Abstract
Hydrogen peroxide (H2O2) generated in response to wounding can be detected at wound sites and in distal leaf veins within 1 hr after wounding. The response is systemic and maximizes at about 4–6 hr in both wounded and unwounded leaves, and then declines. The timing of the response corresponds with an increase in wound-inducible polygalacturonase (PG) mRNA and enzyme activity previously reported, suggesting that oligogalacturonic acid (OGA) fragments produced by PG are triggering the H2O2 response. Systemin, OGA, chitosan, and methyl jasmonate (MJ) all induce the accumulation of H2O2 in leaves. Tomato plants transformed with an antisense prosystemin gene produce neither PG activity or H2O2 in leaves in response to wounding, implicating systemin as a primary wound signal. The antisense plants do produce both PG activity and H2O2 when supplied with systemin, OGA, chitosan, or MJ. A mutant tomato line compromised in the octadecanoid pathway does not exhibit PG activity or H2O2 in response to wounding, systemin, OGA, or chitosan, but does respond to MJ, indicating that the generation of H2O2 requires a functional octadecanoid signaling pathway. Among 18 plant species from six families that were assayed for wound-inducible PG activity and H2O2 generation, 14 species exhibited both wound-inducible PG activity and the generation of H2O2. Four species, all from the Fabaceae family, exhibited little or no wound-inducible PG activity and did not generate H2O2. The time course of wound-inducible PG activity and H2O2 in Arabidopsis thaliana leaves was similar to that found in tomato. The cumulative data suggest that systemic wound signals that induce PG activity and H2O2 are widespread in the plant kingdom and that the response may be associated with the defense of plants against both herbivores and pathogens.
The production of reactive oxygen species (ROS), called the oxidative burst (including O2−, H2O2) (1–4) is one of the earliest responses of incompatible interactions between pathogens and plants and is caused by the activation of a membrane-bound NADPH oxidase (5–7). Several roles for ROS during pathogen infections have been proposed: as direct antimicrobial agents (1), as activators of defense genes (1–3), as agents for crosslinking proteins to limit pathogen infections (8), and as producers of the hypersensitive response (HR), cell death, salicylic acid production, and systemic acquired resistance (SAR) (1–3). ROS alone apparently is not capable of activating the latter responses, and recently NO has been suggested to act in concert with ROS to orchestrate HR, cell death, and SAR (9). ROS also have been associated with plant herbivore interactions, and oxidative changes in the plants correspond with oxidative damage in the midguts of insects feeding on previously wounded plants (10).
Oligogalacturonic acid (OGA) fragments produced by degradation of plant cell walls by polygalacturonases (PGs) have been shown to cause the oxidative burst in plant cell suspension cultures (2, 3, 11). OGA and systemin, an 18-aa polypeptide signal, activated the induction of proteinase inhibitors in leaves of tomato plants (12–14), but systemin did not generate an oxidative burst when added to tomato cell suspension cultures (15). However, within 9 hr of the addition of systemin to the suspension cultures, the generation of ROS in response to OGA was increased 16-fold over the response in the absence of systemin (15). The addition of cycloheximide to the cells before systemin addition inhibited the potentiation, suggesting that systemin may be inducing synthesis of the oxidase or of a cell component that enhances the activity of the oxidase in the presence of OGA. How OGA causes the generation of ROS is not known.
We recently reported that PG mRNA is systemically wound-inducible in tomato leaves (16) and that PG activity is inducible by systemin and methyl jasmonate (MJ). This finding suggested that the PG gene is regulated through the octadecanoid pathway like other systemic wound response genes (17) and PG may play a role either in signaling or in defense against herbivores as well as against pathogens. Although the induction of PG mRNA and enzyme activity could be measured in leaf extracts of both wounded and unwounded leaves of wounded plants, it was not known whether the enzyme was active in planta, or if it was compartmented away from the cell wall substrates and remained inactive until the plants were wounded. If PG was sequestered away from the cell walls until wounded, then H2O2 should be generated only at wound sites. If PG was actively degrading cell walls in planta, then it would be expected to generate H2O2 both locally and systemically. We report herein that H2O2 is generated systemically in tomato leaves in response to wounding and that the activity is likely the result of the systemically wound-inducible PG activity (16). The production of H2O2 in tomato leaves is activated by systemin, OGA, chitosan, and MJ, all inducers of PG (16), and is mediated through the octadecanoid pathway. PG and H2O2 also are shown to be wound-inducible in leaves of species from several plant families.
MATERIALS AND METHODS
Plant Growth.
Tomato (Lycopersicon esculentum), potato (Solanum tuberosum), petunia (Petunia hybrida), tobacco (Nicotiana tabacum), pepper (Capsicum annum), squash (Cucurbita maxima), cucumber (Cucumis sativus), corn (Zea maize), barley (Hordeum vulgare), wheat (Triticum aestivum), peas (Pisum sativum), soybeans (Glycine max), lentils (Lens culinaris), chickpea (Cicer arietinum), alfalfa (Medicago sativa), and cotton (Gossypium hirsutum) were grown in peat pots and maintained under 17 hr of light (30 μEm−2⋅s−1) at 28°C and 7 hr of dark at 18°C. For all experiments 15- to 18-day-old plants were used. Arabidopsis was grown under constant light (15 μEm−2⋅s−1) at 21°C, and rice (Oryza sativa) was grown with days of 10-hr light (30 μEm−2⋅s−1) and 14-hr darkness, maintained at 26°C.
In Vivo Detection of H2O2 in Plants.
H2O2 was visually detected in the leaves of plants by using 3,3-diaminobenzidine (DAB) as substrate (18). Briefly, plants were excised at the base of leaves or at the base of stems with a razor blade and supplied through the cut petioles or stems with a 1 mg/ml solution of DAB, pH 3.8, for 8 hr under light at 25°C. Leaves of DAB-treated plants were wounded 1–3 times perpendicular to the main vein by crushing with a hemostat. After wounding, the plants were continually supplied with DAB solutions until the experiments were terminated by immersion of the plants or leaves in boiling ethanol (96%) for 10 min. This treatment decolorized the leaves except for the deep brown polymerization product produced by the reaction of DAB with H2O2. After cooling, the leaves were extracted at room temperature with fresh ethanol for 4 hr and were preserved at room temperature in ethanol and photographed.
Systemin (25 nM), OGA (0.5 mg/ml), and chitosan (125 μg/ml) were supplied to the excised plants or leaves in solutions of DAB as described above and incubated under light until the experiments were terminated. DAB-treated plants were exposed to MJ vapors for the times indicated in a closed Plexiglas box containing a cotton swab on which had been placed 1 μl of MJ. Diphenylene iodonium chloride (DPI, Sigma), an inhibitor of NADPH oxidase (5, 6, 18) was supplied at a final concentration of 250 μM to excised plants previously incubated in DAB for 8 hr. The plants were wounded and continually supplied with the DAB-DPI solution for 4 hr, when the plants were treated to visualize H2O2. Excised plants were treated in the same manner, but supplied with systemin, OGA, chitosan, or MJ and then assayed for H2O2 generation.
PG and Protein Assays.
At times after wounding, extracts from leaves were prepared for PG assays as described (16). Twenty grams of leaves were homogenized in a Sorvall Omnimizer (Sorvall, DuPont) in 60 ml of ice-cold 0.1 M sodium citrate buffer, pH 6.0, containing 1 M NaCl, 4 mM ascorbic acid, 5 mM DTT, 2% polyvinylpyrrolidone, and 0.1% BSA. The extracts were filtered through four layers of cheesecloth and incubated at 4°C for 3 hr, centrifuged at 10,000 g for 20 min at 4°C. Proteins were precipitated at 4°C by the slow addition of solid ammonium sulfate to a final concentration of 80% and stirred for 1 hr at 4°C. The precipitates were recovered by centrifugation at 10,000 g for 20 min at 4°C, the supernatant was discarded, the pellet was dissolved in 20 ml of 1 M NaCl, and the solution was dialyzed against 1 M NaCl at 4°C for 24 hr. Aliquots equivalent to 0.1 g fresh weight were assayed for PG (16). Protein was analyzed with a BCA protein assay kit (Pierce).
RESULTS AND DISCUSSION
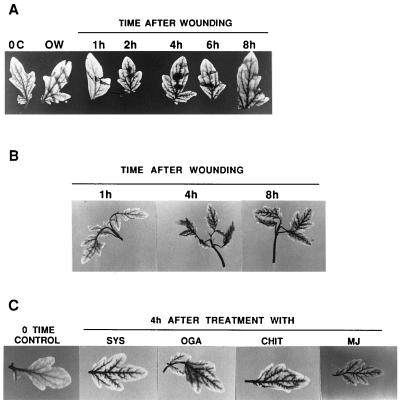
To investigate whether a recently discovered wound-inducible PG enzyme in tomato leaves promotes the generation of ROS in leaf tissues, we assayed tomato plants for the production of H2O2 in response to wounding. Excised plants were allowed to imbibe a 1 mg/ml solution of DAB for 8 hr, then wounded or supplied with systemin, OGA, and chitosan, or exposed to MJ vapors. DAB polymerizes and turns deep brown in the presence of H2O2, and the intensity of the coloration and its localization can be qualitatively assessed and photographed. The development of the DAB-H2O2 reaction product in tomato leaves in response to wounding is shown in Fig. 1A. H2O2 is detectable as early as 1 hr after wounding, with the color deepening for about 4–6 hr, then declining. The color initially was visible at the wound site, deepened in tissue surrounding the wound site, and then appeared in major and minor veins throughout the plants (Fig. 1A). The time course of development of H2O2 closely correlated with the timing of induction of PG in leaves in response to wounding (16). The induction of both PG and H2O2 maximize within about 4–6 hr, suggesting that cell wall fragments produced by wound-inducible PG were generating H2O2. In Fig. 1B, H2O2 is shown to be present in the upper unwounded leaves of young tomato plants that were wounded on the lower leaves, indicating that the generation of H2O2 production was systemic and localized in the vascular tissues. In lettuce tissue, H2O2 was reported to be localized within the cell walls of xylem vessels with secondary thickening and within walls of surrounding cells (18), and DAB detected H2O2 in barley epidermal cells (19) and in microbursts in Arabidopsis leaf periveinal cells in response to pathogen attacks (4). DAB can detect H2O2 in leaves at levels as low as 0.1 μM concentrations, but strong color develops only at higher concentrations of about 1–10 μM (18).
Figure 1.
The generation of H2O2 in leaves of tomato plants in response to wounding and wound signals. Fourteen-day-old plants were excised at the base of the stems and supplied with DAB for 8 hr. The plants then were either wounded, supplied with solutions of systemin, OGA, or chitosan in DAB through the cut stems, or exposed to MJ vapors in a closed Plexiglas box as described in Materials and Methods. (A) The time course of H2O2 production in tomato leaves in response to wounding. (B) The systemic production of H2O2 in leaves of tomato plants wounded only on the lower leaf. (C) The production of H2O2 in leaves of tomato plants 4 hr after treatment with systemin (SYS), OGA, chitosan (CHIT), or MJ.
Supplying tomato plants with systemin, OGA, and chitosan had been shown to increase the levels of jasmonic acid in leaves (18) and to induce the synthesis of PG (16). Systemin, OGA, chitosan, and MJ also induce the production of H2O2 in leaf veins, similar to wounding (Fig. 1C). The similarities between the signaling for the production of H2O2 and the synthesis of PG support the hypothesis that PG activity is resulting in the production of H2O2. Additionally, the decline in H2O2 levels about 4–6 hr after wounding (Fig. 1A) occurs at the same time that the activity of the PG enzyme declines (16).
To confirm that the color developed in the leaves was the result of the product of an oxidase, a specific inhibitor of NADPH oxidase, DPI, was supplied to DAB-treated plants for 1 hr before wounding. No H2O2 was generated within 4 hr after wounding (Fig. 2), indicating that the oxidase causing the generation of H2O2 in response to wounding was inhibited. DPI also blocked the production of H2O2 induced in excised tomato leaves by systemin (data not shown).
Figure 2.
Inhibition of wound-inducible H2O2 production by the NADPH oxidase inhibitor DPI. Fourteen-day-old tomato plants were excised and treated as in Fig. 1A, except that at the time of wounding DPI and DAB were supplied to the plants.
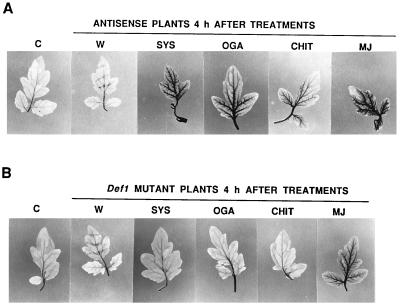
Tomato plants transformed with an antisense prosystemin gene constitutively driven by the cauliflower mosaic virus-35S promoter do not produce systemic wound response proteins, including PG, when wounded (16, 20). These plants do not generate H2O2 in response to wounding (Fig. 3A). The antisense prosystemin transgenic plants do respond to exogenously supplied systemin, the product of prosystemin, by activating defense genes. As expected, supplying systemin to the antisense plants through their cut stems strongly generated H2O2 (Fig. 3A). OGA, chitosan, and MJ also induce the synthesis of proteinase inhbitors when supplied to the antisense plants (unpublished data), and they also cause the generation of H2O2 (Fig. 3A).
Figure 3.
The induction of H2O2 production by wounding in leaves of (A) tomato plants transformed with a prosystemin antisense prosystemin gene and (B) a mutant tomato line (def1) that is compromised in the octadecanoid signaling pathway. C, control; W, wounded; SYS, systemin; CHIT, chitosan. The plants were treated as in Fig. 1C.
The octadecanoid defense signaling pathway in a mutant tomato line, called def1 (21), is compromised because of a single mutation and cannot activate defense genes in response to wounding, systemin, OGA, or chitosan, and the plants have lost their ability to defend themselves against Manduca sexta larvae. These plants do not synthesize defense proteins, including PG, in response to wounding, systemin, OGA, or chitosan, but they do produce the defense proteins in response to MJ. The def1 mutant plants do not produce H2O2 in response to wounding, systemin, or OGA (Fig. 3B), but H2O2 was produced in response to MJ, consistent with a role for the octadecanoid pathway in the signaling pathway. OGA, products of PG action on cell walls and the suspected signal for H2O2 generation, was expected to produce H2O2 when supplied to def1 mutant plants. However, OGA did not produce H2O2 in these plants (Fig. 3B), suggesting that the oxidase activated by OGA in wild-type plants is either not present or not activated by OGA in the def1 plants. Because OGA activates H2O2 in wild-type plants, it must require a functional octadecanoid pathway, perhaps to induce the synthesis of the oxidase, or a component necessary for activity, or to produce endogenous OGA fragments in a location not available to exogenous OGA.
Transgenic plants transformed with the prosystemin gene constitutively express an array of wound-inducible genes in the absence of wounding (22, 23). These plants constitutively exhibit PG mRNA in leaves and PG activity in leaf extracts (16) and a strong constitutive production of H2O2 in leaves (Fig. 4). A constitutive level of H2O2 was found in leaves of both young (14-day-old) and old (3-month-old) tomato plants (Fig. 4), showing that the generation of H2O2 continues throughout the growth and development of the plants. H2O2 was found primarily in major and minor veins of leaves, similar to wounded plants, but in the younger plants the H2O2 appears to have spread throughout the minor veins as well (Fig. 4). The constitutive expression of PG and H2O2 in transgenic tomato plants overexpressing the prosystemin gene apparently does not cause any adverse effects caused by the constant generation of H2O2. It has been reported that H2O2 induces synthesis of salicylic acid (SA) (23), which is known to induce the synthesis of acidic pathogenesis-related (PR) proteins in tomato. However, no PR proteins have been identified among the systemin-inducible proteins in the prosystemin transgenic plants, suggesting that the H2O2 induced by systemin is not directly involved in the synthesis of SA.
Figure 4.
Assays of H2O2 levels in leaves of wild-type tomato plants and transformed tomato plants constitutively expressing prosystemin (ProSYS). The upper terminal leaflet of 14-day-old tomato plants and the terminal leaflet of the third leaf from the apex of 3-month-old tomato plants were excised and assayed by using DAB as described in Materials and Methods.
A survey of the wound induction of both PG and H2O2 in 18 species of plants from six plant families was conducted. In Table 1, species that exhibit at least a 1.3-fold increase in wound-inducible PG activity exhibit visually observable H2O2 generation, assessed by DAB coloration. Four species from the Fabaceae family (soybeans, chickpeas, lentils, and alfalfa) exhibited little or no wound-inducible PG in leaves and did not generate H2O2. On the other hand, peas, another member of the Fabaceae family, produced a 1.9-fold increase in PG in response to wounding and generated a strong H2O2 response. The reason for the lack of response of four other legume species is not known, but it may be related to some characteristic of their nitrogen fixing systems.
Table 1.
PG activity and H2O2 production in leaves from unwounded control plants and wounded plants from several plant families
| Plants | PG activity, units*
|
Wound-inducible H2O2 | ||
|---|---|---|---|---|
| Control | Wounded** | Fold increase | ||
| Solanaceae | ||||
| Tomato | ||||
| Wild type | 0.74 | 2.38 | 3.2 | + |
| Def1 Mutant | 0.05 | 0.04 | 0 | – |
| Antisense | 0.05 | 0.03 | 0 | – |
| Sense | 2.07 | nd | – | nd |
| Potato | 0.20 | 1.60 | 8.0 | + |
| Petunia | 0.30 | 2.94 | 9.0 | + |
| Tobacco | 0.18 | 1.18 | 6.5 | + |
| Pepper | 0.66 | 1.96 | 3.0 | + |
| Curcubitaceae | ||||
| Squash | 0.83 | 1.83 | 2.2 | + |
| Cucumber | 0.76 | 1.26 | 1.7 | + |
| Poaceae | ||||
| Corn | 0.98 | 1.48 | 1.5 | + |
| Barley | 1.43 | 2.03 | 1.4 | + |
| Wheat | 1.50 | 2.00 | 1.3 | + |
| Rice | 1.50 | 2.35 | 1.6 | + |
| Fabaceae | ||||
| Peas | 0.87 | 1.67 | 1.9 | + |
| Soybeans | 0.72 | 0.80 | 1.1 | – |
| Lentils | 0.15 | 0.14 | 0 | – |
| Alfalfa | 0.64 | 0.66 | 0 | – |
| Chickpea | 0.42 | 0.47 | 1.1 | – |
| Brassicaceae | ||||
| Arabidopsis | 1.43 | 2.63 | 1.8 | + |
| Malvaceae | ||||
| Cotton | 1.47 | 2.20 | 1.5 | + |
PG and H2O2 were assayed as described in Materials and Methods.
nd, not determined.
1 unit = 0.1 OD change at 520 nm/30 min at 37°C/0.1 g fresh weight
4–6 hr after wounding.
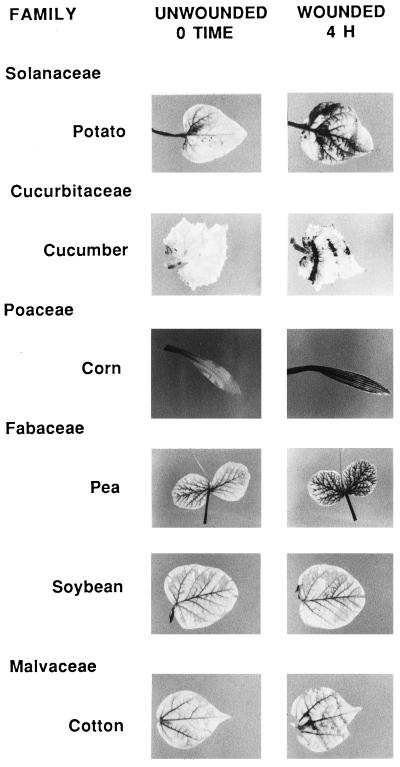
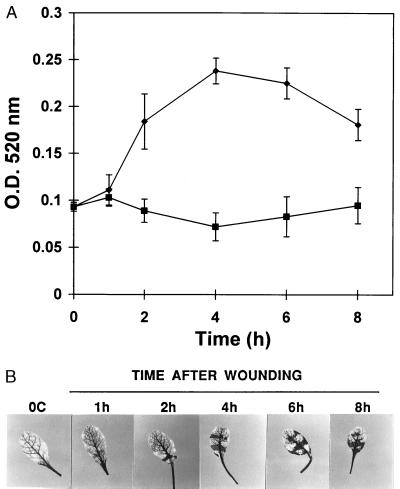
Typical responses to wounding in representative species from six plant families are shown in the photographs in Fig. 5. In leaves of cucumber, the response is found primarily at wound sites, whereas in corn, pea, cotton, and potato the response is strongly systemic. A time course of wound-induced H2O2 generation in leaves of Arabidopsis thaliana (Fig. 6B) is similar to that found with tomato (Fig. 1A). This induction correlated with the time course of induction of PG activity in the leaves induced by wounding (Fig. 6A), which is similar to the time course of wound-inducible PG activity previously found in tomato (16). Parsley (Petroselinum crispum) from the family Umbelliferae also has been reported to produce H2O2 in leaves in response to jasmonic acid (25). However an association with PG activity was not noted.
Figure 5.
Assay for wound-inducible H2O2 production in leaves of selected species from several plant families. Leaves of plants were excised and supplied with a solution of DAB for 8 hr and assayed (0 time) or wounded and assayed after an additional 4-hr incubation with DAB (4H). Details of the experiments are described in Materials and Methods.
Figure 6.
Wound-induced PG activity and H2O2 production in leaves of A. thaliana plants. (A) Time course of PG activity in leaves from wounded plants (●) and leaves from unwounded plants (■). (B) Time course of H2O2 production in the wounded leaves. Details of the experiments are in Materials and Methods.
Constitutive levels of both PG activity and H2O2 were found to be present in control plants of most species (Table 1 and Fig. 5). The Poaceae species appear to have the highest constitutive levels of PG activity, but they exhibit low constitutive H2O2 in the absence of wounding. In other plants the levels of PG found in the absence of wounding are accompanied by the presence of H2O2. Both PG and H2O2 may have resulted from watering and handling of the plants in the growth rooms. This conclusion is supported by the total lack of PG activity and H2O2 in leaves of both the antisense transgenic plants and def1 mutant plants that are compromised in their wound signaling pathways. In each case, no constitutive PG or H2O2 was found in unwounded control plants. Different plants species may differ both in their constitutive levels of PG and H2O2 and in their abilities to generate them in response to external stimuli, including touch and wounding. The overall patterns of wound inducibility of PG and H2O2 among the plants assayed are suggestive of a common mechanism of signal transduction, perhaps using polypeptide signals similar to systemin, which may be widespread in the plant kingdom.
In response to herbivores, H2O2 levels are likely to be elevated as long as the attacks persist. The levels of H2O2 in the prosystemin transgenic tomato plants are constitutively elevated (Fig. 4) and may provide an early defense barrier. Bi and Felton (10) have proposed that herbivore attacks can cause a localized oxidative response in soybean leaves and have identified some potential functions of ROS that might affect plant-herbivore interactions. These include direct oxidative injury to insect midguts and damage to the nutritive and antioxidant components of the plants. But interestingly, three of the Fabaceae species assayed for wound-inducible H2O2 generation, including soybeans, did not exhibit a response (Table 1).
The wound-generated H2O2 in the veins also could have a defense role against bacteria, fungi, or viruses as they invade leaves wounded by herbivores. The elevated H2O2 levels also may potentiate the plants’ defense responses against invading pathogens, in which ROS play an important role. The presence of H2O2 in the plant in response to herbivory, before the pathogen invasion, could be advantageous because timing of the induction of defense responses can be an important factor in the success or failure of plants to defend against pathogen attacks (26). Wu et al. (27, 28) transformed potatoes with a constitutive glucose oxidase from bacteria that generated a low level of H2O2 in cells throughout the plants. These plants exhibited elevated levels of plant defense proteins (27), and the tubers were strongly resistant to a bacterial soft rot and late blight (28). Whether the H2O2 generated in wounded wild-type tomato plants, or in the transgenic plants overexpressing prosystemin, is a factor in resistance against herbivores and pathogens remains to be determined.
Acknowledgments
We thank Amanda Vaughn for technical assistance and Sue Vogtman and Thom Koehler for growing and maintaining plants used in this research. This research was supported in part by Washington State University College of Agriculture and Home Economics Project 1791, Grant IBN 9601099 from the National Science Foundation, Grant WNP03153 from the U.S. Department of Agriculture/Competitive Research Grants Program, and a graduate fellowship from the Charlotte Y. Martin Foundation.
Footnotes
Abbreviations: PG, polygalacturonase; OGA, oligogalacturonic acid; ROS, reactive oxygen species; MJ, methyl jasmonate; DAB, 3,3-diaminobenzidine; DPI, diphenylene iodonium chloride.
References
- 1.Doke N, Miura Y, Sanchez L M, Park H-J, Noritake T, Yoshioka H, Kawakita K. Gene. 1996;179:45–51. doi: 10.1016/s0378-1119(96)00423-4. [DOI] [PubMed] [Google Scholar]
- 2.Lamb C, Dixon R A. Annu Rev Plant Physiol Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 3.Low P S, Merida J R. Plant. 1996;96:533–542. [Google Scholar]
- 4.Alvarez M E, Pennell R I, Meijer P-J, Ishikawa A, Dixon R A, Lamb C. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- 5.Auh C-K, Murphy T M. Plant Physiol. 1995;107:1241–1247. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desikan R, Jancock J T, Coffey M J, Neill S J. FEBS Lett. 1996;382:213–217. doi: 10.1016/0014-5793(96)00177-9. [DOI] [PubMed] [Google Scholar]
- 7.Keller T, Damude H G, Werner D, Doener P, Dixon R A, Lamb C. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisson L F, Tenhaken R, Lamb C. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delledonne M, Xia Y, Dixon R A, Lamb C. Nature (London) 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- 10.Bi J L, Felton G W. J Chem Ecol. 1995;21:1511–1530. doi: 10.1007/BF02035149. [DOI] [PubMed] [Google Scholar]
- 11.Legendre L, Rueter S, Heinstein P F, Low P S. Plant Physiol. 1993;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop P, Makus D J, Pearce G, Ryan C A. Proc Natl Acad Sci USA. 1981;78:3536–3640. doi: 10.1073/pnas.78.6.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce G, Strydom D, Johnson S, Ryan C A. Science. 1991;253:895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 14.Doares S, Syrovets T, Weiler E W, Ryan C A. Proc Natl Acad Sci USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stennis M J, Chandra S, Ryan C A, Low P S. Plant Physiol. 1998;117:1031–1036. doi: 10.1104/pp.117.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergey D R, Orozco-Cardenas M, de Moura D S, Ryan C A. Proc Natl Acad Sci USA. 1999;96:1756–1760. doi: 10.1073/pnas.96.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farmer E E, Ryan C A. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thordal-Christensen H, Shang Z, Wei Y, Collinge D B. Plant J. 1997;11:1187–1194. [Google Scholar]
- 19.Bestwick C S, Brown I R, Bennett M G R, Mansfield J W. Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGurl B, Pearce G, Orozco-Cardenas M, Ryan C A. Science. 1992;255:1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- 21.Howe G, Lightner J, Browse J, Ryan C A. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGurl B, Orozco-Cardenas M, Pearce G, Ryan C A. Proc Natl Acad Sci USA. 1994;91:9799–9802. doi: 10.1073/pnas.91.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergey D, Howe G, Ryan C A. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leon J, Lawton M A, Raskin I. Plant Physiol. 1995;108:1637–1678. doi: 10.1104/pp.108.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauss H, Jeblick W, Zeigler J, Krabler W. Plant Physiol. 1994;105:89–94. doi: 10.1104/pp.105.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dangl J L, Dietrich R A, Richberg M H. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu G, Shortt B J, Lawrence E B, Leon J, Fitzsimmons K C, Levine E B, Raskin I, Shaw D M. Plant Physiol. 1997;115:427–435. doi: 10.1104/pp.115.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G, Shortt B J, Lawrence E B, Leon J, Levine E B, Fitzsimmons K C, Shaw D M. Plant Cell. 1995;7:1357–1368. doi: 10.1105/tpc.7.9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]