Abstract
The role of naturally occurring regulatory T cells (Treg), known to be phenotypically heterogeneous, in controlling the expression of systemic lupus erythematosus (SLE) is incompletely defined. Therefore, different subpopulations of CD4+ FoxP3+ Tregs in patients with active or inactive SLE were investigated and compared with those of healthy subjects and patients with ankylosing spondylitis (AS). Characterization of different subsets of circulating CD4+ FoxP3+ Tregs was examined using flow cytometry. CD4+ CD25high T cells were sorted and examined for suppressive activity in vitro. The results showed first that a significant decrease in the frequency of CD4+ CD25high FoxP3+ T cells was present in patients with active SLE (n = 58), compared with healthy controls (n = 36) and AS patients (n = 23). In contrast, the frequencies of CD25low FoxP3+ and CD25− FoxP3+ CD4+ T cells were significantly increased in patients with active SLE by comparison with the control subjects. The elevation of these two putative Treg subpopulations was associated with lower plasma levels of complement C3 and C4 in patients with SLE. In addition, the ratios of the three subsets of CD4+ FoxP3+ Tregs versus effector T cells (CD4+ CD25+ FoxP3−) were inversely correlated with the titer of anti-double-stranded DNA IgG in patients with inactive, but not active, SLE. These results suggest that the pathogenesis of SLE may be associated with a defect in the homeostatic control of different Treg subsets.
Keywords: anti-dsDNA IgG, FoxP3, regulatory T cells, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE), a chronic and often debilitating autoimmune disease, is characterized by a loss of immune tolerance to self-antigens (Ags) and by the persistent production of pathogenic autoantibodies.1 While the exact pathogenic mechanisms remain to be elucidated, recent studies have suggested dysregulation of the regulatory T cells (Tregs), particularly the naturally occurring Tregs, as one of the major factors conferring risk for the expression of human autoimmune diseases, including SLE. For example, defective suppressor function in CD4+ Treg cells with relatively high levels of CD25 (CD25high) has been demonstrated in patients with multiple sclerosis and autoimmune polyglandular syndrome Type II.2,3 Also, while some studies have suggested that the frequency of CD4+ CD25+/high T cells is decreased in both adult 4–6 and paediatric 7 patients with SLE, the contribution of various subsets of CD4+ FoxP3+ T cells has not been investigated. Moreover, there are conflicting data regarding the function of CD4+ CD25high T cells in patients with SLE,6,8 which may be a result of the heterogeneity of the Treg population.
Two subsets of CD4+ Tregs have been classified as natural and adaptive Tregs.9,10 Naturally occurring Tregs develop during normal T-cell maturation in the thymus and represent 1–2% of CD4+ T cells in the peripheral blood.11,12 They typically express high levels of CD25, as well as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and glucocorticoid-induced tumour necrosis factor receptor-related protein (GITR).13 Most importantly, natural Tregs express a specific transcription factor, forkhead box P3 (FoxP3), which is critical in the generation and development of the regulatory function of CD4+ CD25+ Tregs.14
Similarly to natural Tregs, adaptive Tregs, including transforming growth factor-β (TGF-β)-expressing T-helper 3 (Th3) cells and interleukin (IL)-10-producing T-regulatory 1 (Tr1) cells, originate from the thymus, but they are developed throughout the course of the immune response in vivo. Of interest is the finding that naïve CD4+ T cells can be converted, de novo, into CD25+ FoxP3+ and CD25− FoxP3+ suppressor T cells upon subimmunogenic stimulation.15 Also, it has been shown that TGF-β can induce FoxP3 expression, blurring the distinctions between the different adaptive Treg subsets.16 Therefore, alteration of the tissue and cytokine milieu may tip the balance in the composition of Treg subpopulations, which may, in turn, contribute to the pathogenesis of autoimmune diseases.
While the detailed mechanisms are under active investigation, natural and adaptive Treg-cell subsets may differ functionally in their mechanism of suppression. Natural Tregs primarily mediate suppression by CTLA-4, whereas adaptive Tregs initiate the suppressive cascade in a cytokine-dependent manner.9,17 In addition, the degree to which T-effector cells (Teffs) are resistant to Treg suppression is also important in immune regulation. For example, not only is GITR constitutively expressed by CD4+ CD25+ Tregs but it is also expressed by activated Teffs.18 Previous studies showed that ligation of GITR leads to an Ag-non-specific proliferation and activation of CD4+ CD25+ Tregs,13,19 while the engagement of GITR renders Teffs resistant to Treg suppression.20
In this study, we used intracellular FoxP3, together with CD25 staining, to distinguish different subsets of CD4+ FoxP3+ T cells and Teffs (CD4+ CD25+ FoxP3−), clearly, in human peripheral blood mononuclear cells (PBMCs). We found a decrease in CD4+ CD25high FoxP3+ T cells and a dramatic increase of CD25low FoxP3+ and CD25− FoxP3+ T cells in patients with active SLE. We also demonstrated that CD4+ CD25high FoxP3+ T cells from SLE patients exhibit a potent ability to inhibit activated naïve CD4+ T cells in vitro. Interestingly, the ratio of CD4+ CD25low/− FoxP3+ T cells to Teffs was significantly associated with disease activity and autoantibody levels in SLE patients. This study shows a comprehensive quantification of FoxP3-expressing CD4+ Treg subsets in SLE patients and examines their relevance to disease progression. This study will help us to understand, in greater detail, the role of different subsets of Tregs, and potentially to optimize novel therapies based on the expansion of Tregs in SLE.
Materials and methods
Subjects
We enrolled individuals who were 18 years of age or older, and used a protocol approved by the institutional review board at the Kaohsiung Medical University. Informed consent was provided according to the Declaration of Helsinki. The study subjects included patients with inactive SLE[SLE disease activity index (SLEDAI) score ≤ 3], patients with active SLE (SLEDAI score > 3), healthy controls and subjects with ankylosing spondylitis (AS) as patient controls. Healthy volunteers with no history of autoimmune diseases were enrolled in the study. Any normal donors or patients with signs of infection were excluded before the study start. Patients with SLE fulfilled the American Rheumatism Association revised criteria for SLE.21 Treatment regimens in the patients with inactive and active SLE were as follows: prednisolone alone (n = 10 and 14, respectively); prednisolone + hydroxychloroquine (n = 14 and 33, respectively); and prednisolone + hydroxychloroquine + azathioprine (n = 2 and 5, respectively).
The clinical information of the SLE patients is shown in Table 1. The following were measured in the SLE patients in the clinical immunology laboratory of the Kaohsiung Medical University Hospital: differential white cell count; the titer of anti-double-stranded DNA (dsDNA) immunoglobulin G (IgG) (Pharmacia & Upjohn, Freiburg, Germany); and the level of plasma complement C3 and C4 (Beckman Coulter, Fullerton, CA). These were all performed in parallel with the analysis of Treg-cell subpopulations.
Table 1.
Clinical information for control subjects and patients with systemic lupus erythematosus (SLE) included in this study
| Normal control | Inactive SLE | Active SLE | AS | |
|---|---|---|---|---|
| Patient no. | 36 | 29 | 58 | 23 |
| Age 1 (range) | 37 ± 12 (22–70) | 40 ± 11 (21–70) | 36 ± 11 (21–61) | 43 ± 12* (21–70) |
| Gender (female/male) | 34/2 | 27/2 | 52/6 | 4/19** |
| SLEDAI score1 | NA | 1·5 ± 1·0 | 7·5 ± 5·3 | NA |
| Prednisolone (users/non-users) | NA | 26/3 | 54/4 | NA |
| Hydroxychloroquine (users/non-users) | NA | 16/13 | 40/18 | NA |
| Azathioprine (users/non-users) | NA | 2/27 | 5/53 | NA |
| Drug (users/non-users) | NA | 26/3 | 54/4 | NA |
AS, ankylosing spondylitis; NA, not applicable; SLEDAI, systemic lupus erythematosus disease activity index.
P <0·05 compared with active SLE, results analyzed using the Mann–Whitney U-test
P <0·05 compared with normal control, results analyzed using Fisher's exact test.
Mean ± standard deviation.
Cell isolation and flow cytometry
To diminish non-specific staining by monocytes, total T cells were negatively selected from the peripheral blood from study subjects (StemCell Tech., Vancouver, BC, Canada). For fluorescence-activated cell sorter (FACS) analysis (BD Biosciences, Mountain View, CA), the following conjugated antibodies were used: CD4 (RPA-T4), human FoxP3 (PCH101), HLA-DR (LN3), CD25 (B1.49.9), CD127 (hIL-7R-M21), CD45RO (UCHL1), CD45RA (HI100), CTLA-4 (BNI3), GITR (110416) and isotype controls. All antibodies were used at concentrations titrated for optimal staining. The samples were run on a FACScan or an LSRII flow cytometer, collecting data on 105 lymphocytes (gated by forward-scatter and side-scatter properties), and were analyzed using FCS express software (De Novo Software, Thornhill, ON, Canada) and cellquestpro software (BD Biosciences).
For cell sorting, PBMCs were purified by Ficoll–Hypaque gradient centrifugation and this was followed by CD4+ T-cell isolation. CD4+ CD25high T cells, CD4+ CD45RAhigh naïve T cells and CD4+ CD25− T cells were separated on a FACSVantage™ SE cell sorter to a purity of > 97%. CD4+ CD25high T cells contained only 1–2% of CD4+ T cells with the highest CD25 expression, as previously reported.12 The purity and phenotype of samples were further tested by intracellular FoxP3 staining that revealed at least > 94% FoxP3+ cells in the sorted CD4+ CD25high T-cell population and < 1% FoxP3+ cells in sorted naïve T cells.
Proliferation assays
For the assessment of CD4+ CD25high T-cell suppressive activity, different numbers of sorted CD4+ CD25high T cells were cocultured with 2500 autologous naïve responder T cells (CD4+ CD45RAhigh) and 50 000 autologous mitomycin C-treated, CD4+ T-cell-depleted PBMCs in 96-well U-bottom plates coated with 0·5 μg/ml of anti-CD3 (UCHT1). Cells were cultured in RPMI-1640 supplemented with 5% human AB serum. After 5 days of culture, 1 μCi of [3H]thymidine was added and the cells were cultured for a further 16 hr. [3H]Thymidine incorporation was measured in counts per minute (c.p.m.) using a scintillation counter. To compare independent assays, proliferation of responder T cells was set at 100%. Thus, per cent suppression of Tregs was defined as 1 − (c.p.m. incorporated in the coculture of CD4+ CD25high T cells with responder T cells/c.p.m. of responder cells alone) × 100%.
Statistical analysis
To adjust for the gender and age effect on Treg percentages in different groups, the general linear model was adopted to compare the group differences. The frequencies of different Treg subsets were considered as response variables, individually. The variables of group, gender and age were included in each general linear model. The significant level of group variables was then considered as significantly different after adjusting for gender and age effects. Statistical comparisons of data among groups of normal and SLE subjects (without the AS group) were performed using the non-parametric Mann–Whitney U-test. Correlations were determined by non-parametric Spearman's correlation. Probability (P) values of <0·05 were considered significant. All statistical tests were performed using spss for Windows, version 13.0. (SPSS Inc., Chicago, IL).
Results
Demographic and clinical information of study subjects
The healthy volunteers were matched with the inactive and active SLE patients for both age and gender. In addition, we chose AS patients as patient controls because a previous study had mentioned that the function of CD4+ CD25high T cells from AS patients was normal.2 Within the study group, the mean age of patients with active SLE was significantly younger than that of AS patients. Patients with AS were predominantly male, while patients with SLE were primarily female (Table 1). Therefore, statistical comparisons of data among groups of study subjects were performed using a general linear model to adjust for age and gender effects, as described in the Materials and methods.
Definition and phenotypic characterization of Treg subsets and Teff in patients and controls
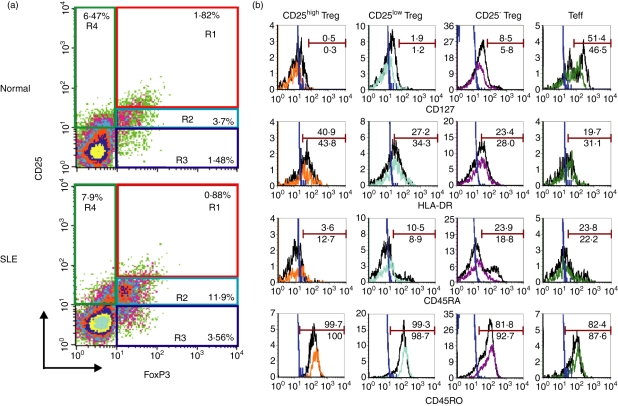
To clarify the proportions of different populations of Tregs in the peripheral blood of study subjects, including SLE patients, the expression of CD25 and FoxP3 on CD4+ T cells was examined to define the CD25high FoxP3+, CD25low FoxP3+ and CD25− FoxP3+ subsets. As shown in Fig. 1a, most CD4+ CD25high T cells are FoxP3+ cells (termed CD25high Tregs).12 In the phenotypic analysis of the cells from SLE patients (Fig. 1a lower panel), the population of CD25low and CD25− cells among CD4+ T cells contained a substantial number of the cells co-expressing FoxP3+ (termed CD25low Tregs and CD25− Tregs, respectively). CD4+ CD25+ FoxP3− T cells may represent recently activated CD4+ T cells (termed Teffs).22
Figure 1.
Characterization of different subsets of regulatory T cells (Tregs) and effector T cells (Teffs) in patients with systemic lupus erythematosus (SLE) and in controls. (a) Purified T cells from peripheral blood mononuclear cells (PBMCs) were stained with monoclonal antibodies specific for CD4, CD25 and intracellular forkhead box P3 (FoxP3) and analyzed using a FACScan. Representative dot-plots show the expression of CD25 and FoxP3 on gated CD4+ T cells from one healthy donor (upper panel) and from one patient with SLE (lower panel). Quadrants were established using appropriate isotype controls. CD4+ CD25high FoxP3+ (R1), CD4+ CD25low FoxP3+ (R2) and CD4+ CD25− FoxP3+ (R3) cells were termed as CD25high Tregs, CD25low Tregs and CD25− Tregs, respectively. CD4+ CD25+ FoxP3− (R4) cells were termed as Teffs. (b) PBMCs were stained with monoclonal antibodies specific for CD4, CD25, CD127, HLA-DR, CD45RO, CD45RA and intracellular FoxP3 and analyzed using LSRII. Histograms show surface marker expression on different Treg subsets and Teffs of one healthy donor (black line) and one patient with SLE (color line). The blue lines represent the isotype control. Data are representative of five independent controls and four independent patients.
We further characterized these putative Treg subsets and Teffs using CD127 and other surface markers associated with activation and Ag presentation. Using multicolor analysis (Fig. 1b), most CD4+ FoxP3+ T cells, irrespective of their CD25 expression, were CD127low/−, whereas CD4+ CD25+ FoxP3− T cells contained around 50% of CD127+ cells and at least 80% of CD45RO+ cells, both in the controls and patients. As CD127 could be used to discriminate between Tregs and activated T cells,23,24 our data support that CD4+ FoxP3+ T cells, irrespective of their CD25 expression, represent Treg subsets and that CD4+ CD25+ FoxP3− T cells represent Teffs.
As to other markers, CD25high Tregs are mainly HLA-DRlow, CD45RO+ and CD45RAlow/−, both in the controls (black line) and in patients (color line). In addition, CD25− Tregs in the controls may consist of memory and naïve subsets, based on their CD45RO and CD45RA expression, as shown in Fig. 1b. However, CD25− Tregs in patients with SLE were mainly CD45RO+ and CD45RAlow/−, possibly reflecting the recent activation of patient's CD25− Tregs by self-Ags in vivo.
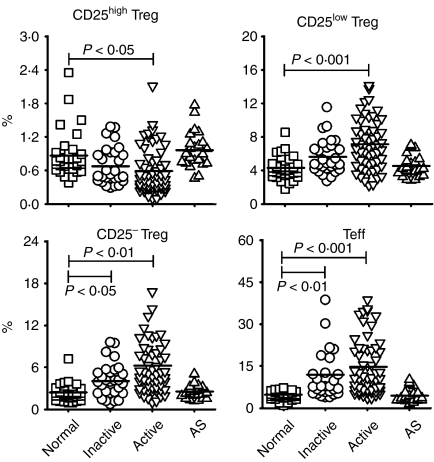
Frequencies of circulating CD4+ CD25high FoxP3+ T cells (CD25high Tregs) were significantly decreased in patients with active SLE
As shown in Fig. 2, the percentage of circulating CD25high Tregs was significantly lower in patients with active SLE (0·61 ± 0·41%) that in healthy donors (0·86 ± 0·39%). Although no significant difference in Treg preponderance was observed between subjects with inactive SLE (0·69 ± 0·33%) and healthy controls, the percentage of CD25high Tregs in patients with inactive SLE tended to be lower than that in healthy controls. We also found that the absolute numbers of CD25high Tregs in patients with inactive (3·64 ± 3·39 × 103/ml) and active (2·33 ± 2·06 × 103/ml) SLE were significantly lower than those found in healthy controls (5·58 ± 2·11 × 103/ml).
Figure 2.
The percentages of different regulatory T-cell (Treg) subsets and effector T cells (Teffs) in patients with systemic lupus erythematosus (SLE) and in healthy controls. The results are shown as percentages of CD25high Tregs, CD25low Tregs, CD25− Tregs and Teffs in normal controls (n = 36), in patients with inactive SLE (n = 29), in patients with active SLE (n = 58) and in patients with ankylosing spondylitis (AS) (n = 23). Symbols represent the frequencies of Treg subsets and Teffs in individual samples. The line within the vertical points marks the mean for each group. A P-value of<0·05 was considered as significant following analysis of the results using a general linear model adjusted for gender and age. See Fig. 1 for other definitions.
The percentages of CD25low FoxP3+, CD25− FoxP3+ and CD25+ FoxP3− populations were increased among CD4+ T cells in SLE patients
In contrast to the reduction of CD25high Tregs, the proportions of CD25low Tregs, CD25− Tregs and Teffs were significantly elevated in SLE patients (Fig. 2). The proportions of circulating CD25low Tregs, as well as CD25− Tregs, were significantly increased in patients with inactive SLE (5·62 ± 2·02%, 4·14 ± 2·38%, respectively) and active SLE (7·1 ± 2·92%, 6·26 ± 4·87%, respectively) compared with the proportions in healthy donors (4·31 ± 1·29%, 2·37 ± 1·18%, respectively). However, the absolute counts of CD25low Tregs and CD25− Tregs were similar among these three subject groups (CD25low Tregs: normal, 2·8 ± 1·12 × 104/ml, inactive, 2·62 ± 2·01 × 104/ml, active, 2·57 ± 1·68 × 104/ml; CD25− Tregs: normal, 1·49 ± 0·7 × 104/ml, inactive, 1·85 ± 1·77 × 104/ml, active, 2·19 ± 2·19 × 104/ml). The apparently normal absolute Treg numbers found in patients with SLE may be partly the result of lymphopenia associated with the disease, which led to a decrease in the number of total CD4+ T cells in patients with both inactive (4·93 ± 3·62 × 105/ml) and active (4·24 ± 3·78 × 105/ml) SLE, compared with that seen in normal controls (6·69 ± 1·93 × 105/ml). As expected, when compared with healthy controls (4·68 ± 1·54%), patients with SLE showed a significantly increased percentage of Teffs (inactive, 11·97 ± 10·1%; active, 14·71 ± 9·8%) (Fig. 2). Absolute counts of Teffs were also significantly elevated in patients with active (5·44 ± 5·24 × 104/ml) but not inactive (4·5 ± 3·07 × 104/ml) SLE, compared with those in healthy controls (3·15 ± 1·44 × 104/ml).
Regarding patients with AS, the percentages of CD25high Tregs, CD25low Tregs, CD25− Tregs and Teffs (0·97 ± 0·33%, 4·56 ± 1·36%, 2·51 ± 1·06%, 4·43 ± 2·39%, respectively) did not significantly differ from those found in normal controls (Fig. 2).
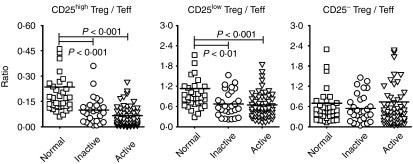
Altered relative ratios of CD25high Tregs and CD25low Tregs versus Teffs in patients with SLE
Owing to the importance of the delicate balance between Tregs and Teffs, the ratios of these two populations were further analyzed and the results are shown in Fig. 3. There was a significantly lower ratio of both CD25high Tregs and CD25low Tregs versus Teffs in patients with inactive and active SLE than in healthy individuals (CD25high Tregs/Teffs: inactive, 0·1 ± 0·08, active, 0·07 ± 0·06, normal, 0·23 ± 0·24; CD25low Tregs/Teffs: inactive, 0·67 ± 0·37, active, 0·65 ± 0·38, normal, 1·13 ± 0·86). However, there was no significant difference in the ratio of CD25− Tregs to Teffs between patients with inactive or active SLE and healthy controls (inactive, 0·54 ± 0·42, active, 0·74 ± 1·26, normal, 0·7 ± 0·78).
Figure 3.
The comparisons of ratios of regulatory T-cell (Treg) subsets versus effector T cells (Teffs) in patients with SLE and in healthy controls. The ratio was defined as the percentage of the indicated Treg subset divided by the percentage of Teffs from the same individual. The ratio was calculated from individual data shown in Fig. 2. A P-value of<0·05 was considered significant following analysis of the results using the Mann–Whitney U-test. See Fig. 1 for other definitions.
The imbalance between Tregs and Teffs correlated with levels of anti-dsDNA IgG in patients with inactive SLE
We next examined whether the change in the distribution of the Treg subsets is correlated with the clinical and serologic features of SLE. First, the titer of anti-dsDNA IgG was, as expected, positively correlated with the percentage of Teffs in patients with inactive SLE (Fig. 4a), but this correlation was not seen in active SLE (data not shown). Second, it was found that the ratios of CD25high Tregs, CD25low Tregs, or CD25− Tregs versus Teffs were inversely correlated with the titer of anti-dsDNA IgGs in patients with inactive SLE (Fig. 4a), but not in those with active disease (data not shown). In addition, we compared the percentages of different Treg subsets in SLE patients exhibiting normal complement levels or low complement levels. The percentages of CD25low Tregs and CD25− Tregs in patients with low complement levels (6·9 ± 2·8%, 6·06 ± 4·58%, respectively) were significantly higher than those in patients with normal complement levels (5·16 ± 1·94%, 3·61 ± 2·28%, respectively). However, no significant differences in the percentages of CD25high Tregs and Teffs were found in these two groups (Fig. 4b). We also compared the percentages of different Treg subsets and the ratios of Tregs versus Teffs in SLE patients with or without nephritis; however, we did not find any statistically significant differences between these two groups (data not shown).
Figure 4.
The relationships between disease activity and the frequencies or relative ratios of regulatory T-cell (Treg) subsets and effector T cells (Teffs) in patients with systemic lupus erythematosus (SLE). (a) The ratios of different Treg subsets versus Teffs were negatively correlated with the titre of anti-double-stranded DNA immunoglobulin G (anti-dsDNA IgG) in patients with inactive SLE (n = 18). (b) The results are shown as the percentage of CD25high Tregs, CD25low Tregs, CD25− Tregs and Teffs in healthy controls (n = 36); SLE patients exhibiting normal complement levels (Non-low C’) (n = 18); and SLE patients exhibiting low complement levels (Low C’) (n = 69). Lines within the vertical points mark the mean for each group. A P-value of<0·05 was considered as significant following analysis of the results using the Mann–Whitney U-test. *P <0·05 compared with normal subjects; δP <0·05 compared to SLE subjects with normal complement levels. See Fig. 1 for other definitions.
CD25high Tregs displayed normal function in patients with SLE
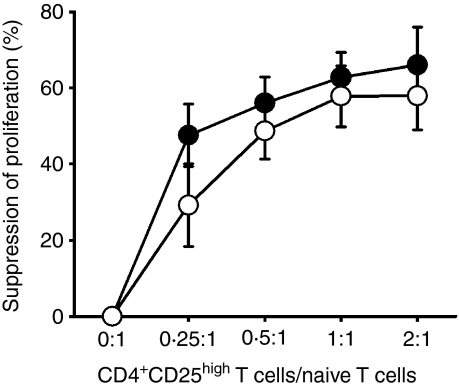
Previous studies examined SLE patient Treg function using CD4+ CD25− T cells as responding T cells.6,8 However, our data showed that CD4+ CD25− T cells contained significantly higher levels of FoxP3+ cells in patients with inactive (4·14 ± 2·38%) and active (6·26 ± 4·87%) SLE compared with control donors (2·37 ± 1·18%) (Fig. 2). To avoid the contamination of FoxP3+ cells in responder T cells, we sorted only highly pure naïve T cells (CD4+ CD45RAhigh) as responder cells. When activated with a plate-bound anti-CD3, these naïve T cells responded with robust proliferation, although the magnitude of the proliferative response was lower than that induced by CD4+ CD25− cells in the same culture conditions.
We cocultured sorted CD4+ CD25high T cells and autologous naïve T responder cells in the presence of autologous antigen-presenting cells (APCs) at different ratios. As shown in Fig. 5, CD4+ CD25high T cells from both healthy individuals and patients with SLE significantly inhibited T-cell proliferation in a dose-dependent manner. In fact, CD4+ CD25high T cells from normal individuals and SLE patients demonstrated similar capacities for suppressing the proliferative responses of naïve T cells at varying ratios of Treg cells/naïve T cells.
Figure 5.
The CD25high regulatory T-cell (Treg) suppressive function in patients with systemic lupus erythematosus (SLE) appears normal in vitro. Naïve T cells (CD4+ CD45RAhigh) were stimulated alone or cocultured with autologous CD4+ CD25high T cells in the presence of mitomycin C-treated autologous antigen-presenting cells (APCs) at the indicated ratios. SLE patients (○, n = 7, three inactive and four active) and age-and gender-matched healthy individuals (•, n = 6) were tested for CD25high Treg activity. The per cent suppression of proliferation was calculated as described in the ‘Materials and methods’. The average proliferative response of APCs was 583 counts per minute (c.p.m.) in the control group and 1224 c.p.m. in the patient group. The average stimulation index of responder T cells was 8·3 in the control group and 9·9 in the patient group. Sorted CD4+ CD25high T cells proliferated poorly after activation with monoclonal anti-CD3, while the average stimulation index of CD25high Tregs in both groups was < 2·0. See Fig. 1 for other definitions.
Discussion
The role of Tregs in the pathogenesis of SLE has been suggested, but the exact mechanism remains to be defined. This study provides, to our knowledge, the first evidence for an increased prevalence of circulating CD25low and CD25− of CD4+ FoxP3+ T cells in SLE patients. The results also showed that quantitative imbalances between different CD4+ FoxP3+ subsets and Teffs were associated with disease activity and autoantibody production in patients with SLE. While the causal relationship remains to be established, this study suggests that the delicate balance between Tregs and Teffs may be important in the pathogenesis of human lupus.
Several studies demonstrated that CD25low FoxP3+ cells are Tregs with a naive phenotype, which is reflected in their low expression of CD45RO and CD95, as well as their resistance to CD95 ligand (CD95L)-mediated apoptosis. In contrast, CD25high FoxP3+ Tregs exhibited a predominantly memory phenotype with high expression of CD45RO and CD95, and displayed sensitivity to CD95L-induced apoptosis.22,25,26 As a corollary, Miyara et al.6 suggested that enhanced CD95-mediated apoptosis of CD4+ CD25high Tregs could result in reduced frequencies of Treg in active SLE. The sensitivity to apoptosis may thus determine, in part, the relative frequency of the Treg subpopulations. This possibility would be consistent with our finding of a decreased frequency in the CD25high Tregs in patients with active SLE. Furthermore, the increased frequency of CD25low Tregs, as seen in our study patients, may compensate for the loss of CD25high Tregs in active SLE. However, this compensation may not be enough to regulate the autoimmune response because the ratio of CD25low Tregs versus Teffs in both inactive and active SLE still remains significantly lower than that in normal controls (Fig. 3). Thus, our data suggest that the pathogenesis of SLE might be associated with not only a reduction in CD25high Tregs, but also the quantitative imbalance between CD25low, and CD25− Tregs, and Teffs.
Active systemic inflammation against self-Ags, as observed in patients with SLE, may induce T-cell activation, resulting in increased CD25 expression. Our data indeed showed a significantly higher frequency of CD4+ CD25+ FoxP3− T cells in SLE patients compared with normal controls (Fig. 2). In addition, systemic inflammation may induce the differentiation of naïve CD4+ T cells into CD25+ FoxP3+ and CD25− FoxP3+ Tregs, or so-called adaptive Tregs.15,16,27 Liu et al.23 also found that that FoxP3 was expressed in 8–10% of the human CD4+ T cells, independently of CD25 expression in normal individuals. Although it is currently unclear whether such differentiation of adaptive Tregs exists in human inflammatory diseases in vivo, it is tempting to speculate that CD4+ CD25− FoxP3+ T cells in SLE patients might represent adaptive Tregs induced by a systemic autoimmune response. It is also possible that CD25low Tregs in SLE patients may contain not only thymically derived Tregs with a naïve phenotype,22,25 but also peripherally converted Tregs.
In addition to the disproportionate numbers of Tregs and Teffs, the functions of different Treg subsets in SLE still need further clarification. The in vitro suppressive data of CD4+ CD25high T cells (Fig. 5) support the presence of normally functioning, suppressive CD4+ CD25high T cells in SLE patients. The data are consistent with those of Miyara et al.,6 but not with those of Valencia et al.8 This discrepancy might be a result of the different monoclonal antibodies and types of APCs used for in vitro stimulation. Regarding the functions of CD25low Tregs and CD25− Tregs in normal individuals, Liu et al. demonstrated that CD4+ CD127low/− T cells, which include both CD25low FoxP3+ and CD25− FoxP3+ subsets, could suppress the allogeneic mixed-lymphocyte response as well as classical ‘CD4+ CD25high’ Tregs.23 However, our phenotypic analysis of different Treg subsets showed a reduced frequency of CTLA-4-expressing, CD25low/− Tregs in SLE patients compared with those in normal controls (data not shown). On the other hand, it should be taken into account that activation-induced expression of FoxP3 mRNA has been reported for in vitro-stimulated CD4+ T cells.28–30 Furthermore, acetylation of several lysines in the forkhead domain of the FoxP3 protein determines the optimal Treg function.31 These observations raise questions about whether CD25low Tregs and CD25− Tregs in patients with SLE exhibit suppressive activity.
Immune regulation is not only controlled by Tregs but also by the status of responder T cells. A previous study demonstrated that activated responder T cells from the synovial fluid of patients with rheumatoid arthritis were more resistant to suppression mediated by CD4+ CD25+ T cells than responder cells from the peripheral blood of these patients.32 This study also found an increased percentage of CD4+ CD25+ T cells in synovial fluid, but not in the peripheral blood, of patients with rheumatoid arthritis.32 Therefore, the degree to which Teffs are resistant to Treg suppression in SLE patients requires further investigation.
One important characteristic of SLE is the production of anti-dsDNA IgG. Although the role of Tregs on T-cell-mediated immunity has been widely explored, the effects of Tregs on humoral immunity are less clear. Seo et al.33 have shown that the tolerance breakdown of autoreactive B cells in lpr/lpr mice requires the help of CD4+ T cells and the overcoming of suppression by CD4+ CD25+ Tregs. In agreement with the previous study,33 our data showed that the ratios of CD4+ FoxP3+ subsets versus Teffs were inversely correlated with the titer of anti-dsDNA IgG in patients with inactive SLE (Fig. 4a). This suggests that as the number and/or function of Tregs decline relative to those of Teffs, tolerance begins to be breached in SLE patients. Thus, for autoimmune diseases with altered frequencies of Tregs, an analysis of Teffs should be carried out in parallel.
On the other hand, the imbalance between Treg subsets and Teffs did not correlate with the level of anti-dsDNA IgG in patients with active SLE (data not shown). We speculate that the high dose of drug treatment may affect the levels of autoantibodies in patients with active SLE. The main treatment approach for lupus patients in this study was the combination of prenisolone, hydroxychloroquine and azathioprine. Among these drugs, corticosteroids, such as prednisolone, can control inflammation through the suppression of multiple inflammatory genes that are activated in chronic inflammatory diseases. Prednisolone also suppresses T-lymphocyte proliferation by inhibiting nuclear factor-κB, which eventually down-regulates IL-2 expression.34,35 With a lack of T-cell help, IgG autoantibody production would significantly decrease. Thus, the higher dose of prenisolone used in active SLE may interfere with the titre of anti-dsDNA IgG, and therefore no correlation was found between Tregs versus Teffs ratios and the anti-dsDNA IgG titer in those study patients. This possibility clearly needs to be further investigated. As to the effect of treatment on the distribution of Tregs, the percentage of those three Treg subsets in the patients did not correlate with daily prednisolone dose (data not shown).
In summary, our study examined the frequencies of different subpopulations of CD4+ FoxP3+ T cells, which may represent a comprehensive Treg pool in human PBMCs from normal individuals and patients with SLE. Our study also found that altered homeostasis, as evidenced by an imbalance between different CD4+ FoxP3+ subsets and Teffs, were associated with disease activity, complement consumption and the production of anti-dsDNA IgG in SLE patients. Expanding the frequencies of Tregs, especially in an Ag-specific manner, may be a potential therapeutic strategy for treating autoimmune diseases such as SLE.
Acknowledgments
This work was supported by grants from the National Science Council (NSC 94-2320-B-037-030 and NSC 95-2320-B-037-009) and by the funds KMU-EM-97-1.2b. We sincerely thank Dr Shau Ku Huang for critical review of the manuscript. We also thank the Center for Resources, Research and Development of KMU and the Department of Medical Research, KMUH for providing LSRII and FACSVantage, respectively.
Glossary
Abbreviations:
- Ag
antigen
- APC
antigen-presenting cell
- AS
ankylosing spondylitis
- c.p.m.
counts per minute
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- dsDNA
double-stranded DNA
- FACS
fluorescence-activated cell sorter
- FoxP3
forkhead box P3
- IgG
immunoglobulin G
- GITR
glucocorticoid-induced tumour necrosis factor receptor-related protein
- PBMCs
peripheral blood mononuclear cells
- SLE
systemic lupus erythematosus
- SLEDAI
SLE disease activity index
- Teff
effector T cell
- Treg
regulatory T cell
References
- 1.Tan EM. Autoantibodies and autoimmunity: a three-decade perspective. A tribute to Henry G. Kunkel. Ann N Y Acad Sci. 1997;815:1–14. doi: 10.1111/j.1749-6632.1997.tb52040.x. [DOI] [PubMed] [Google Scholar]
- 2.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285–91. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–6. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 5.Liu MF, Wang CR, Fung LL, Wu CR. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Wang LC, Lin YT, Yang YH, Lin DT, Chiang BL. Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus. Immunology. 2006;117:280–6. doi: 10.1111/j.1365-2567.2005.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 9.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 10.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–42. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 12.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 14.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 18.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100:15059–64. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 20.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–20. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 22.Fritzsching B, Oberle N, Pauly E, et al. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood. 2006;108:3371–8. doi: 10.1182/blood-2006-02-005660. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–62. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–9. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 27.Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006;212:149–62. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 28.Morgan ME, van Bilsen JH, Bakker AM, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Allan SE, Passerini L, Bacchetta R, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–84. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 32.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 33.Seo SJ, Fields ML, Buckler JL, et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–46. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 34.Ferron GM, Jusko WJ. Species-1and gender-related differences in cyclosporine/prednisolone/sirolimus interactions in whole blood lymphocyte proliferation assays. J Pharmacol Exp Ther. 1998;286:191–200. [PubMed] [Google Scholar]
- 35.Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148:245–54. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]