Abstract
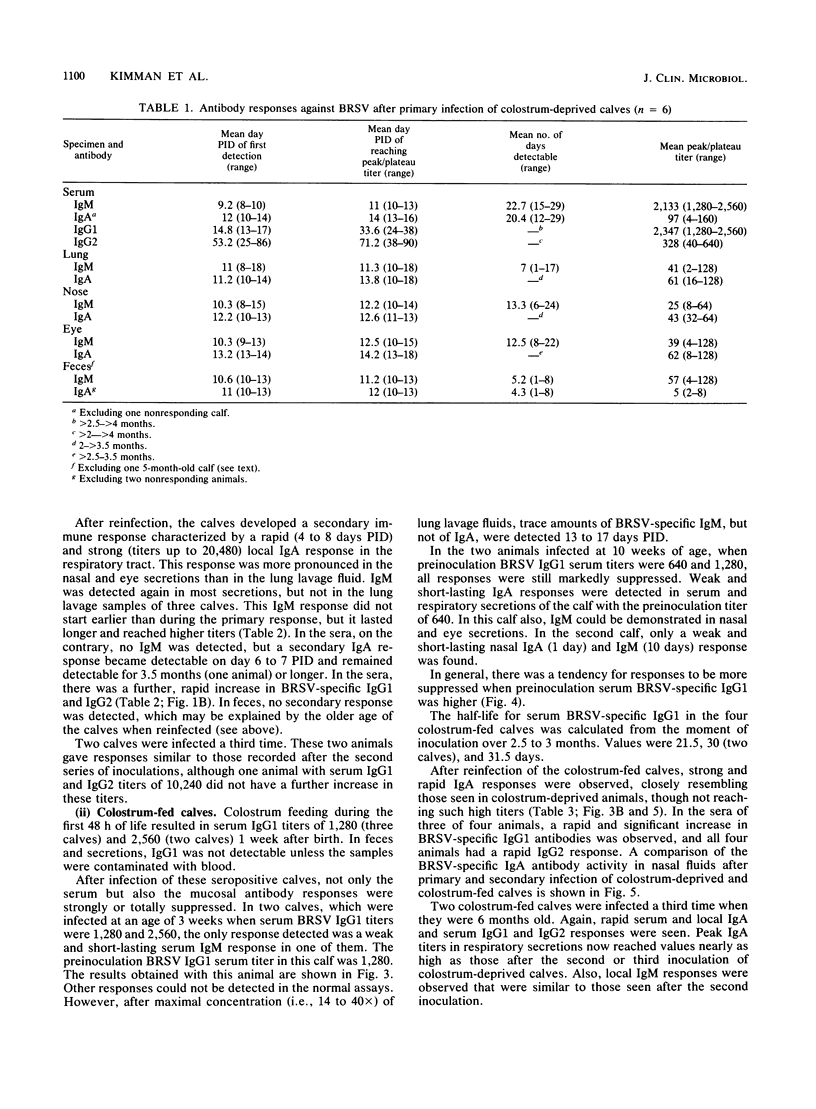
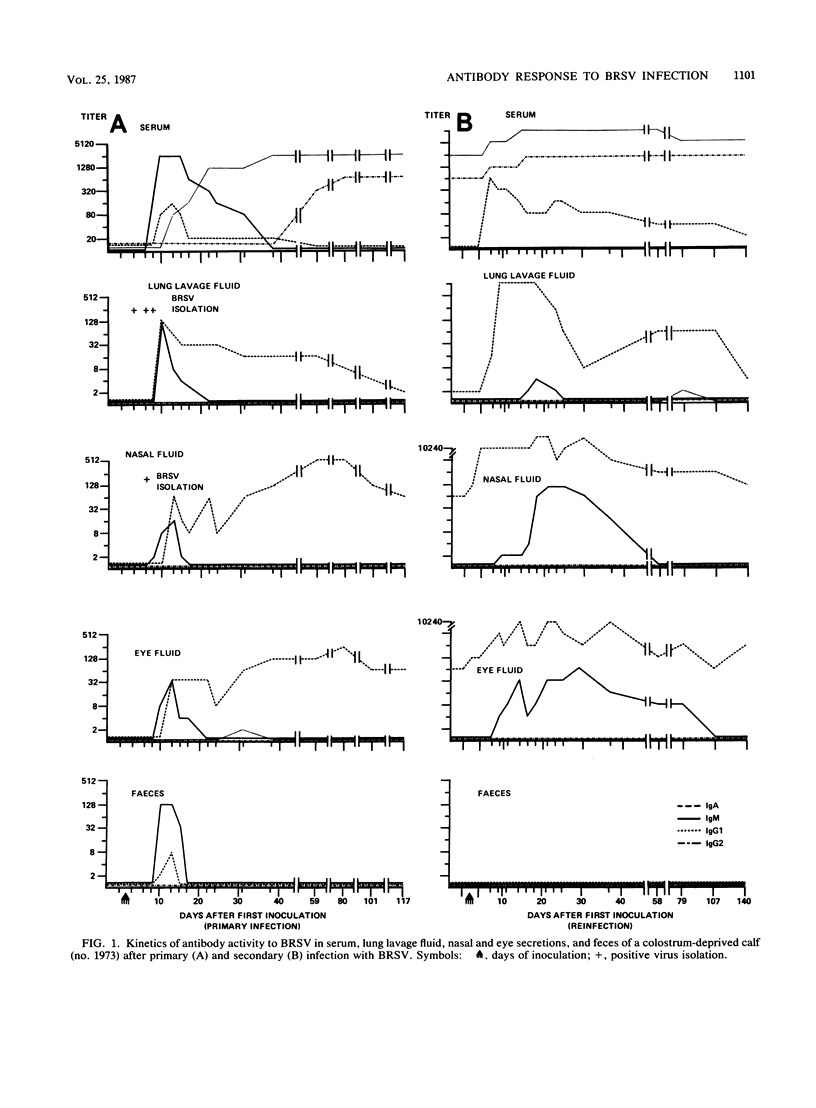
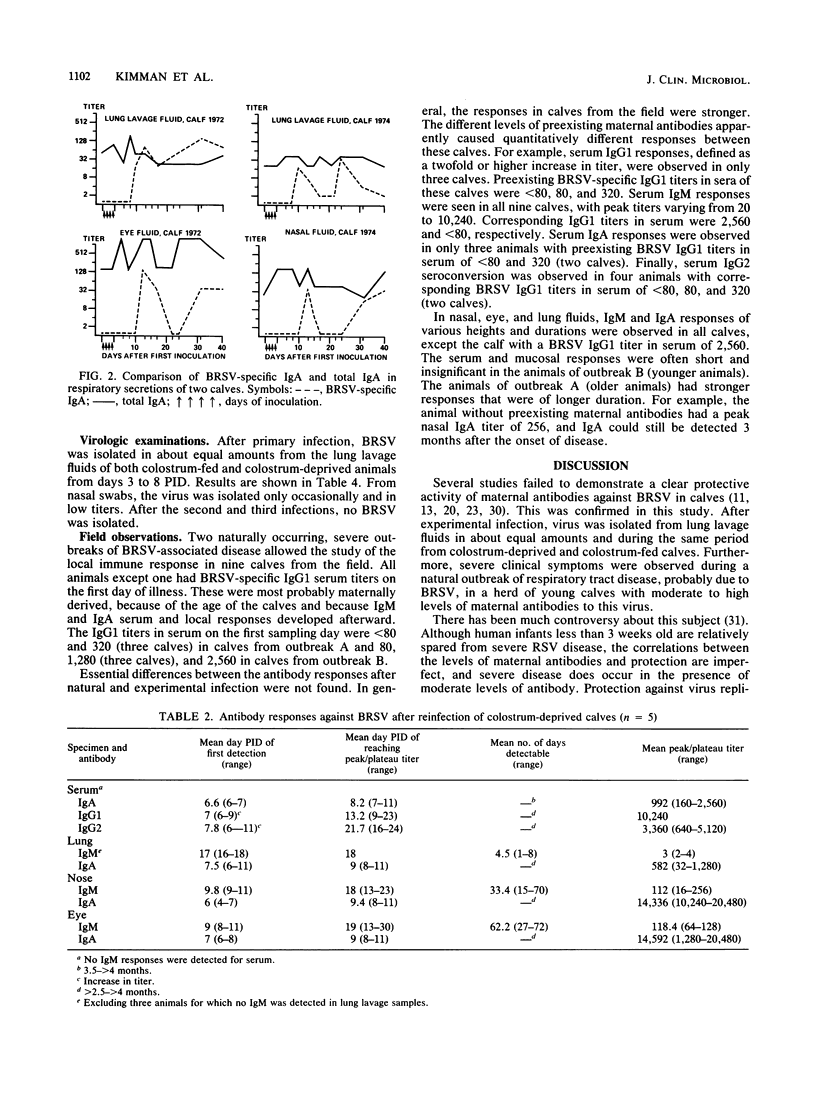
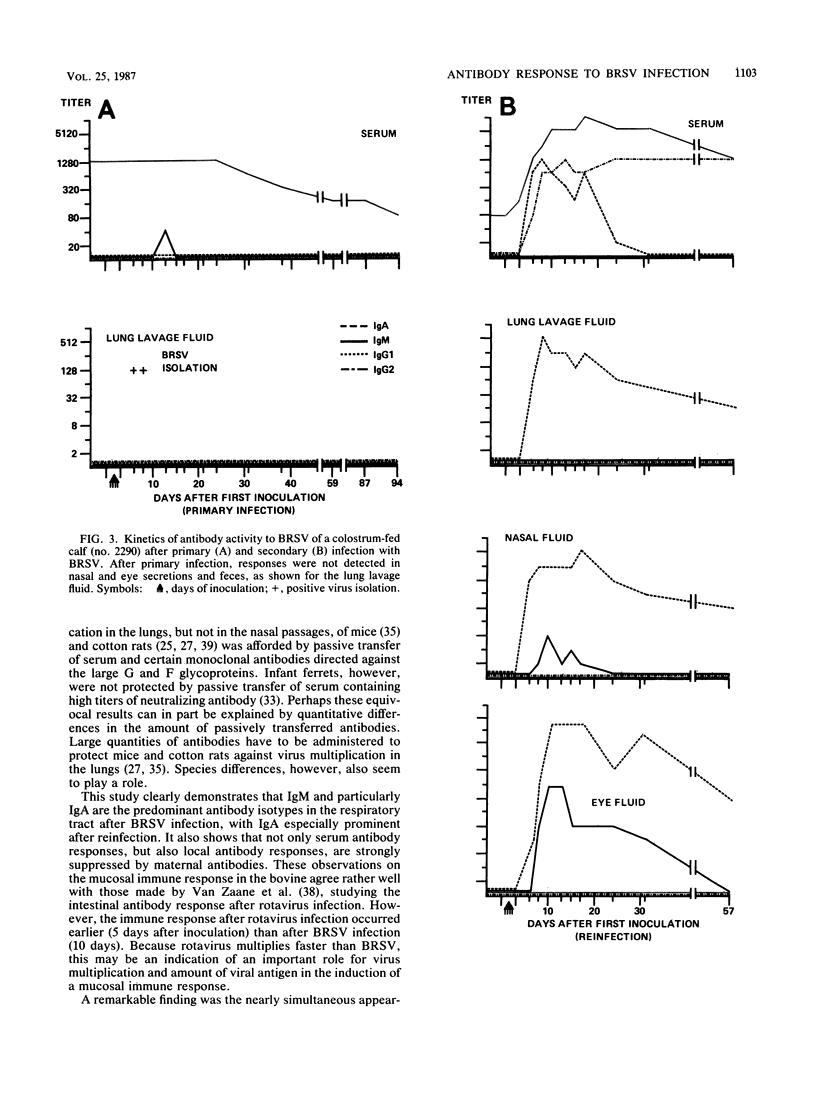
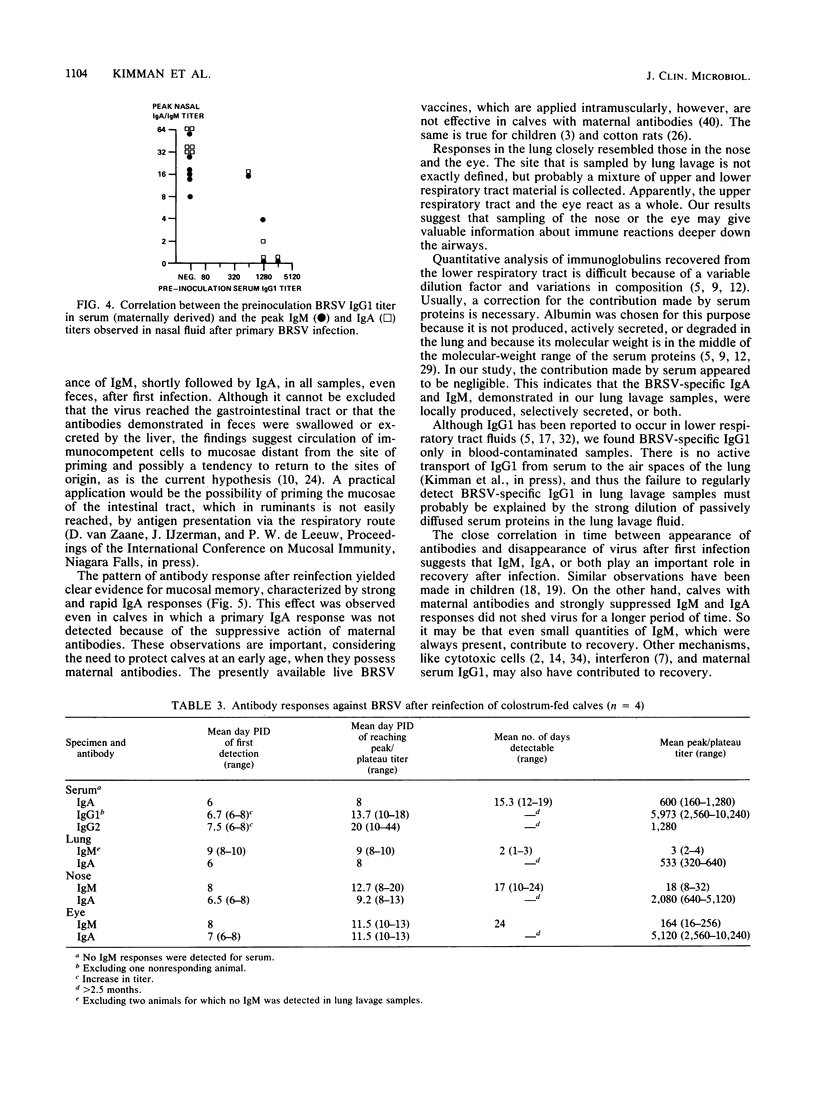
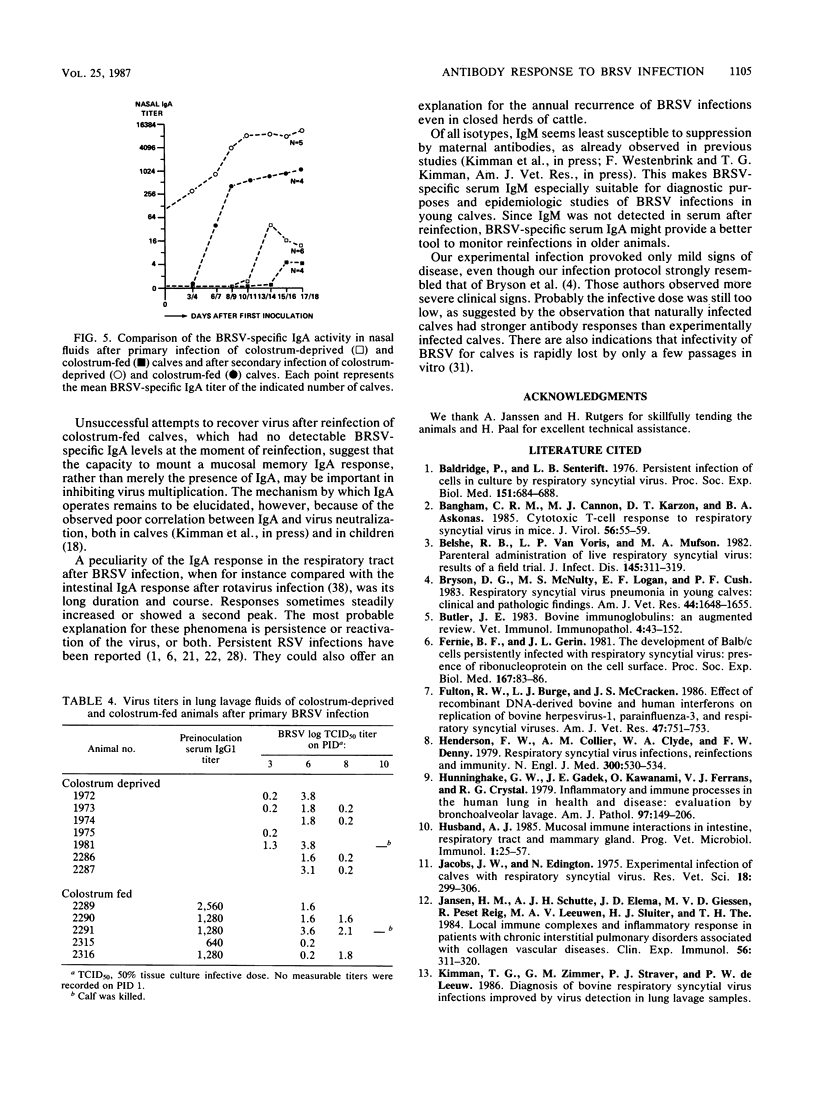
Enzyme-linked immunosorbent assays for the detection of immunoglobulin M (IgM), IgA, IgG1, and IgG2 antibodies against bovine respiratory syncytial virus (BRSV) were used to measure antibody responses of calves after experimental or natural infection with BRSV. Serially collected sera, lung lavage samples, nasal and eye secretions, and feces were tested for the presence of these antibodies. Lung lavage fluids and nasal secretions were further examined for the presence of virus. After experimental infection of 3- to 4-week-old, colostrum-deprived (seronegative) calves, the virus was detected from days 3 to 8 post-initial inoculation day (PID). An immune response was first detected 8 to 10 days PID, when BRSV-specific IgM and IgA appeared nearly simultaneously in serum, secretions, and feces. BRSV-specific IgG1 appeared only in serum on days 13 to 17 PID, and IgG2 was first detected in sera from 1 to 3 months PID. Specific IgM and IgA were detectable in the different samples for various periods. In the respiratory and eye secretions, IgA usually remained detectable for long periods, that is, for up to 3.5 months or longer. In lung lavage samples, BRSV-specific IgG1 was only incidentally demonstrated and appeared to be blood derived. The immune response of a 5-month-old calf strongly resembled that of the 3- to 4-week-old calves (feces excepted), indicating that an age effect on the immune response to BRSV is unlikely. After experimental infection of colostrum-fed, seropositive calves, both local and systemic antibody responses were largely or totally suppressed. The degree of suppression seemed to be related to the level of preinoculation virus-specific serum IgG1. Of all isotypes, IgM was least affected. Colostrum-fed animals shed virus in about equal amounts and for the same length of time as colostrum-deprived calves. Clinical signs were mild in both groups. After reinfection, no virus shedding was detected in either colostrum-deprived or colostrum-fed calves. In both groups, a secondary immune response developed, characterized by strong and rapid (from about day 6 PID) mucosal and systemic IgA responses, but reaching higher titers in colostrum-deprived calves. Also, strong mucosal, but not serum, IgM responses were observed, which, however, did not develop faster than those observed after primary infection. Naturally infected calves, showing severe signs of respiratory disease, had various levels of, most likely, maternally derived antibodies on the first day of illness. Mucosal and systemic antibody responses of various heights and durations were observed, but in general these responses were stronger than those observed after experimental infection. The results point to an important role for local IgA, rather for serum IgG1, in the protection against BRSV infection. The capacity to mount a local memory IgA response seems especially important. Priming for such a mucosal memory response is possible even when the primary immune response is severely suppressed because of the presence of material antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldridge P., Senterfit L. B. Persistent infection of cells in culture by respiratory syncytial virus. Proc Soc Exp Biol Med. 1976 Apr;151(4):684–688. doi: 10.3181/00379727-151-39286. [DOI] [PubMed] [Google Scholar]
- Bangham C. R., Cannon M. J., Karzon D. T., Askonas B. A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985 Oct;56(1):55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Mufson M. A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982 Mar;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- Bryson D. G., McNulty M. S., Logan E. F., Cush P. F. Respiratory syncytial virus pneumonia in young calves: clinical and pathologic findings. Am J Vet Res. 1983 Sep;44(9):1648–1655. [PubMed] [Google Scholar]
- Butler J. E. Bovine immunoglobulins: an augmented review. Vet Immunol Immunopathol. 1983 Mar;4(1-2):43–152. doi: 10.1016/0165-2427(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Ford E. C., Gerin J. L. The development of Balb/c cells persistently infected with respiratory syncytial virus: presence of ribonucleoprotein on the cell surface. Proc Soc Exp Biol Med. 1981 May;167(1):83–86. doi: 10.3181/00379727-167-41129. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Burge L. J., McCraken J. S. Effect of recombinant DNA-derived bovine and human interferons on replication of bovine herpesvirus-1, parainfluenza-3, and respiratory syncytial viruses. Am J Vet Res. 1986 Apr;47(4):751–753. [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Clyde W. A., Jr, Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979 Mar 8;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Husband A. J. Mucosal immune interactions in intestine, respiratory tract and mammary gland. Prog Vet Microbiol Immunol. 1985;1:25–57. [PubMed] [Google Scholar]
- Jacobs J. W., Edington N. Experimental infection of calves with respiratory syncytial virus. Res Vet Sci. 1975 May;18(3):299–306. [PubMed] [Google Scholar]
- Jansen H. M., Schutte A. J., Elema J. D., Giessen M. V., Reig R. P., Leeuwen M. A., Sluiter H. J., The T. H. Local immune complexes and inflammatory response in patients with chronic interstitial pulmonary disorders associated with collagen vascular diseases. Clin Exp Immunol. 1984 May;56(2):311–320. [PMC free article] [PubMed] [Google Scholar]
- Kumagai T., Wong D. T., Ogra P. L. Development of cell-mediated cytotoxic activity in the respiratory tract after experimental infection with respiratory syncytial virus. Clin Exp Immunol. 1985 Aug;61(2):351–359. [PMC free article] [PubMed] [Google Scholar]
- Lamprecht C. L., Krause H. E., Mufson M. A. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J Infect Dis. 1976 Sep;134(3):211–217. doi: 10.1093/infdis/134.3.211. [DOI] [PubMed] [Google Scholar]
- Martin H. T. Indirect haemagglutination test for the detection and assay of antibody to bovine respiratory syncytial virus. Vet Rec. 1983 Sep 24;113(13):290–293. doi: 10.1136/vr.113.13.290. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Befus A. D., Bienenstock J. The structural basis for immunity in the respiratory tract. Int Rev Exp Pathol. 1982;23:47–112. [PubMed] [Google Scholar]
- McIntosh K., Masters H. B., Orr I., Chao R. K., Barkin R. M. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978 Jul;138(1):24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- McIntosh K., McQuillin J., Gardner P. S. Cell-free and cell-bound antibody in nasal secretions from infants with respiratory syncytial virus infection. Infect Immun. 1979 Feb;23(2):276–281. doi: 10.1128/iai.23.2.276-281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty M. S., Bryson D. G., Allan G. M. Experimental respiratory syncytial virus pneumonia in young calves: microbiologic and immunofluorescent findings. Am J Vet Res. 1983 Sep;44(9):1656–1659. [PubMed] [Google Scholar]
- Mills B. G., Singer F. R., Weiner L. P., Holst P. A. Cell cultures from bone affected by Paget's disease. Arthritis Rheum. 1980 Oct;23(10):1115–1120. doi: 10.1002/art.1780231007. [DOI] [PubMed] [Google Scholar]
- Mills B. G., Singer F. R., Weiner L. P., Holst P. A. Immunohistological demonstration of respiratory syncytial virus antigens in Paget disease of bone. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1209–1213. doi: 10.1073/pnas.78.2.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S. B., Lillie M. G., Ingling A. L. Effect of serum and nasal neutralizing antibodies on bovine respiratory syncytial virus infection in calves. J Infect Dis. 1976 Oct;134(4):409–413. doi: 10.1093/infdis/134.4.409. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr Cellular dissemination of priming for a mucosal immune response to cholera toxin in rats. J Immunol. 1981 Dec;127(6):2461–2464. [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Camargo E., Koenig D., Chanock R. M. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983 Oct;42(1):81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Camargo E., Suffin S. C., Chanock R. M. Parenteral immunization with live respiratory syncytial virus is blocked in seropositive cotton rats. Infect Immun. 1982 Sep;37(3):1074–1078. doi: 10.1128/iai.37.3.1074-1078.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Chanock R. M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985 Sep;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Shirodaria P. V., Gimenez H. B., Levine S. Antigen and polypeptide synthesis by temperature-sensitive mutants of respiratory syncytial virus. J Gen Virol. 1981 May;54(Pt 1):173–183. doi: 10.1099/0022-1317-54-1-173. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Smith M. H., Frey M. L., Dierks R. E. Isolation, characterization, and pathogenicity studies of a bovine respiratory syncytial virus. Arch Virol. 1975;47(3):237–247. doi: 10.1007/BF01317811. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Taylor G. Respiratory syncytial virus. Brief review. Arch Virol. 1985;84(1-2):1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Thomas L. H., Taylor G., Collins A. P., Jebbett J., Crouch S. A comparison of three vaccines against respiratory syncytial virus in calves. J Hyg (Lond) 1984 Oct;93(2):251–261. doi: 10.1017/s0022172400064779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffin S. C., Prince G. A., Muck K. B., Porter D. D. Immunoprophylaxis of respiratory syncytial virus infection in the infant ferret. J Immunol. 1979 Jul;123(1):10–14. [PubMed] [Google Scholar]
- Sun C. S., Wyde P. R., Wilson S. Z., Knight V. Cell-mediated cytotoxic responses in lungs of cotton rats infected with respiratory syncytial virus. Am Rev Respir Dis. 1983 Apr;127(4):460–464. doi: 10.1164/arrd.1983.127.4.460. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D., Ijzerman J., De Leeuw P. W. Intestinal antibody response after vaccination and infection with rotavirus of calves fed colostrum with or without rotavirus antibody. Vet Immunol Immunopathol. 1986 Jan;11(1):45–63. doi: 10.1016/0165-2427(86)90087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984 Feb;43(2):756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwstadt A. P., Verhoeff J. Serology for diagnosis and epizootiological studies of bovine respiratory syncytial virus infections. Res Vet Sci. 1983 Sep;35(2):153–159. [PubMed] [Google Scholar]
- van Zaane D., Ijzerman J. Monoclonal antibodies against bovine immunoglobulins and their use in isotype-specific ELISAs for rotavirus antibody. J Immunol Methods. 1984 Sep 4;72(2):427–441. doi: 10.1016/0022-1759(84)90011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


