Abstract
Life span can be extended in rodents by restricting food availability (caloric restriction [CR]) or by providing food low in methionine (Meth-R). Here, we show that a period of food restriction limited to the first 20 days of life, via a 50% enlargement of litter size, shows extended median and maximal life span relative to mice from normal sized litters and that a Meth-R diet initiated at 12 months of age also significantly increases longevity. Furthermore, mice exposed to a CR diet show changes in liver messenger RNA patterns, in phosphorylation of Erk, Jnk2, and p38 kinases, and in phosphorylation of mammalian target of rapamycin and its substrate 4EBP1, HE-binding protein 1 that are not observed in liver from age-matched Meth-R mice. These results introduce new protocols that can increase maximal life span and suggest that the spectrum of metabolic changes induced by low-calorie and low-methionine diets may differ in instructive ways.
Keywords: Methionine, Litter size, Caloric restriction, TOR, ERK
THERE is now ample evidence that aging can be slowed in rodents, and life span extended, by caloric deprivation (1), by diets low in methionine (2,3), and by at least three single-gene mutations for which extension of maximum life span has been documented in two or more laboratories (4–6). There is much to be learned, however, about the pathways by which these diets and mutations slow aging and, in particular, whether the antiaging pathways induced in these animal models overlap with one another. The demonstration (7) that caloric restriction (CR) extends life span in Ames dwarf mice supports the idea that the CR and the df/df genotype extend life span through at least partly different means, but this inference has been questioned on theoretical grounds (8), and the observation that a CR diet does not extend life span of the long-lived growth hormone receptor knockout (GHRKO) mutant (9) further complicates interpretation of these results. Genetic studies in Caenorhabditis elegans (10) have suggested that mutations that extend life span of this species may fall into classes with different modes of action: one of these has homologies to the insulin and insulin-like growth factor-I (IGF-I) responses lacking in Ames and GHRKO mice and another that appears to play a role in nematode responses to CR. Studies of long-lived invertebrates provide important clues to the potential role of homologous genetic and cellular elements in mammals but are unlikely to serve as an infallible guide to developmental biology and aging in such a distant clade.
The ability of CR diets to retard many aspects of aging in multiple species has been well documented, but studies of methionine-restricted (Meth-R) diets have been less widely discussed. Studies in rats have shown that Meth-R diets can extend mean and maximal longevity by 40% or more, similar to the effect produced by CR diets (2,11). The Meth-R diet extends longevity of several strains of rats (3). Rats on Meth-R diets are smaller than control rats, but they consume more food, per gram body weight, than controls, and rats allowed to consume normal chow at the rate consumed by rats on a Meth-R diet do not show a similar life-span extension (2), showing that the longevity of the Meth-R rat is not due simply to diminished caloric intake. Mice placed on a Meth-R diet at the age of 6 weeks show extended mean and maximal longevity compared with controls, despite relatively low survival in the first year of life (12). Mice on a Meth-R diet show a slower rate of age-related change in T-cell subsets and slower development of cataracts, consistent with the generalization that this diet may slow the pace of age-related change in multiple tissues in addition to its overall effect on lethal illnesses. Meth-R mice resemble CR mice in several respects, including low serum levels of insulin, glucose, thyroid hormone T4, and IGF-I, and dramatically higher resistance to the hepatotoxic effects of injected acetaminophen (APAP) (12).
Although CR begun in young adult animals has the most dramatic impact on aging and life span, there is some evidence to suggest that CR can have beneficial effects when begun in adulthood (13–16). In some settings, these effects seem to be reversible, in that cessation of CR feeding results in a rapid return to the “normal” state (17), with a concomitant reduction in life span relative to animals maintained on lifelong CR (14). However, the age at which the switch from CR back to normal feeding occurs may be important. In particular, Yu and colleagues demonstrated that 4.5 months of CR, from 1.5 to 6 months of age, in male F344 rats was sufficient to significantly extend both median and maximum life span in conjunction with a delayed onset of age-related disease (16,18), and similar studies in other rat strains corroborate these results (19,20). This suggests that the life-extending effects of CR (21) may depend on both the timing and the duration of the period of dietary intervention. Studies in mice have been more ambiguous (22–24), but there is some evidence to suggest that a period of restriction early in life can have lasting effects on aging and life span (25,26).
This article reports results of two experiments on mouse longevity. The first experiment shows that Meth-R in mice can extend life span even when initiated as late as 12 months of age. The second shows that a nutritional intervention, litter enlargement (LE) limited to the 3-week period between birth and weaning, can extend mean and maximal life span in mice. We also present results of gene expression and signal transduction experiments that document multiple differences between the effects of Meth-R and those of CR. Taken together, these results show that longevity can be altered by multiple nongenetic pathways in mice imposed at different stages of the life cycle.
MATERIALS AND METHODS
Meth-R Experiment
Male (BALB/c × C57BL/6)F1 (CB6F1) mice were purchased from the National Institute on Aging (NIA) contract colony at Charles River Laboratories at 11 or 12 months of age and thereafter housed in a specific pathogen vivarium as described by Miller and colleagues (12). Serological testing was used every 3 months, as described (12), to monitor the pathogen-free status of the colony, and all such tests were negative throughout the experimental period. Mice were housed three per cage, with care taken to assure that they were shipped from the supplier as cage mates, and then housed in these original groups at our own site. After a 7-day adaptation period, during which the animals received standard laboratory chow, 17 control cages (51 mice, “Meth-C”) were shifted to a diet containing a synthetic mixture of amino acids in lieu of natural sources of protein and including methionine at 0.43% per weight. An equal number of mice (Meth-R) were given an equivalent diet containing 0.23% methionine by weight and after two further weeks were shifted to a diet containing 0.15% methionine; these mice remained on this diet for the rest of the experiment. The control diet, prepared by Purina Test Diets, Inc. (Richmond, IN), was based upon the AIN-76 formulation. It was compounded using 43% cornstarch, 20% sucrose, 8% corn oil, 5% dextrin, 5% cellulose, 3.6% glutamic acid, vitamin and mineral mix, choline, and a set of defined amino acids in lieu of a protein source. Amino acid concentrations were as follows, expressed as weight of amino acid per weight of total diet: 0.93% arginine, 0% cystine, 2.31% glycine, 0.27% histidine, 0.82% isoleucine, 1.11% leucine, 1.15% lysine, 0.43% methionine, 1.16% phenylalanine, 0% tyrosine, 0.82% threonine, 0.18% tryptophan, and 0.82% valine. A similar diet containing 0.15% methionine was also prepared by the same company for our use, in which cornstarch was substituted for the diminished methionine; this reduced-methionine diet is available as Catalogue No. 52501 (58MK). At 18 months of age, eight Meth-R and eight Meth-C mice were euthanized to provide tissues for biochemical analyses of Erk, Jnk, p38, and target of rapamycin (TOR) activities. The remaining 43 mice in each group were evaluated at least daily to determine the date of death.
CR Protocol
Male CB6F1 mice were obtained at age 11 or 12 months from the NIA colony as described for the Meth-R and Meth-C groups and housed in the same room as these two groups. After a 1-week adaptation period, mice in the CR group were given an amount of food equal to 90% of the amount consumed by mice in the ad libitum control group (“AL” group) for 2 weeks. They were then shifted to 75% food availability for 2 weeks and then shifted to 60% food intake for the remainder of the experiment. All mice in the CR and AL groups were euthanized at 18 months of age for biochemical analyses.
Liver RNA Samples for Gene Expression Analysis
Liver samples for messenger RNA (mRNA) studies were derived from a separate population of Meth-R and Meth-C CB6F1 mice, exposed to these diets from 6 weeks of age; these mice were developed in parallel to the groups used for the longevity experiment previously described by Miller and colleagues (12). CR and AL samples for mRNA analysis were derived from CB6F1 mice that had been housed along with the Meth-R and Meth-C mice from 6 weeks of age. Samples were obtained at the same time of day (8–10 am), and on the same day for controls and experimental animals in both groups, and were processed in parallel.
Maternal Protein Restriction and Litter Expansion Experiments
Rather than CB6F1 mice, this experiment used mice of the UM-HET3 stock (27); they were the offspring of crosses between CB6F1 mothers and (C3H/HeJ × DBA/2J)F1 fathers and thus genetically heterogeneous in that any two mice were full sibs, sharing a random 50% of their nuclear genes. First litters of the CB6F1 mothers were used for other purposes, and the offspring from the second litters used for this study. The dams were fed a diet containing 20% protein (Harlan Teklad TD91352) for the duration of their pregnancy. Litters were culled to eight, and the dams were randomly assigned to one of three groups: (a) one that remained on the 20% control diet during lactation (control); (b) one that was switched to an isocaloric 8% protein diet (Harlan Teklad TD93033) during lactation (protein restriction [PR]); or (c) one in which the dams received the control diet (with 20% protein) and four additional pups from another litter, resulting in a 50% increase in total litter size (LE). To control for the potentially confounding effect of differing levels of nutrition experienced in utero, litters with less than 8, or more than 10, original pups were not used. At 20 days of age, pups were weaned onto a normal chow diet (Purina 5001, 23% protein) and were periodically weighed and bled for the quantification of circulating hormone levels. Some of the animals were challenged with intraperitoneal injections of APAP at 2, 4, 8, and/or 18 months old, using a method previously described (28).
Life Span Analysis
Differences in survival between Meth-R and control mice were evaluated using the log-rank statistic. All mice were dead at the time of the analysis, so no censoring was required. Differences in the proportion of mice alive at the age at which 90% of the pooled population had died were evaluated as recommended by Wang and colleagues (29), using the Fisher exact statistic; this provides a useful estimate of differences in “maximal longevity.” The log-rank test was also used to evaluate differences between LE mice (or PR mice) and controls. To examine the potentially confounding effect of APAP exposure on life span, a second analysis was performed using Cox regression with treatment group (control, PR, LE) and number of APAP exposures (0, 1, or 2) as covariates in the model.
Quantification of mRNA Levels Using Multiplex Reverse Transcription–Polymerase Chain Reaction
RNA samples were isolated from the livers of eight CR and eight AL mice, as well as nine Meth-R and seven Meth-C mice using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. An Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA) was used to determine the concentration and quality of each RNA isolate. Complementary DNA (cDNA) was generated from 2 μg of each RNA sample using the High Capacity cDNA kit (Applied Biosystems, Foster City, CA). In total, 1:1 mixtures of the master mix and each cDNA reaction product were loaded onto Applied Biosystems’ multiplex (microfluidics-based) reverse transcription–polymerase chain reaction (RT-PCR) cards custom made to contain four replicate sets of 96 primers, that is, 1 control primer and 95 primers for genes relevant to aging and stress resistance. Each card received RNA from four mice, one from each of the two control and two experimental populations. The cards were run on ABI Prism's 7900HT Sequence Detection System and analyzed with version 2.1.1 of the system's software. The relative RNA expression levels were inferred from the Ct values.
mRNA Analysis: Statistics
To adjust for variation among RT-PCR microfluidics cards, a normalization constant was defined for each card based upon the mean Ct value of the 20 mRNA species that had the highest mRNA abundance (lowest Ct values) across the entire series of cards. Each individual Ct value was then adjusted by adding in this card-specific normalization factor, so that each card had the same average estimate of mRNA for the 20 most abundant mRNAs. The size of the effect of the CR or Meth-R diet was then estimated, for each mRNA, by subtracting the mean Ct values of the respective control mice from mean Ct values for the experimental mice, raised to the power of two because each change of one Ct unit reflects a twofold change in mRNA abundance. Statistical significance was evaluated using a false discovery rate (FDR) algorithm as implemented in SAM (Statistical Analysis for Microarray) software, as described by Tusher and colleagues (30); a collection of mRNAs for which FDR = 0.05 is expected to include only 5% of false-positive elements were included by chance alone.
Antibodies
Antibodies for immunoblotting were obtained as follows: p38 mitogen-activated protein kinase (MAPK), phospho-p38 MAPK (Thr180/Tyr182), ERK, phospho-ERK (Thr202/Tyr204), JNK, and phospho-JNK (Thr183/Tyr185), phospho-Akt(Ser473), Akt(Thr308), mammalian target of rapamycin (mTOR), 4EBP1, HE-binding protein, and S6K from Cell Signaling Technology (Beverly, MA); ß-actin from Sigma-Aldrich Corp. (St Louis, MO); and goat anti-rabbit and goat anti-mouse antibodies from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Western Blot Analysis
Total proteins were obtained from whole-tissue homogenates. Approximately 100 mg liver samples were homogenized in 500 μL ice-cold homogenizing buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton 100, with protease inhibitors cocktail and phosphatase inhibitors cocktails [Sigma-Aldrich Corp.]) and spun at 12,000 rpm for 40 minutes. The supernatants were removed and stored at –70°C. Protein concentrations were determined using the bicinchoninic acid assay (Pierce Corp., Rockford, IL) according to the manufacturer's instructions. For Western blotting, protein extracts were mixed with Sample Buffer (Bio-Rad Laboratories, Inc., Hercules, CA) and boiled for 5 minutes. Forty micrograms of total protein were separated electrophoretically according to size by sodium dodecyl sulfate–polyacrylamide gel electrophoresis using Criterion XT Precast Gel (Bio-Rad Laboratories) for 60 minutes at 150 V. Subsequently, proteins were wet transferred for 2 hours at 100 V onto nitrocellulose membranes. Membranes were rinsed briefly in Tris-buffered saline (TBS; pH 7.6) and blocked with 5% dry milk (or 3% bovine serum albumin for phosphorylated proteins) in TBS plus 0.05% Tween 20 (TBST) for 1 hour at room temperature. Blots were washed with TBST and incubated with the primary antibody diluted in the appropriate blocking solution at 4°C overnight with shaking. After incubation, blots were washed three times (15 minutes each) with TBST and incubated with an appropriate secondary antibody. For visualization of specific bands in the chemiluminescence assays, the membrane was exposed to X-OMAT film; for chemifluorescence, the membrane was incubated with enhanced chemifluorescence substrate and a digital image was generated with the Molecular Dynamics Storm system. Quantification of immunoblot signals was performed using the ImageQuant software package (Molecular Dynamics, Sunnyvale, CA).
Hormone Measures
Immediately prior to weaning (Day 20), mice were bled via puncture of the retroorbital sinus for the quantification of serum hormone (IGF-I, insulin, leptin, adiponectin, insulin-like growth factor binding protein-2 [IGFBP-2]) and glucose levels. Adiponectin and leptin levels were quantified via commercially available radioimmunoassay kits (LINCO Research, St Charles, MO) according to the manufacturer's instructions except that sample volumes were reduced by a factor of two and four for the adiponectin and leptin assays, respectively. For leptin, serum samples were diluted 2.75-fold prior to assay. Serum IGF-I levels were quantified via a double-antibody RIA kit (Diagnostic Systems Laboratories, Webster, TX) run at one-quarter volume according to the manufacturer's instructions. Prior to assay, 5–10 μL of serum from each individual was subjected to an acid–ethanol extraction procedure using the materials provided in the kit. Serum insulin levels were quantified via an enzyme immunoassay (Alpco Diagnostics, Windham, NH) run according to the manufacturer's instructions expect that samples were diluted 1.83-fold prior to assay, and serum IGFBP-2 levels were quantified via an enzyme immunoassay according to the manufacturer's instruction (Alpco Diagnostics). All samples were assayed in duplicate and were run in parallel for each hormone. Blood glucose was quantified at the time of sample collection in whole blood using the One Touch Basic Diabetes Monitoring System (Lifescan, Milpitas, CA).
RESULTS
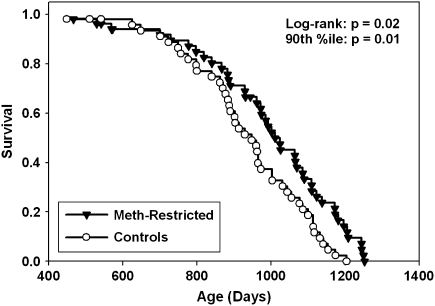
Figure 1 shows survival curves for two groups of male CB6F1 mice: a control group receiving a diet containing 0.43% methionine by weight and an experimental group consuming food with 0.15% methionine from age 12 months onward. Early mortality noted in our previous experiment, in which the Meth-R diet had been administered to mice starting from 6 weeks of age, was not apparent in the current protocol in which the diet was initiated at a later age. Median longevity was 948 days in the control group (95% confidence interval 884–1,003 days) compared with 1,011 days (95% confidence interval 931–1,085 days) in the Meth-R group, an increase of 7%. The log-rank test showed that the survival of Meth-R mice was significantly better than the controls (p = .02). As a test for maximal longevity, we used the approach of Wang and Allison (29) to compare the proportion of live mice in each group at the age (1,175 days) at which only 10% of the pooled population remained alive. At this age, 8 of 43 Meth-R mice were alive (19%) compared with 1 of 43 control mice (2%); this difference is significant at p = .01. We conclude that the Meth-R diet, initiated at 12 months of age, leads to an increase in longevity in CB6F1 mice.
Figure 1.
Survival curves for mice placed on a methionine-restricted diet at 12 months of age compared with controls. Each symbol represents one mouse. Statistical results are shown for the log-rank test and for a test of the proportion of mice still alive at the 90th percentile of the joint survival distribution.
Meth-R mice resemble CR mice in that they have lower serum IGF-I, insulin, thyroxin, and glucose levels and show a similar resistance to the hepatoxic effects of injected APAP (12,28). Meth-R mice also resemble CR mice in that both kinds of animals are lighter in weight than their respective controls, although food intake per gram body weight is higher in Meth-R rats than in control Meth-C rats (2), and limiting rats to the amount of food (per animal) consumed by Meth-R rats does not extend longevity. Because the mechanisms of life-span extension by Meth-R and CR diets are both unknown, it is a matter of speculation as to whether these two interventions promote health and survival by identical, overlapping, or distinct pathways. To gain insight into this question, we conducted two sorts of experiments: one based on mRNA expression patterns in liver of CR and Meth-R mice and the other on measurement of protein kinases thought to participate in stress resistance pathways.
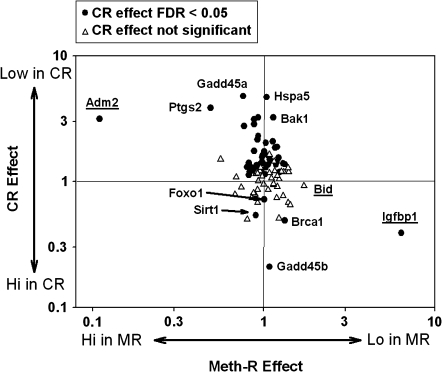
The mRNA profiles were derived from female CB6F1 mice, aged 18 months, which had been exposed to the Meth-R diet (0.15% methionine) from 6 weeks of age. Survival analysis showed (12) that this diet extended life span by the log-rank test and also extended maximal life span compared with mice on a control diet with 0.43% methionine. Liver samples were removed at 18 months of age from nine Meth-R and seven Meth-C mice, as well as from eight CR mice and eight mice on an AL diet that had been housed from 6 weeks of age in the same room as the Meth-R and Meth-C mice. A multiplex RT-PCR method was used to estimate the levels of 95 mRNAs, selected for their potential involvement in resistance to cellular stress. Figure 2 summarizes the results of this analysis. In this graphic, the horizontal axis shows the contrast between Meth-R and Meth-C mice; high numbers, toward the right side, indicate lower expression in Meth-R mice. The vertical axis shows the contrast between CR and AL mice, with higher values (top) representing lower mRNA levels in the CR animals. Filled symbols indicate the 48 mRNAs that were significantly higher or lower in CR mice, compared with AL mice, using FDR greater than 0.05 as significance criterion. If the effects of CR on liver gene expression were similar to those of the Meth-R diet, most of the symbols would be on a diagonal line from the lower left corner to the upper right corner of the plot, but in fact there is little or no relationship between the effects of CR diet and those of the Meth-R diet. Only three of the mRNAs tested differed significantly between Meth-R and Meth-C mice. Igpbp1 was significantly lower in Meth-R mice but was elevated significantly by the CR diet. Conversely, Adm2 was elevated to a significant extent in liver of Meth-R mice but significantly depressed by CR. The third gene affected by Meth-R, Bid, was lower in Meth-R mice but unchanged by the CR diet. The figure also shows many mRNAs that were elevated or depressed by CR but showed little or no change in the Meth-R mice. A complete list of mean values for each of the 95 genes in CR and Meth-R mice is shown in Supplementary Table 1. Although it is not clear which, if any, of these changes in gene expression levels contribute to good health in Meth-R and CR mice, it is clear that the changes in hepatic metabolism induced by the two diets have little in common.
Figure 2.
Gene expression results for a set of 94 messenger RNAs (mRNAs) evaluated by multiplex reverse transcription–polymerase chain reaction in groups of seven to nine mice. The horizontal axis reports the ratio of expression in liver of methionine-restricted (Meth-R) compared with Meth-C mice. The vertical axis shows the ratio of expression in liver of caloric restriction (CR) compared with ad libitum (AL) control mice. Filled symbols show genes for which a test of false discovery rate showed p < .05 for the comparison of CR with AL mice. Underlined gene names show the three mRNA for which the false discovery statistic reached p < .05 for the comparison between Meth-R and Meth-C mice. Gene names are given for 11 arbitrarily selected genes; a complete listing is included in Supplementary Table 1.
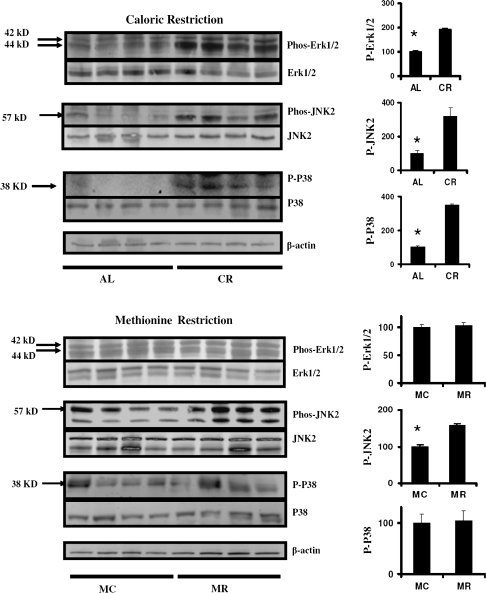
Figure 3 shows analyses of three protein kinases known to participate in cellular responses to injury; these experiments used liver tissue from mice exposed to the Meth-C, Meth-R, AL, or CR diets from 12 months of age, as in Figure 1. Figure 3 (top), comparing CR with AL mice, shows significant increases in hepatic levels of phosphorylation of Erk, Jnk2, and p38 kinases, roughly two- or threefold in each case. In contrast, liver tissue from Meth-R mice shows no change in phosphorylation of Erk or p38 and only a small (though significant) elevation in phosphorylation of Jnk2 compared with Meth-C animals (Figure 3, bottom).
Figure 3.
Evaluation of Erk, JNK, and p38 status in liver of caloric restriction (CR) and methionine-restricted (Meth-R) mice. Each group of immunoblots shows a representative experiment involving four ad libitum and four CR mice (top set) or four Meth-C and four Meth-R mice (bottom set), stained (top to bottom) for phosphorylated Erk1/2, total Erk1/2, phosphorylated JNK2, total JNK2, phosphorylated p38, total p38, and actin. The bar graphics at the right show means and SEs of the mean for eight mice of each type. Asterisks indicate significance at p < .05 by an unpaired t-test for the indicated comparison.
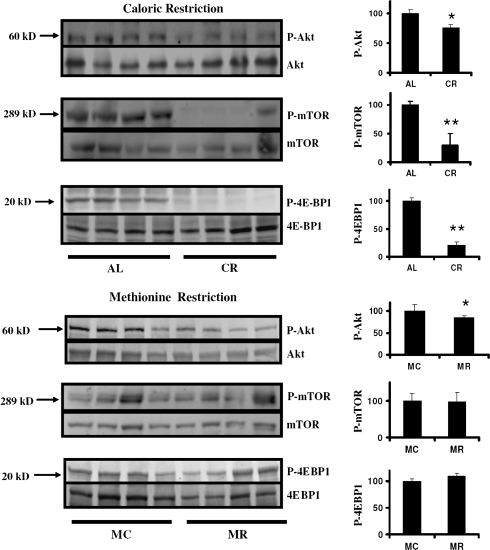
Figure 4 shows analyses, using the same liver samples, of three enzymes in the TOR pathway, which participates in cellular responses to nutrient availability and stress. The top panels show data comparing CR with AL mice and document dramatically lower levels of mTOR phosphorylation and phosphorylation of the mTOR substrate 4EBP1 in the CR liver tissue. The level of phosphorylated Akt, an activator of mTOR, is also significantly diminished in the CR mice, though the amount of diminution is far less than the decline of phospho-mTOR and phospho-4EBP1 in the same tissue. Meth-R mice (Figure 4, bottom) show a similar, significant but very slight, decline in phospho-Akt, but unlike CR mice show no change in phosphorylation of either mTOR or 4EPB1. The results of the Erk, Jnk2, p38, and TOR analyses all suggest that the cellular changes induced by the Meth-R diet are distinct from those induced by CR diets in mice.
Figure 4.
Evaluation of enzymes in the target of rapamycin pathway in liver of caloric restriction (CR) and methionine-restricted (Meth-R) mice. Each group of immunoblots shows a representative experiment involving four ad libitum and four CR mice (top set) or four Meth-C and four Meth-R mice (bottom set), stained (top to bottom) for phosphorylated Akt, total Akt, phosphorylated mammalian target of rapamycin (mTOR), total mTOR, phosphorylated 4EBP1, and total 4EBP1. The bar graphics at the right show means and SEs of the mean for eight mice of each type. Asterisks indicate significance at p < .05 (*) or p < .01 (**) by an unpaired t-test for the indicated comparison.
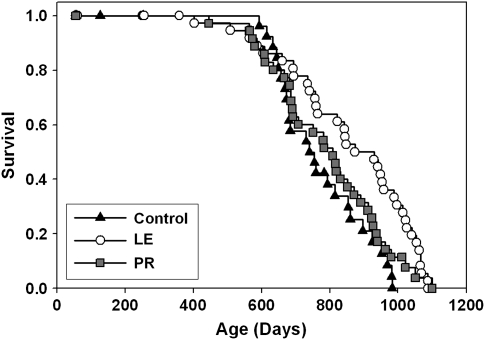
The data of Figure 1 show that maximal life span can be increased by a nutritional intervention started as late as 12 months of age. To see if very early, but transient, nutritional intervention can also extend maximal life span, we conducted an experiment to evaluate two models in which access to nutrients was limited only for the first 3 weeks of postnatal life. Figure 5 shows survival curves for LE or maternal PR mice compared with simultaneous controls. All the UM-HET3 mice in this experiment came from once-bred females with litter sizes of 8, 9, or 10 pups. In the control group, each female nursed her own offspring, with litter size trimmed within 24 hours of birth to N = 8 pups. In the LE group, each litter was first trimmed to N = 8 pups and then supplemented with 4 additional pups from another litter born on the same day to a total size of N = 12 pups. In the PR group, nursing mothers received a protein-poor diet. At 20 days of age, all pups were weaned and thereafter housed in same-sex cages containing two or three weanlings.
Figure 5.
Survival plots for litter enlargement (LE) mice (litters expanded from 8 to 12 pups at birth), PR mice (mothers given a low-protein diet during lactation), and control mice (litters of eight pups, mothers receiving standard diet during lactation). Each symbol represents one mouse. The LE group differs from controls by log-rank test (p = .0007) and by the Wang and Allison test for survival past the 90th percentile (p = .03).
Median survival was 740 days (95% confidence interval 672–854 days) in the control mice and was 874 days (95% confidence interval 760–988 days) in the LE group, an increase of 18%. A log-rank test indicated a significant difference between LE and control mice (p = .0007) pooled across sexes. When males were considered alone, the LE group had a median survival that was only 7% higher than controls (874 vs 815 days) and the log-rank test was marginal at p = .07. When females were considered alone, the LE group had median survival 16% higher than controls (848 vs 731 days) and the log-rank test indicated a significant difference at p = .003. We also used the Wang and Allison method (29) to evaluate survival to the age (1,060 days) at which only 10% of the pooled population remained alive. At this age, 6 of 36 LE mice were still alive (17%) compared with 0 of 25 control mice (0%); this difference is significant at p = .03. We conclude that LE from 8 to 12 pups, between birth and weaning, is sufficient to lead to increased longevity in UM-HET3 mice.
The survival curve for PR mice, whose mothers were provided unusually low levels of dietary protein while nursing the PR offspring, is also shown in Figure 5. There is no significant difference between PR and control mice, either pooled across gender (Figure 5) or when males or females are considered separately (not shown). Cox regression analyses (not shown) that included APAP exposure (no exposure, one lifetime exposure, or two exposures) documented a significant effect of the LE treatment (p = .004), consistent with the log-rank test, but again failed to demonstrate any effect of the PR treatment (p = .21).
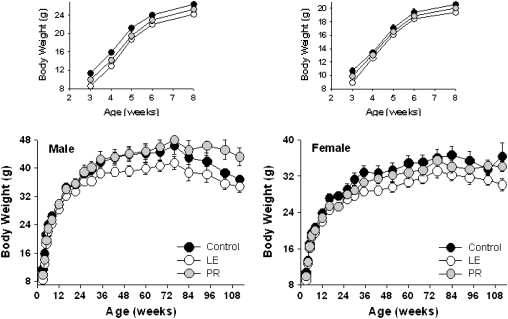
Figure 6 shows the body weights of the LE, PR, and control groups across the life span. As expected, offspring in the PR and LE groups are significantly lighter than controls at weaning, presumably because of diminished access to milk in the first 20 days of life. LE mice of both sexes remain smaller than controls at least through 112 weeks. The lower weight of the LE mice was statistically significant (p = .01) in a repeated measures analysis of variance model incorporating effects of age, sex, treatment, and interaction. In the PR groups, female weights are similar to controls at all ages. PR males weigh as much as control mice by 12 weeks of age and, by 72 weeks of age, are actually heavier than the controls, exhibiting “catch-up” growth.
Figure 6.
Body weight curves for male (left) and female (right) mice exposed to differing levels of nutrition during early postnatal development. Each point represents the mean ± SE body weight of 7–26 individuals dependent upon sex and treatment with one exception: only two female control mice were alive at Week 112. For clarity, the inset top panels show body weight curves for mice aged 3–8 weeks.
Hormone Assays in PR and LE Mice
Both serum IGF-I and nonfasted glucose levels were lower, at weaning, in both LE and PR mice relative to controls (p < .05 by Dunnett's post hoc test); the data were pooled across sex because there was no sex effect on either end point. Leptin levels in weanling PR mice were significantly higher (p < .03) than in LE or controls. There were no significant differences among these groups in insulin, adiponectin, or IGFBP2.
DISCUSSION
Our data show that a diet low in methionine can extend mean and maximal life span in mice even if started as late as 12 months of age, consistent with our previous report of extended life span in mice exposed to a low-methionine diet from 6 weeks of age (12) and several reports of life-span extension in multiple strains of inbred rats (2,3,11). Early imposition of a low-methionine diet led, in our earlier report, to death of approximately 25% of the test mice within the first year of life, with rectal prolapse frequently a contributing cause of these early deaths. The present experiment was conducted to see if diminished methionine, begun after the attainment of near-maximal body weight, might have a beneficial effect without the heightened mortality risk seen in the first year of life. The extension of maximal life span seen in the current population is very similar to that seen in mice exposed to the Meth-R diet from 6 weeks of age; thus, the beneficial effects do not seem to be proportional to the length of time during which the mice receive low levels of this amino acid. In the previous experiment, the mean longevity of the longest lived 10% of control mice was 1,144 days compared with 1,153 days in the current protocol, showing that the relative increase in life span in our current group of Meth-R mice does not reflect unusually poor survival of their contemporaneous controls. We do not have sufficient necropsy data to decide if the spectrum of late-life illness differs between Meth-R and Meth-C control mice, although a limited study of mice in our earlier study (12) found a similar set of mostly neoplastic illnesses in both groups of animals. We have noted that mice in both the Meth-R and the Meth-C groups have unusually high levels of fat deposits in the liver, presumably due to a dietary formulation in which virtually all protein has been replaced by an artificial mixture of synthetic amino acids. It is possible that adjustments of the ratios of the various amino acids might diminish the extent of fatty change in the liver, possibly with additional health benefits.
Low-methionine diets might postpone aging and extend life span by any of several plausible mechanisms, including alterations in translation rate or accuracy, modulation of DNA methylation patterns and hence gene expression, changes in glutathione levels or distribution (11), changes in endocrine levels, including changes in IGF-I, insulin, glucose, migration inhibition factor, and thyroxine (12), or hormesis (31).
In weighing the merits of these different mechanistic ideas, it would be particularly helpful to know if life-span extension could be induced by restriction of any essential amino acid or whether instead Meth-R was unique in this effect. Timiras and her colleagues have reported a series of studies focused on tryptophan restriction (see (32) and references cited therein), which suggest that diets low in tryptophan may delay the onset of age-dependent pathology in many organs and extend survival in Long-Evans rats. The most recent survival study (32), for example, noted that a diet with 70% less tryptophan than standard control diets increased the proportion of rats alive at 3 years of age from 7% (3 of 43) to 24% (10 of 42), significant at p = .03. The low-tryptophan diet, imposed at 21 days of age, led to substantially higher risks of death in the first year of life, and it would be helpful to see if delayed onset of a diet low in tryptophan or other essential amino acids led to a greater overall benefit in survival risk. The Timiras study has several design features that complicate its interpretation, including restoration of many (but not all) mice in the low-tryptophan group to control diets at ages between 23 and 36 months, post hoc pooling of controls from a chow-only and a tryptophan-supplemented synthetic diet, and a lack of information as to whether the colony was or was not specific pathogen free. Despite these uncertainties, the evidence that low-tryptophan diets, like low-methionine diets, may be able to extend rodent life span argues against mechanisms based on the special role of methionine per se in intermediary metabolism and suggests that mechanisms based on altered translation, altered hormonal profiles, or hormesis deserve special attention.
Meth-R mice and rats are smaller than those on control diets and thus require less energy (and food intake) to maintain thermal equilibrium. The decline in food intake per animal has raised the possibility that their extended life span might be caused by CR per se, despite the evidence that rodents on a Meth-R diet consume more calories per gram body weight than control animals and the finding that rats given the same amount of control food as that consumed, per animal, by rats on a low-methionine diet are not longer lived (3). In this report, we show data from gene expression analyses, studies of the Erk pathway, and studies of TOR kinase and its substrates, all of which suggest that the effects of the Meth-R diet and the CR diet are quite distinct, at least with respect to hepatic biochemistry and physiology. Much more work will be needed to learn how CR and Meth-R diets delay pathology and extend life span in rodents, and the hypothesis that they have parallel effects on one or more critical tissues is still highly plausible.
The effects of CR diets on activation of MAP kinases appear to be cell and tissue specific. For example, ERK and p38 enzyme activity have been reported to be impaired in the brain of aged rats, a change that was completely prevented by lifelong CR (33). Similarly, an age-related decline in Erk induction in splenic T cells was prevented by a CR diet in rats (34). CR led to elevation of p38 phosphorylation in liver of normal (and long-lived GHR-null mutant) mice (35), consistent with our own data in Figure 4; this effect is, however, not seen in our tests of liver from mice on the Meth-R diet. Phosphorylation of JNK is depressed by the CR diet in muscle tissue (36), although our data show a dramatic increase of JNK phosphorylation in the liver of CR mice. Thus, although our studies of Erk, JNK, and p38 in liver tissue show a clear disparity between the effects of the CR and Meth-R diets, it may well be that analyses of other tissues would document parallel changes of the two interventions on stress kinase activation.
The TOR molecule is a well-conserved serine/threonine kinase present in organisms from yeast to mammals (37). mTOR is a target of insulin/IGF-I signals and acts as a sensor of intracellular nutrient status and a regulator of protein synthesis, and of cell growth and proliferation. Diminution of TOR kinase function can lead to delayed aging and/or life-span extension in flies, worms, and yeast cells (38–40). Transcriptional analysis in heart and adipose tissue has suggested a decline with age in mTOR activity that is opposed by a CR diet (41). Our data suggest that diminution of mTOR activity in liver is characteristic of CR but not of Meth-R mice, but it is not yet known if mTOR activity in this or other tissues contributes to the antiaging effects of these interventions.
As shown in Figure 2 and Supplementary Table 1, CR and Meth-R appear to have very different effects on the expression of those genes evaluated in this project. The experimental design replicated that used for published longevity protocols: control mice for the CR animals received a natural product diet, and control mice for the Meth-R population used a diet in which a mixture of synthetic amino acids was used instead of a natural source of protein. The Meth-R experiment is likely to modify gene expression patterns compared with natural product control diets, and indeed 10 of the 95 genes tested varied by twofold or more when AL and Meth-C control mice are compared (not shown). The current protocol was not designed to evaluate the effects of semisynthetic versus natural product diets but to address the idea that CR and Meth-R diets produce similar physiological changes potentially relevant to their effects on life span. For this reason, mRNA levels for CR and Meth-R mice cannot be compared directly, and therefore the gene expression data in Figure 2 present the effects of each diet in comparison with its respective control. The gene expression results of Figure 2, along with the biochemical data of Figures 3 and 4, suggest that it is not safe to assume that CR and Meth-R diets affect mice in similar ways.
In addition to this general point, two of the specific disparities deserve special note: (a) CR downregulates the expression of cytoplasmic and ER chaperone mRNAs in the liver (Hsf1, Hspa5, Hspb2, Hspca, and Hspcb), a finding in agreement with the previous reports in the literature (42,43). In contrast, the Meth-R diet did not modulate expression of these genes. (b) CR, but not Meth-R, lowers the expression of the genes for proapoptotic proteins Bak1, Bax, Caspase 8, and Caspase 12. The question of whether CR diets have pro- or antiapoptotic effects in the liver is controversial. Proponents of the proapoptotic view base their assertion on the increased expression of Bak1 and Vdac genes in mouse liver samples (42). In our own data set, there are several findings that support the idea that CR diets are antiapoptotic, including decreased expression for Caspase 8, Caspase 12, and Bak1. We also note diminished mRNA for Bax, whose heterodimer with Bak1 instigates apoptosis, as well as an increase in the expression of SIRT1 mRNA, which sequesters Bax away from mitochondria (44). Nonetheless, the functional significance of these alterations in gene expression remains unknown and may vary with the specific model employed (45–47).
It has long been realized that events early in life can have lasting effects on physiology, and human epidemiological studies have established a clear link between birth weight and the subsequent onset and progression of late-life disease (48). To explain this phenomenon, Hales and Barker (49) proposed the “thrifty phenotype hypothesis” that suggests metabolism is “set” at a beneficial level for periods of poor nutrition but that this metabolic set point becomes detrimental if nutrition returns to an adequate level. This is thought to be due to morphological and functional alterations of the pancreatic islets, as well as the altered expression of the insulin receptor and its downstream signaling cascades (50,51), which in turn leads to impaired glucose tolerance and insulin resistance with advancing age (52).
In contrast to models of gestational undernutrition, a period of undernutrition confined solely to the suckling period may actually be beneficial in terms of life span and disease. For example, imposing maternal PR during lactation has been reported to extend the life span of both rats (53) and mice (25,26). Each of these previous studies, however, left some points in doubt. The mouse study, for example, compared six groups of C57BL/6 mice exposed to various combinations of pregnancy diet (high or low protein), lactation diet (high or low protein), and postweaning diet (chow or cafeteria diet; the latter leads to obesity). The contrast between control mice (high protein in pregnancy and lactation; chow diet thereafter) and mice whose dams received low protein only during lactation is the closest to our own design and produced a 6% increase in mean age at death (765 vs 814 days). Low maternal protein during lactation also protected offspring mice against the harmful effects of the obesity-inducing cafeteria diet on life span. Our own data on mice whose dams received low-protein diets during lactation (Fig. 5) also show a small increase in longevity, which was not statistically significant in our population.
Some of the early studies of food restriction in mice (22–24) included a period of food deprivation prior to weaning as a part of a more complex protocol. In some cases, for example, mice destined for assignment to a low-calorie diet in adult life were deliberately taken from litters that were larger than normal, in an attempt to reduce availability of calories prior to weaning to the low-calorie postweaning diet. These studies did not produce evidence that diminished nutrition prior to weaning, in itself, led to a sizeable or significant increase in longevity. A study of early life nutrition in rats (53) included a group of animals taken from litters 75% larger than those of a control group, but the dams in this group were also subjected to a low-protein diet, making it impossible to decide whether the life-span effect seen in this study was the result of reduced maternal protein intake or increased litter size. An earlier study, also using rats, had reported that a 75% reduction in litter size can shorten the life span of Wistar rats compared with controls (54) but did not provide any information about the effects of litter sizes larger than normal.
Our design involved evaluating, separately, the effects of reduced maternal nutrient supply and those of litter expansion, using a protocol that did not involve postweaning alterations in either food quality or availability. It is also noteworthy that whereas the PR mice appeared to exhibit a period of “catch-up” growth, the LE mice remained lighter than the controls for at least the first 112 weeks of life. Because the detrimental effects of rapid early growth on life span are well known (25,55), the relatively steep postweaning growth trajectory of the PR mice might have contributed to the small size of the longevity effect compared with mice in the LE group. More detailed studies of GH/IGF-1 signals and possible alterations in insulin, adipokines, and other mediators of glucose homeostasis in adult animals from the LE group may shed light on the mechanisms of life-span extension in this protocol.
Modifications of the LE protocol, including designs in which food availability remains limited for a short interval after weaning, might lead to larger effects and might also help to define the intervals during postnatal and postweaning life during which the aging process and timing of late-life illnesses are most susceptible to manipulation. It is not clear from our design whether the beneficial effects of the LE approach are related to nutritional deprivation per se or to neuroendocrine changes that might be produced by early life competition with littermates for limited maternal resources and attention or to other factors entirely. Cavigelli and McClintock (56), for example, have shown that genetically identical rats exhibit psychological characteristics, as early as 4 weeks of age, that are stable throughout life, predict adult adrenocorticoid responses to stress, and strongly predict life span. It is a plausible idea that the mechanism of life-span extension in the LE group overlaps with the antiaging mechanisms produced by adult CR, but neither mechanism has yet been defined well enough to make discussion of this point very fruitful. Although it is clear from prior work that pups in larger litters do indeed receive less food per pup than members of small litters (57–60), it would be worthwhile to conduct additional experiments in which the effects of nutrient availability can be compared directly with possible benefits from the social stresses related to large litter size and litter admixture. Our demonstration, in this article, that mean and maximal life span can indeed be increased by interventions in the first 3 weeks of life should provide a stimulus and justification for further work along these lines.
The Meth-R data suggest that postponement of lethal illnesses can also be achieved by interventions started as late as 1 year of age in mice. Although Meth-R mice resemble CR mice in some of their characteristics (12), our new data show clearly that these two antiaging interventions lead to very different effects on liver gene expression and signal transduction cascades. Other dietary approaches, for example, those that involve reduction of other amino acids or combinations of amino acids, or periodic exposures to low-methionine diets, might prove more beneficial than the protocol we chose, somewhat arbitrarily, for this study. Evaluations of the effects of the LE protocol, and the Meth-R diet, on age-related changes in multiple tissues and parallel evaluations of the effects of these and related interventions on injury and repair pathways postulated to be affected by CR diets or antiaging mutations will together help to fill in the currently sketchy picture of the plasticity of aging rate and life span control in mice.
Supplementary Material
Supplementary material can be found at: http://biomed.gerontologyjournals.org/
FUNDING
The work was supported by NIA grants AG024824 and AG023122 and an American Federation for Aging Research Grant (A06048).
Acknowledgments
We thank Melissa Burns, Maggie Lauderdale, and Jessica Sewald for technical assistance.
References
- 1.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- 2.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends lifespan. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman J, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 4.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the aging process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 5.Coschigano KT, Holland AN, Riders ME, List EO, Flyvberg ALLA, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin and IGF-I levels and increased lifespan. Endocrinology. 2003;174:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 6.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Longevity: extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 8.Gems D, Pletcher SD, Partridge L. Interpreting interactions between treatments that slow aging. Aging Cell. 2002;1:1–9. doi: 10.1046/j.1474-9728.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonkowski M, Rocha J, Masternak M, Al Regaiey K, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panowski S, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 11.Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- 12.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci U S A. 2004;101:5524–5529. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merry BJ, Kirk AJ, Goyns MH. Dietary lipoic acid suplementation can mimic or block the effect of dietary restriction on life span. Mech Ageing Dev. 2008;129:341–348. doi: 10.1016/j.mad.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Pugh TD, Oberley TD, Weindruch R. Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res. 1999;59:1642–1648. [PubMed] [Google Scholar]
- 16.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 17.Spindler SR. Rapid and reversible induction of the longevity, anticancer and genomic effects of caloric restriction. Mech Ageing Dev. 2005;126:960–966. doi: 10.1016/j.mad.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- 19.Nolen GA. Effect of various restricted dietary regimens on the growth, health, and longevity of albino rats. J Nutr. 1972;102:1477–1494. doi: 10.1093/jn/102.11.1477. [DOI] [PubMed] [Google Scholar]
- 20.Ross MH. Length of life and caloric intake. Am J Clin Nutr. 1972;25:834–838. doi: 10.1093/ajcn/25.8.834. [DOI] [PubMed] [Google Scholar]
- 21.Jennings BJ, Ozanne SE, Hales CN. Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol Genet Metab. 2000;71:32–42. doi: 10.1006/mgme.2000.3077. [DOI] [PubMed] [Google Scholar]
- 22.Cheney KE, Liu RK, Smith GS, Meredith PJ, Mickey MR, Walford RL. The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice. J Gerontol. 1983;38:420–430. doi: 10.1093/geronj/38.4.420. [DOI] [PubMed] [Google Scholar]
- 23.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 24.Weindruch RH, Kristie JA, Cheney KE, Walford RL. Influence of controlled dietary restriction on immunologic function and aging. Fed Proc. 1979;38:2007–2016. [PubMed] [Google Scholar]
- 25.Ozanne SE, Hales CN. Catch-up growth and obesity in mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- 26.Ozanne SE, Hales CN. Poor fetal growth followed by rapid postnatal catch-up growth leads to premature death. Mech Ageing Dev. 2005;126:852–854. doi: 10.1016/j.mad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Miller RA, Burke D, Nadon N. Announcement: four-way cross mouse stocks: a new, genetically heterogeneous resource for aging research. J Gerontol A Biol Sci Med Sci. 1999;54:B358–B360. doi: 10.1093/gerona/54.8.b358. [DOI] [PubMed] [Google Scholar]
- 28.Harper JM, Salmon AB, Chang Y, Bonkowski M, Bartke A, Miller RA. Stress resistance and aging: influence of genes and nutrition. Mech Ageing Dev. 2006;127:687–694. doi: 10.1016/j.mad.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Ooka H, Segall PE, Timiras PS. Histology and survival in age-delayed low-tryptophan-fed rats. Mech Ageing Dev. 1988;43:79–98. doi: 10.1016/0047-6374(88)90099-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhen X, Uryu K, Cai G, Johnson GP, Friedman E. Age-associated impairment in brain MAPK signal pathways and the effect of caloric restriction in Fischer 344 rats. J Gerontol A Biol Sci Med Sci. 1999;54:B539–B548. doi: 10.1093/gerona/54.12.b539. [DOI] [PubMed] [Google Scholar]
- 34.Pahlavani MA, Vargas DM. Influence of aging and caloric restriction on activation of Ras/MAPK, calcineurin, and CaMK-IV activities in rat T cells. Proc Soc Exp Biol Med. 2000;223:163–169. doi: 10.1046/j.1525-1373.2000.22322.x. [DOI] [PubMed] [Google Scholar]
- 35.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor I/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 36.Bartke A, Masternak MM, Al-Regaiey K, Bonkowski M. Effects of dietary restriction on the expression of insulin-signaling-related genes in long-lived mutant mice. Interdiscip Top Gerontol. 2007;35:69–82. doi: 10.1159/000096556. [DOI] [PubMed] [Google Scholar]
- 37.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 41.Linford NJ, Beyer RP, Gollahon K, et al. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell. 2007;6:673–688. doi: 10.1111/j.1474-9726.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 42.Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A. 2001;98:10630–10635. doi: 10.1073/pnas.191313598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spindler SR, Dhahbi JM. Conserved and tissue-specific genic and physiologic responses to caloric restriction and altered IGFI signaling in mitotic and postmitotic tissues. Annu Rev Nutr. 2007;27:193–217. doi: 10.1146/annurev.nutr.27.061406.093743. [DOI] [PubMed] [Google Scholar]
- 44.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 45.Apte U, Limaye P, Desaiah D, Bucci T, Warbritton A, Mehendale H. Mechanisms of increased liver tissue repair and survival in diet-restricted rats treated with equitoxic doses of thioacetamide. Toxicol Sci. 2003;72:272–282. doi: 10.1093/toxsci/kfg021. [DOI] [PubMed] [Google Scholar]
- 46.Higami Y, Shimokawa I, Tomita M, et al. Aging accelerates but life-long dietary restriction suppresses apoptosis-related Fas expression on hepatocytes. Am J Pathol. 1997;151:659–663. [PMC free article] [PubMed] [Google Scholar]
- 47.Muskhelishvili L, Hart RW, Turturro A, James SJ. Age-related changes in the intrinsic rate of apoptosis in livers of diet-restricted and ad libitum-fed B6C3F1 mice. Am J Pathol. 1995;147:20–24. [PMC free article] [PubMed] [Google Scholar]
- 48.Petry CJ, Ozanne SE, Hales CN. Programming of intermediary metabolism. Mol Cell Endocrinol. 2001;185:81–91. doi: 10.1016/s0303-7207(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 49.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 50.Ozanne SE, Dorling MW, Wang CL, Nave BT. Impaired PI 3-kinase activation in adipocytes from early growth-restricted male rats. Am J Phyiol Endocrinol Metab. 2001;280:E534–E539. doi: 10.1152/ajpendo.2001.280.3.E534. [DOI] [PubMed] [Google Scholar]
- 51.Ozanne SE, Olsen GS, Hansen LL. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol. 2003;177:235–241. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- 52.Holness MJ, Sugden MC. Antecedent protein restriction exacerbates development of impaired insulin action after high-fat feeding. Am J Physiol Endocrinol Metab. 2004;276:E85–E93. doi: 10.1152/ajpendo.1999.276.1.E85. [DOI] [PubMed] [Google Scholar]
- 53.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determine longevity in male rats and may be related to telomere shortening in the kidney. FEBS Letters. 1999;448:4–8. doi: 10.1016/s0014-5793(99)00336-1. [DOI] [PubMed] [Google Scholar]
- 54.Frolkis VV, Grigorov YG, Pisarchuk KL. Long-term effect of litter size in early postnatal period on metabolism, aging and life span in rats. Arch Gerontol Geriatr. 1993;17:65–73. doi: 10.1016/0167-4943(93)90018-d. [DOI] [PubMed] [Google Scholar]
- 55.Hales C, Ozanne S. The dangerous road of catch-up growth. J Physiol. 2002;547:5–10. doi: 10.1113/jphysiol.2002.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci U S A. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.López-Soldado I, Munilla MA, Herrera E. Long-term consequences of under-nutrition during suckling on glucose tolerance and lipoprotein profile in female and male rats. Br J Nutr. 2006;96:1030–1037. doi: 10.1017/bjn20061949. [DOI] [PubMed] [Google Scholar]
- 58.McCance RA. Food, growth and time. Lancet. 1962;280:621–626. doi: 10.1016/s0140-6736(62)92539-4. [DOI] [PubMed] [Google Scholar]
- 59.Plagemann A, Harder T, Rake A, et al. Obeservations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999;11:541–546. doi: 10.1046/j.1365-2826.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- 60.Velkoska E, Cole TJ, Dean RG, Burrell LM, Morris MJ. Early undernutrition leads to long-lasting reductions in body weight and adiposity whereas increased intake increases cardiac fibrosis in male rats. J Nutr. 2008;138:1622–1627. doi: 10.1093/jn/138.9.1622. [DOI] [PubMed] [Google Scholar]