tree cover from remotely sensed data and on 126 field survey sites. Note some of the densely wooded parts of the study area were public land. (A) Cumulative proportion of the study area occurring at different levels of tree cover within a 2-ha moving window (e.g., ≈75% of the study area had ≤30% tree cover). (B) Percent tree cover from remote sensing versus the number of trees measured at ground sites (R2 = 0.81; P < 0.001). Circles denote primary survey sites, and crosses denote validation sites. The dashed line is the 95% confidence interval for the predicted relationship. (C) Based on A and B, predicted number of trees occurring at different densities in the study area (e.g., ≈3 million trees occurred at densities ≤30%).
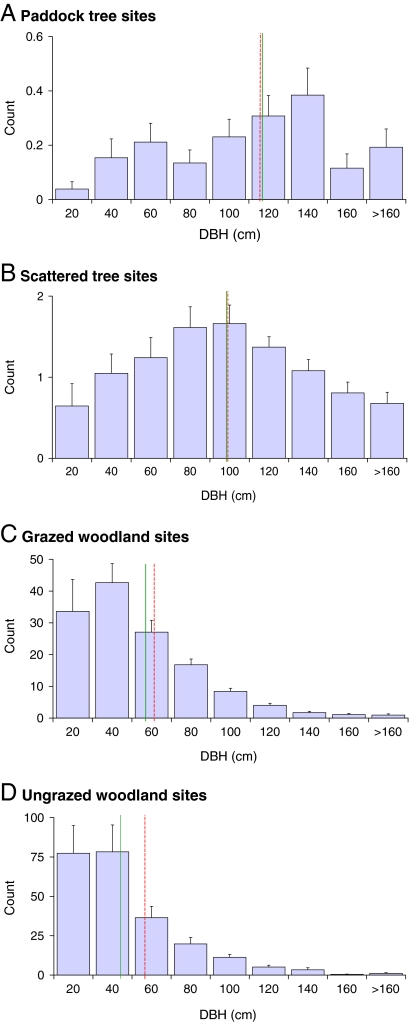
The diameters of all tree species were standardized, so that sites with different species could be compared (see SI Methods). Tree diameter distributions varied systematically across sites with different tree densities (Fig. 2). In paddock sites and scattered tree sites, the mean diameters of trees were highest. In both site types, the median tree diameter was approximately equal to the mean diameter, indicating a symmetrical spread of diameters (Fig. 2). Smaller mean and median diameters were found in grazed woodlands, and even more so in ungrazed woodlands. Diameter distributions in these sites were right-skewed, with mean diameters smaller than median diameters (Fig. 2).
Fig. 2.
Mean count of trees (and standard error) in different diameter classes across the 4 site types [paddock (A), scattered (B), grazed (C), and ungrazed (D)], regardless of farm or grazing regime. The approximate mean diameter of all trees, across all farms and grazing regimes, in a given site type is indicated by a dotted red line; the approximate median diameter is indicated by a solid green line. Diameters were standardized before analysis to compare sites with different tree species (see SI Methods).
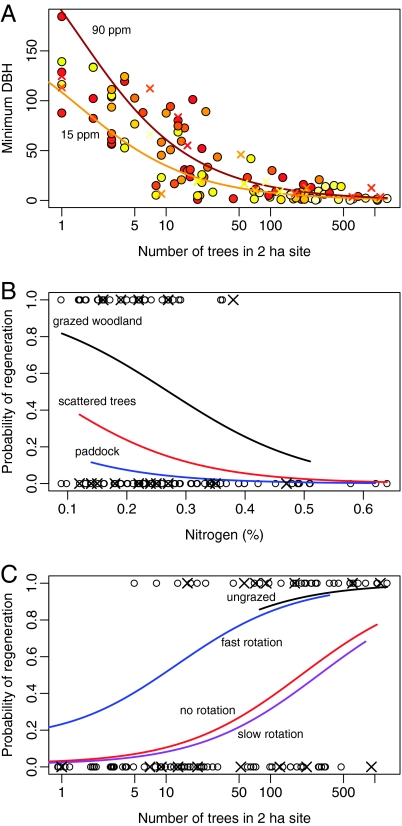
The minimum tree diameter at a site significantly decreased with an increasing number of trees in the site, and also decreased with decreasing levels of available phosphorus (Fig. 3A and Table S2). That is, regeneration had occurred more recently in areas with many trees and low soil phosphorus. These variables were significant whether or not validation sites were included, suggesting the model was robust. Based on an approximately constant diameter growth rate of 0.8 cm per year until the age of 100 years (23), a tree with a diameter of 50 cm is more than 60 years old. None of our paddock sites with 5 or fewer trees had regenerated within the last 60 years (Fig. 3A). Under high-phosphorus conditions, the expected minimum age of trees in paddock sites exceeded 100 years (Fig. 3A).
Fig. 3.
Significant predicted relationships from generalized linear mixed models (confidence intervals are not shown because methods for their calculation in the presence of random effects are controversial in statistical science; points denote primary survey sites, and crosses denote validation sites). (A) Minimum (standardized) diameter varied significantly in response to the number of trees at a site and available soil phosphorus (see Table S2 for details). Darker points indicate sites with higher phosphorus concentrations. The 2 lines denote low- and high-phosphorus conditions. (B) The probability of seedling presence varied significantly with the number of trees at a site and with the amount of soil nitrogen. The predicted relationship for the mean number of trees at the 3 grazed site types is shown, assuming continuous grazing (see Table S3 for details). (C) The probability of seedling presence also varied significantly between livestock rotation regimes. Pairwise differences were significant between either of the upper 2 predicted lines, and between either of the lower 2 predicted lines (P = 0.004; see Table S3 for details). The predicted relationship is based on mean total nitrogen concentrations (0.26%).
Recent Regeneration.
The probability of seedlings occurring at a site (i.e., recent regeneration) significantly increased with the number of trees present, and it significantly decreased with the amount of total soil nitrogen (Fig. 3B and Table S3). The probability of regeneration also depended significantly on the degree of stock rotation (Fig. 3C and Table S3). Ungrazed sites had the highest probability of regeneration, followed by fast-rotation sites, continuously grazed sites, and slow-rotation sites (Fig. 3C). These variables were significant whether or not validation sites were included, suggesting the model was robust. Investigation of the standard errors of all pairwise differences showed that the differences between no grazing and fast rotation, and between slow rotation and continuous grazing, were not significant (differences <1 standard error), whereas the difference between the two groupings was significant (difference >2 standard errors; overall significance P = 0.004; Table S3).
Discussion
Extent and Implications of the Crisis.
Our findings highlight the extent of the tree regeneration crisis and the urgency with which conservation policy must address it if ongoing ecological decline is to be avoided. Especially in locations with scattered and isolated trees, “average” trees typically are more than 100 cm in diameter (Fig. 2) or more than 120 years old (23), and even the youngest trees are often more than 60 years old (Fig. 3A).
Locations with scattered or isolated trees account for ≈75% of our 800,000-ha study area and currently support some 3 million individual trees (Fig. 1). The probability of regeneration at these sites is extremely low under conventional, continuous livestock grazing and regular fertilizer use (Fig. 3). Regeneration probabilities in much of the more densely wooded parts of the study area were also significantly lower than under more “natural” conditions without livestock grazing and with low soil nutrient levels (Fig. 3). Extrapolating these findings across southeastern Australia's grazing region suggests that millions of hectares currently supporting tens of millions of trees will be treeless in the future under conventional management. For example, within 50 years from now, the abundance of scattered Yellow Box (Eucalyptus melliodora) has been modeled to decline to half of its current level (10).
The ecological repercussions of further losses of tree cover in an already overcleared and endangered ecoregion would be substantial (19). The total amount of tree cover in the landscape and the heterogeneity provided by scattered trees are essential drivers of species diversity, for example, of birds (24). Given the potential for thresholds (25), cumulative effects (26), and a disproportionate influence of scattered trees on ecosystem processes (11), native species are likely to be lost at an accelerating rate if tree cover continues to decline. We estimate conservatively that more than 100 bird, 25 mammal, and 25 reptile species in our study area depend on tree-derived habitat features. We estimate that scattered trees are used as primary or complementary habitat by well over half of these species (Table S4).
The patterns reported here mirror those in other parts of the world (10, 27). For example, in southern European oak systems (Quercus spp.), scattered trees often fail to regenerate (8). As in our study area, the diameters of scattered oak trees in anthropogenic systems were found to be distributed symmetrically (12), indicating insufficient recruitment of young trees to replace existing mature trees (10). Nevertheless, scattered trees can persist in some anthropogenic landscapes. For example, in central Nicaragua, of ≈80 tree species scattered throughout pastures, more than half have been reported to regenerate under commercial cattle grazing (9). Our data suggest that in temperate Australia, natural regeneration also can be achieved under some forms of commercial grazing.
Reversing the Crisis.
Contrary to common wisdom, scattered trees are not doomed to be the living dead. Although low seed supply (8, 21, 28) and a history of intensive land use (16) impose constraints on tree regeneration in heavily cleared areas, reducing nutrient inputs and applying fast-rotational grazing can substantially enhance regeneration (Fig. 3).
The widespread application of superphosphate fertilizer is detrimental to many Australian plants (22). Especially in combination with livestock grazing, high phosphorus levels are associated with a shift from native perennial ground cover species to introduced annual species (22, 29–31). Introduced species, in turn, can compete with tree seedlings, thereby limiting tree regeneration (32). Superphosphate application is also associated with increased nitrogen levels because it facilitates enhanced nitrogen fixation (29) and higher stocking rates (31, 33). Given the relationships between phosphorus and nitrogen, it is not surprising that increases in both variables were associated with tree regeneration failure (Fig. 3 A and B).
Reducing fertilizer use would not immediately reduce soil nutrient levels (34), but soil nutrient levels most likely would drop substantially after several decades. In the medium term, reducing nutrient inputs therefore would enhance tree regeneration and also would have positive consequences for other ecological processes. For example, mature trees are healthier in low-nutrient environments (35, 36). Maximizing their survival is vital to bridge an already unavoidable bottleneck in the future availability of mature trees (10, 19). Native ground cover species (22, 30) and native arthropod diversity (31) also would benefit from reduced nutrient levels.
Regeneration probabilities were low under continuous grazing and slow livestock rotation, but they were significantly higher if livestock were excluded or fast-rotational grazing was practiced (Fig. 3C). This finding supports existing evidence that the exclusion of livestock grazing enhances natural regeneration (37, 38). However, livestock exclusion will only be feasible in a small part of the region because it imposes high opportunity costs (exceeding AU $10,000 per 100 ha per year; ref. 39). In contrast, the benefits of fast-rotational grazing for tree regeneration have not been recognized to date. Because land managed under fast-rotational grazing remains economically productive, fast-rotational grazing provides a win–win opportunity for tree regeneration and commercial livestock grazing. For example, among scattered trees, regeneration probabilities were approximately 4-fold higher under fast-rotational grazing than under conventional management (Fig. 3C). Fast-rotational grazing may have had benefits for tree regeneration because the disturbance associated with high-intensity, short-duration grazing created favorable conditions for germination, because water infiltration was more effective, or because once seedlings had established, they were less likely to die from trampling or browsing than under conventional management (Fig. S2).
Fast-rotational grazing may have additional benefits. It typically increases ground cover, litter cover, and the cover of economically desirable pasture species (40, 41), and it facilitates improved water infiltration (42). It also can have benefits for microarthropods (43) and for soil chemistry, leading to reduced nitrate and phosphorus levels (41). Despite these benefits, fast-rotational grazing has limitations (44), and like all grazing systems, it depends on appropriate management decisions. Most importantly, although it improves pasture composition from an economic perspective, rotational grazing may not improve ground species diversity per se (45–48). In addition, not all studies of fast-rotational grazing have reported increases in litter cover or water infiltration (49).
Management and Policy Implications.
Trees influence patterns in pasture productivity via the net effects of stimulatory and competitive processes. Stimulatory processes include enhanced water infiltration, nutrient accumulation, and microclimate regulation, whereas competitive processes relate to competition between trees and pasture grasses for light and nutrients (50). As a result of this balance, pasture productivity and profitability peak at intermediate levels of tree cover (13, 14).
Despite demonstrable economic benefits of trees in pastures, under current policy settings, farm profitability during a 15-year timeframe is highest under conventional management, which inhibits natural tree regeneration (51). That is, short-term economic incentives are at odds with the long-term maintenance of tree cover. Farming systems will lose biodiversity and economic productivity unless new policy settings can encourage the establishment of young trees to replace existing old trees. A bottleneck in the availability of mature trees is unavoidable, even if young trees are established now (52). Therefore, developing new policy settings is a matter of urgency.
Management options include active tree establishment by planting or direct seeding, or passive tree establishment by creating conditions conducive to natural regeneration (53). Specific options are as follows:
The planting or seeding of trees in patches or along fence lines (active). This option already has been used on many farms. Despite local benefits (54), its contribution to regional-scale tree cover has been minimal (55), and it has not addressed the loss of scattered trees within pastures.
The planting of trees in a scattered pattern within grazed pastures by using individual tree guards (active). This option is used by some pioneering farmers. Tree guards can be reused once seedlings are tall enough to withstand grazing, which reduces costs in the medium term.
The exclusion of livestock from entire paddocks before reseeding paddocks at low densities and resting them until trees have been established (active). This option is used successfully by a leading nongovernment organization (39).
The permanent exclusion of livestock from woodland patches to enhance natural regeneration (passive). This option is widely used (37) but does not address the loss of scattered trees from grazed pastures.
The cessation of fertilizer use to enhance conditions for natural regeneration (passive). Despite likely time lags in soil recovery (56, 57), this option has major potential to deliver lasting, regional-scale sustainability outcomes.
Increased uptake of fast-rotational grazing (passive). Rotational grazing is gaining popularity but is currently used by only a small proportion of farmers.
Active tree establishment is particularly appropriate where natural regeneration is unlikely to occur, such as in nutrient-enriched areas with few trees. In the long term, passive management options are desirable because they are likely to create self-perpetuating farm ecosystems. Policy settings can constrain or enable landholders to adopt the above management options. Past incentive and education programs have focused primarily on management options 1 and 4. Future programs must recognize the complementary values of scattered trees in commercially used pastures and must support management options that can ensure their ongoing existence.
Methods
Farm Selection.
Farms ranged from 236 ha to 3,036 ha (median, 900 ha; mean, 1,191 ha) and were selected to provide a wide range of grazing practices. We obtained information from farmers about the annual mean stocking rate of each paddock and the extent to which livestock were rotated. We differentiated between high stocking rates [>5 dry sheep equivalent (DSE)—a 48- to 50-kg wether—per hectare] and low stocking rates (up to 5 DSE per hectare). We identified paddocks grazed continuously (>275 days/year), paddocks under slow rotation (from 91 to 275 days/year), and paddocks under fast rotation (up to 90 days/year; equivalent to high-intensity, short-duration grazing, or cell grazing). Ungrazed locations also were identified.
Continuous grazing is the conventional practice in the region. Rotational grazing is a relatively new practice, and few farmers have used it for >10 years. Fast-rotational grazing is typically used by farmers who have attended courses based on holistic resource management (42). To convert to fast-rotational grazing, conventional farms are subdivided into a larger number of smaller paddocks. The annual mean stocking rate can be the same as on a continuously grazed farm, but livestock are kept in one or few distinct mobs. These mobs typically occupy a given paddock for only a few days at a time before being moved to the next paddock. Between grazing events, paddocks are rested for several weeks. Most fast-rotational farmers make flexible decisions about their stock movement rather than strictly adhering to a time-controlled protocol. Slow-rotational grazing is practiced by several farmers who have adopted aspects of rotational grazing, or who strategically rest paddocks in some months of the year.
For field sites, we only considered paddocks that had been under broadly the same grazing regime since at least mid-2002. We did not consider cropping areas. Native grazing animals and introduced herbivores, such as the European rabbit (Oryctolagus cuniculus), had access to all sites. Their impact was assumed to be constant throughout the study area.
Site Selection.
On each farm, we selected up to 4 primary survey sites of 2 ha each from satellite imagery. These were 1 paddock site (approximately 10 or fewer discernible crowns), 1 scattered tree site (≈10–40 crowns), 1 grazed woodland, and 1 ungrazed woodland (both with dense crown cover). Almost all sites were in separate paddocks and were separated by several hundred meters or more. Not all site types were available on all farms, resulting in a total of 106 primary survey sites. We also selected 20 additional validation sites (5 of each of the 4 site types) located across 20 of the original 33 farms. Validation sites were used to assess the robustness of statistical models (see data analysis section below).
Site-Level Data on Trees.
In spring 2007, at each site we measured the diameters of all individual trees or a representative sample of trees. From this we determined each site's tree diameter profile. We also counted the total number of trees in each site or estimated it via a distance-sampling protocol. In total, we identified more than 4,000 trees and measured their diameters. The detailed protocol for tree measurements is outlined in the SI Methods.
Tree Seedlings.
In spring 2008, we counted the number of seedlings at each site (height ≤ 130 cm). At this time, a given site's management regime had been in place for 6 years or longer. We acknowledge that some “seedlings” may be older than 6 years because eucalypts can resprout from a subterranean lignotuber. This potential source of error means that our estimated rates of regeneration may be too high in treatments where the growth of seedlings is suppressed (e.g., because of continuous livestock grazing).
Other Site-Level Data.
We considered the following covariates in our regression analyses: geological substrate, mean annual rainfall, percent rock cover, percent bare ground cover, pasture type (dominant native, dominant introduced, or mix of both), and livestock type (cattle, sheep, or both).
Data Analysis.
First, we used linear regression to investigate the relationship between the number of trees measured on the ground and remotely sensed tree cover. Second, for each site type, we calculated the mean numbers of trees in 20-cm diameter intervals and their standard errors. We also calculated the mean and median diameters of all trees in each site type. Third, we analyzed minimum tree diameter and seedling presence by using mixed regression models. Mixed models incorporate fixed effects (design variables and covariates) and random effects that account for systematic variability arising from the experimental design (58). Here, the random effect “farm” was fitted, to account for possible dependence between multiple sites located on the same farm. Minimum tree diameter was modeled as a log-transformed, unit-free diameter index (see SI Methods) by using ordinary mixed models (implemented in the package R (Foundation for Statistical Computing) by using the lme function). Seedling presence was modeled by using a generalized linear mixed model, with logit link function, binomial error distribution, and the dispersion parameter fixed at one (implemented in the package Genstat) (VSN International Ltd.).
Model selection occurred as follows. First, only primary survey sites were considered. The first formal model was fitted containing the (log-transformed) number of trees within the site, stocking rate, degree of stock rotation, the interaction between stocking rate and rotation, available phosphorus, and total nitrogen. These variables were fitted first because they were key parts of the experimental design (number of trees and grazing regime) or because we had strong theoretical reasons to expect their significance (soil nutrients). By using backwards selection, the initial model was reduced until only significant variables were retained. The small number of additional covariates (listed above) then was added to the model to test for their significance (none were significant in any of our analyses). Once a final model had been obtained, the same model was specified by using the combination of all primary survey sites plus all validation sites. This was done to assess whether the model was robust when a substantial amount of new data were added.
Number of Seedlings.
We explored whether the same variables that explained seedling presence or minimum tree diameter also were related to the abundance of seedlings at those sites where seedlings were present. We explored these trends graphically instead of using formal analyses because data were limited. See Fig. S4 for results.
Supplementary Material
Acknowledgments.
We thank all participating farmers and everyone who helped us establish contacts in the region, especially G. Fitzhardinge and the Lachlan Catchment Management Authority. We give special thanks to J. Dorrough for his advice during project design. Comments by T. Gardner, J. Ranganathan, K. Rawlings, K. Stagoll, S. Dovers, H. Clayton, and 2 anonymous referees on earlier versions of the paper were invaluable. We thank D. Carroll (SPOT Imaging Services, McMahons Point New South Wales, Australia) for supplying Satellite pour l'Observation de la Terre (SPOT) imagery, K. Stagoll for assistance with field work, and J. Field and L. Fitzsimmons for help with soil analyses. This work was funded by the Australian Research Council and the Commonwealth Environment Research Facilities.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900110106/DCSupplemental.
References
- 1.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 2.Foley JA, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- 3.Tilman D, et al. Forecasting agriculturally driven global environmental change. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 4.Asner GP, Elmore AJ, Olander LP, Martin RE, Harris AT. Grazing systems, ecosystem responses, and global change. Annu Rev Environ Resources. 2004;29:261–299. [Google Scholar]
- 5.Fleischner TL. Ecological costs of livestock grazing in western North America. Conserv Biol. 1994;8:629–644. [Google Scholar]
- 6.Hoekstra JM, Boucher TM, Ricketts TH, Roberts C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol Lett. 2005;8:23–29. [Google Scholar]
- 7.Gibbons P, Boak M. The value of paddock trees for regional conservation in an agricultural landscape. Ecol Manage Restor. 2002;3:205–210. [Google Scholar]
- 8.Acácio V, Holmgren M, Jansen PA, Schrotter O. Multiple recruitment limitation causes arrested succession in mediterranean cork oak systems. Ecosystems. 2007;10:1220–1230. [Google Scholar]
- 9.Esquivel MJ, Harvey CA, Finegan B, Casanoves F, Skarpe C. Effects of pasture management on the natural regeneration of neotropical trees. J Appl Ecol. 2008;45:371–380. [Google Scholar]
- 10.Gibbons P, et al. The future of scattered trees in agricultural landscapes. Conserv Biol. 2008;22:1309–1319. doi: 10.1111/j.1523-1739.2008.00997.x. [DOI] [PubMed] [Google Scholar]
- 11.Manning AD, Fischer J, Lindenmayer DB. Scattered trees are keystone structures-implications for conservation. Biol Conserv. 2006;132:311–321. [Google Scholar]
- 12.Pulido FJ, Diaz M, de Trucios SJH. Size structure and regeneration of Spanish holm oak Quercus ilex forests and dehesas: Effects of agroforestry use on their long-term sustainability. For Ecol Manage. 2001;146:1–13. [Google Scholar]
- 13.Walpole SC. Assessment of the economic and ecological impacts of remnant vegetation on pasture productivity. Pac Conserv Biol. 1999;5:28–35. [Google Scholar]
- 14.Williams DG, et al. Effects of Trees on Native Pasture Production. Canberra, Australia: RIRDC; 1999. RIRDC Publ No 99/165. [Google Scholar]
- 15.Ozolins A, Brack C, Freudenberger D. Abundance and decline of isolated trees in the agricultural landscapes of central New South Wales, Australia. Pac Conserv Biol. 2001;7:195–203. [Google Scholar]
- 16.Dorrough J, Moxham C. Eucalypt establishment in agricultural landscapes and implications for landscape-scale restoration. Biol Conserv. 2005;123:55–66. [Google Scholar]
- 17.Saunders DA, Smith GT, Ingram JA, Forrester RI. Changes in a remnant of salmon gum Eucalyptus salmonophloia and York gum E. loxophleba woodland, 1978 to 1997. Implications for woodland conservation in the wheat-sheep regions of Australia. Biol Conserv. 2003;110:245–256. [Google Scholar]
- 18.Janzen DH. The future of tropical ecology. Annu Rev Ecol Syst. 1986;17:305–324. [Google Scholar]
- 19.Vesk PA, Mac Nally R. The clock is ticking-revegetation and habitat for birds and arboreal mammals in rural landscapes of southern Australia. Agric Ecosyst Environ. 2006;112:356–366. [Google Scholar]
- 20.Vesk PA, Dorrough JW. Getting trees on farms the easy way? Lessons from a model of eucalypt regeneration on pastures. Aust J Bot. 2006;54:509–519. [Google Scholar]
- 21.Windsor DM. In: Temperate Eucalypt Woodlands in Australia: Biology, Conservation, Management and Restoration. Hobbs RJ, Yates CJ, editors. Chipping Norton, UK: Surrey Beatty and Sons; 1999. pp. 271–285. [Google Scholar]
- 22.Dorrough J, Scroggie MP. Plant responses to agricultural intensification. J Appl Ecol. 2008;45:1274–1283. [Google Scholar]
- 23.Banks JCG. In: The Coming of Age - Forest Age & Heritage Values. Dargavel J, editor. Canberra, Australia: Environment Australia; 1997. pp. 17–28. [Google Scholar]
- 24.Haslem A, Bennett AF. Birds in agricultural mosaics: The influence of landscape pattern and countryside heterogeneity. Ecol Appl. 2008;18:185–196. doi: 10.1890/07-0692.1. [DOI] [PubMed] [Google Scholar]
- 25.Fahrig L. Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst. 2003;34:487–515. [Google Scholar]
- 26.Fischer J, Lindenmayer DB. In: Managing and Designing Landscapes for Conservation: Moving from Perspectives to Principles. Lindenmayer DB, Hobbs RJ, editors. Oxford: Blackwell; 2007. pp. 229–244. [Google Scholar]
- 27.Zavaleta ES, Hulvey KB, Fulfrost B. Regional patterns of recruitment success and failure in two endemic California oaks. Divers Distrib. 2007;13:735–745. [Google Scholar]
- 28.Clarke PJ, Davison EA. Experiments on the mechanism of tree and shrub establishment in temperate grassy woodlands: Seedling emergence. Austral Ecol. 2001;26:400–412. [Google Scholar]
- 29.Dorrough J, Moxham C, Turner V, Sutter G. Soil phosphorus and tree cover modify the effects of livestock grazing on plant species richness in Australian grassy woodland. Biol Conserv. 2006;130:394–405. [Google Scholar]
- 30.McIntyre S. The role of plant leaf attributes in linking land use to ecosystem function in temperate grassy vegetation. Agric Ecosyst Environ. 2008;128:251–258. [Google Scholar]
- 31.Oliver I, et al. Effects of fertiliser and grazing on the arthropod communities of a native grassland in south-eastern Australia. Agric Ecosyst Environ. 2005;109:323–334. [Google Scholar]
- 32.Semple WS, Koen TB. Effect of pasture type on regeneration of eucalypts in the woodland zone of south-eastern Australia. Cunninghamia. 2003;8:76–84. [Google Scholar]
- 33.Close DC, Davidson NJ, Watson T. Health of remnant woodlands in fragments under distinct grazing regimes. Biol Conserv. 2008;141:2395–2402. [Google Scholar]
- 34.Prober SM, Lunt ID, Morgan JW. In: New Models for Ecosystem Dynamics and Restoration. Hobbs RJ, Suding K, editors. Washington, DC: Island Press; 2008. pp. 156–168. [Google Scholar]
- 35.Davidson NJ, et al. Eucalypt health and agricultural land management within bushland remnants in the Midlands of Tasmania, Australia. Biol Conserv. 2007;139:439–446. [Google Scholar]
- 36.Landsberg J, Morse J, Khanna P. Tree dieback and insect dynamics in remnants of native woodlands on farms. Proc Ecol Soc Aust. 1990;16:149–165. [Google Scholar]
- 37.Spooner PG, Briggs SV. Woodlands on farms in southern New South Wales: A longer-term assessment of vegetation changes after fencing. Ecol Manag Restor. 2008;9:33–41. [Google Scholar]
- 38.Spooner P, Lunt I, Robinson W. Is fencing enough? The short-term effects of stock exclusion in remnant grassy woodlands in southern NSW. Ecol Manag Restor. 2002;3:117–126. [Google Scholar]
- 39.FiField G, Streatfield S, Vanzella B. Introducing Whole of Paddock Rehabilitation. Australia: Greening Australia Canberra; 2008. [Accessed May, 21, 2009]. Available at www.greeningaustralia.org.au/uploads//General pdfs/ACT_WOPR_Brochure.pdf. [Google Scholar]
- 40.Earl JM, Jones CE. The need for a new approach to grazing management-is cell grazing the answer? Rangeland J. 1996;18:327–350. [Google Scholar]
- 41.Sanjari G, Ghadiri H, Ciesiolka CAA, Yu B. Comparing the effects of continuous and time-controlled grazing systems on soil characteristics in Southeast Queensland. Aust J Soil Res. 2008;46:348–358. [Google Scholar]
- 42.Savory A, Butterfield J. Holistic Management. Washington DC: Island Press; 1999. [Google Scholar]
- 43.Tom N, Raman A, Hodgkins DS, Nicol H. Populations of soil organisms under continuous set stocked and high intensity-short duration rotational grazing practices in the central tablelands of New South Wales (Australia) N Z J Agric Res. 2006;49:261–272. [Google Scholar]
- 44.Bock CE, Bock JH. Response of winter birds to drought and short-duration grazing in southeastern Arizona. Conserv Biol. 1999;13:1117–1123. [Google Scholar]
- 45.Biondini ME, Manske L. Grazing frequency and ecosystem processes in a northern mixed prairie, USA. Ecol Appl. 1996;6:239–256. [Google Scholar]
- 46.Dorrough J, McIntyre S, Stol J, Brown G, Barrett G. Understanding the Interactions Between Biodiversity and the Management of Native Pastures in the Murray Darling Basin. Sydney: Meat and Livestock Australia; 2008. [Google Scholar]
- 47.Dowling PM, et al. Effect of continuous and time-control grazing on grassland components in south-eastern Australia. Aust J Exp Agric. 2005;45:369–382. [Google Scholar]
- 48.Taylor CA, Ralphs MH, Kothmann MM. Vegetation response to increasing stocking rate under rotational stocking. J Range Manag. 1997;50:439–442. [Google Scholar]
- 49.Greenwood KL, McKenzie BM. Grazing effects on soil physical properties and the consequences for pastures: a review. Aust J Exp Agric. 2001;41:1231–1250. [Google Scholar]
- 50.Scanlan JC. A model of woody-herbaceous biomass relationships between eucalypt and mesquite communities. J Range Manag. 1992;45:75–80. [Google Scholar]
- 51.Crostwaite J, Malcolm B, Moll J, Dorrough J. Future investment in landscape change in southern Australia. Landsc Res. 2008;33:225–239. [Google Scholar]
- 52.Vesk PA, Nolan R, Thomson JR, Dorrough JW, Mac Nally R. Time lags in provision of habitat resources through revegetation. Biol Conserv. 2008;141:174–186. [Google Scholar]
- 53.Dorrough J, Vesk PA, Moll J. Integrating ecological uncertainty and farm-scale economics for planning restoration. J Appl Ecol. 2008;45:288–295. [Google Scholar]
- 54.Munro NT, Lindenmayer DB, Fischer J. Faunal response to revegetation in agricultural areas of Australia: A review. Ecol Manag Restor. 2007;8:199–207. [Google Scholar]
- 55.Freudenberger D, Harvey J, Drew A. Predicting the biodiversity benefits of the Saltshaker Project, Boorowa, NSW. Ecol Manag Restor. 2004;5:5–14. [Google Scholar]
- 56.McIntyre S, Lavorel S. A conceptual model of land use effects on the structure and function of herbaceous vegetation. Agric Ecosyst Environ. 2007;119:11–21. [Google Scholar]
- 57.Prober SM, Thiele KR, Lunt ID, Koen TB. Restoring ecological function in temperate grassy woodlands: Manipulating soil nutrients, exotic annuals and native perennial grasses through carbon supplements and spring burns. J Appl Ecol. 2005;42:1073–1085. [Google Scholar]
- 58.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-Plus. New York: Springer; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.