Abstract
Sspg1d, one of endopolygalacturonases, is an important fungal effector secreted by the necrotrophic fungus Sclerotinia sclerotiorum during early infection. Using sspg1d as bait, a small C2 domain protein (designated as IPG-1) was identified by yeast two-hybrid screening of a canola cDNA library. Deletion analysis confirmed that the C-terminus of IPG-1 is responsible for its interaction with sspg1d in the yeast two-hybrid assay. The sspg1d/IPG-1 interaction was further confirmed in plant cells by a biomolecular fluorescence complementation (BiFC) assay. A transient expression assay showed that the IPG-1–GFP fusion protein was targeted to the plasma membrane and nucleus in onion epidermal cells. Following treatment with a Ca2+ ionophore, it was distributed throughout the cytosol. Real-time PCR assay demonstrated that IPG-1 was highly induced by Sclerotinia sclerotiorum in canola leaves and stems. Southern blot analysis indicated the presence of about five homologues of IPG-1 in the canola genome. Two additional members of the IPG-1gene family were isolated by RT-PCR. Their sequence similarity with IPG-1 is as high as 95%. However, they did not interact with sspg1d in the yeast two-hybrid assay. Possible roles of IPG-1 and its association with sspg1d in the defence signalling pathway were discussed.
Keywords: BiFC, C2 domain, endo-PG, PG, Sclerotinia sclerotiorum
Introduction
The necrotrophic fungal pathogen Sclerotinia sclerotiorum exhibits little host specifity and has a range of more than 400 plant species among 75 families, primarily dicotyledons, including many economically important crops such as the grain legumes (soybean, pea, and bean) and oilseeds (canola and sunflower) (Boland and Hall, 1994). Stem rot caused by the fungus is an important disease of canola in China. There is a lot of evidence to show that complete resistance to this pathogen has not been identified in canola germplasm, although partial resistance or tolerance to the pathogen in different breeding lines has been reported (Liu et al., 1991; Chen et al., 1993).
Sclerotinia sclerotiorum secretes several types of effector proteins such as polygalacturonases (PGs) during its development and plant infection. These PGs can be classified into endo-polygalacturonases (endo-PGs) and exo-polygalacturonases (exo-PGs) (Li et al., 2004). Each of them has different expression levels under pathogenic conditions; one of the endo-PGs, named sspg1d, was highly expressed during early infection and thus may play an important role in pathogen development and pathogenicity (Li et al., 2004; Hegedus and Rimer, 2005). Zuppini et al. (2005) reported that an endo-PG from Sclerotinia sclerotiorum can induce calcium-mediated signalling and programmed cell death in soybean cells.
The immediate response of plant cells to pathogen attack is the increase in cytosolic Ca2+ concentration, which can be decoded by various Ca2+-binding proteins (Ca2+ sensors) (Reddy, 2001). Once activated by Ca2+ ions, these Ca2+ sensors interact with downstream effectors that modulate the numerous biochemical and cellular functions involved in defence responses (Lecourieux et al., 2006). Many of these Ca2+-binding proteins contain C2 domains (Ca2+-regulatory domains) (Kretsinger, 1980). In mammals, C2 domains have been found in more than 100 proteins, most of which are involved in lipid metabolism, signal transduction or membrane trafficking (Rizo and Südhof, 1998). In response to Ca2+ being imported, the C2 domains of phospholipases, synaptotagmin I, and protein kinase C were shown to bind Ca2+ and migrate from the cytosol to the plasma membrane, thereby transducing the foreign signal into the cells (Clark et al., 1991; Davletov and Sudhof, 1993; Edwards and Newton, 1997; Lomasney et al., 1999; Pepio and Sossin, 2001; Ananthanarayanan et al., 2002; Teruel and Meyer, 2002). In plants, copine and phospholipase D, two proteins that contain C2 domains, have been shown to be involved in defence responses (Young et al., 1996; Laxalt et al., 2001; Laxalt and Munnik, 2002; Jambunathan and McNellis, 2003). In rice, the small protein OsERG1 contains a single C2 domain and is induced by treatment with a fungal elicitor resulting in protein migration from the cytosol to the plasma membrane in a Ca2+-dependent manner (Kim et al., 2003). In mung beans (Vigna radiata L.), the C2 domain of V3-PLC3 plays an important role in the translocation of the protein to the membrane in response to abiotic stress (Kim et al., 2004). However, only a few C2 domain-containing proteins have been identified or investigated in plants.
Using sspg1d as bait, a C2 domain protein, designated IPG-1, was identified by screening a canola cDNA library with the yeast two-hybrid assay (Wang et al., 2008). In the current report, the sspg1d/IPG-1 interaction was confirmed in living plant cells by bimolecular fluorescent complementation (BiFC) assay (Walter et al., 2004). The C-terminus of IPG-1 was demonstrated to be responsible for its interaction with sspg1d. In addition, the dynamic subcellular localization of IPG-1 protein was analysed in response to Ca2+. Finally, the expression of IPG-1 in different tissues of canola in response to Sclerotinia sclerotiorum inoculation was characterized and the copy number of IPG-1 in the canola genome was investigated. The possible roles of PG and IPG-1, and their interaction in defence signalling, are discussed.
Materials and methods
Construction of IPG-1 deletion mutants and the two-hybrid assay
Two IPG-1 mutants, IPG-1/del-C (contains residues 1–87 aa) and IPG-1/del-N (contains residues 89–168 aa) were produced. The coding sequences of each of the two mutants were fused with the GAL4 activating domain in the pGADT7 vector and individually transformed into yeast AH109 cells. The full-length coding sequence of sspg1d was fused with the GAL4 DNA binding domain in the pGBKT7 vector to generate the pGBKT7–sspg1d construct and it was transformed into yeast Y187 cells. Yeast mating between AH109 and Y187 was used to test the IPG-1 domain that interacts with sspg1d.
BiFC aasay
The vectors (PUC-pSPYNE, PUC-pSPYCE) used in the BiFC assay were kind gifts of Jorg Kudla. For BiFC analysis, the full-length coding sequence of sspg1d was fused with the N-terminal fragment of YFP in PUC-pSPYNE vector to form the YFPN–sspg1d construct. The full-length coding sequence of IPG-1 was cloned into PUC-pSPYCE as a fusion with the C-terminal fragment of YFP to form the YFPC–IPG-1 construct. The plasmids YFPN–sspg1d and YFPC–IPG-1 were cotransformed into onion epidermal cells by bombardment. YFP fluorescence was analysed 16 h later using a Leica TCS SP2 laser confocal scanning microscope.
Dynamic subcellular localization of IPG-1 in plant cells in response to Ca2+
The full-length coding region of IPG-1was cloned into pJIT166GFP vector as a fusion with the N-terminus of GFP to form the IPG-1–GFP construct. The IPG-1–GFP construct was transformed into onion epidermal cells by bombardment, and after GFP was detected, the cells were treated with the calcium ionophore (5 mM Ca2+ and 10 μM ionomycin) and incubated in the dark at 22 °C for 5–8 h and then the GFP fluorescence was monitored using a Leica TCS SP2 laser confocal scanning microscope.
Real-time quantitative RT-PCR
The eukaryotic translation elongation factor 1-α (EF-1α) was used as the internal control in real-time quantitative RT-PCR. The sequences of the forward and the reverse primers were 5′-AGACCACCAAGTACTACTGCAC-3′ and 5′-CCACCAATCTTGTACACATCC-3′, respectively. The primers used to amplify IPG-1 gene were 5′-GAG CCT CGC CAT CAG AGA TA-3′ and 5′-GTC CTC ATG GAC TTG CAC ACT-3′. PCR was performed using Multicolor Real-Time PCR Detection System, iQ™5 (Bio-Rad).
Results
Full-length IPG-1 and its C-terminal region interact with sspg1d in yeast cells
IPG-1 was a small C2 domain protein containing 168 amino acid residues (Wang et al., 2008). BLAST analysis in Genbank predicted that the 1–87 aa is the C2 domain in which there are five conserved aspartic acid residues (D) (Fig. 1), the potential Ca2+-binding sites. The 88–168 aa is the C-terminal region that may be involved in protein–protein interaction. To verify this hypothesis, two deletion mutants were constructed: IPG-1-del-N, which contained the C-terminal domain (89–168 aa) and IPG-1-del-C, which contained the N-terminal domain (C2 domain, 1–87 aa). The coding sequence of the two mutants and the full-length IPG-1 were individually fused with an activating domain (AD) in the pGADT7 vector and transformed into yeast strain AH109 separately. The pGBKT7–sspg1d construct was transformed into yeast strain Y187. The domain of IPG-1 necessary for its interaction with sspg1d was determined by yeast mating between Y187 and AH109 yeast cells.
Fig. 1.
The structure of the IPG-1 protein. The domain consisting of residues 1–87 is the C2 domain, in which the asterisks represent the five conserved aspartatic residues (the positions are 22, 73, 75, 80, and 81, respectively). The C-terminus is shown in black box consisting of residues 88–168.
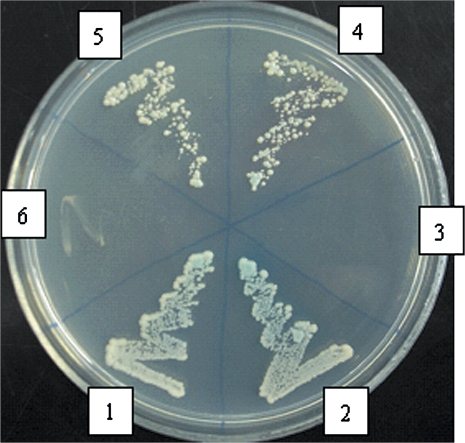
Yeast cells with pGBKT7–sspg1d and IPG-1-del-N plasmids, and cells with pGBKT7–sspg1d and pGADT7–IPG-1 plasmids were able to grow on SD/-Ade/-His/-Leu/-Trp and turned blue with the X-αGal overlay assay, but yeast cells with pGBKT7–sspg1d and IPG-1-del-C plasmids were not (Fig. 2). The experiments were replicated three times and each obtained the same results. It means that the full-length IPG-1 and the C-terminal region of IPG-1 can interact with sspg1d, while the C2 domain was not necessary for the interaction.
Fig. 2.
sspg1d/IPG-1 interaction and the domain of IPG-1 necessary for its interaction with sspg1d analysed by yeast two-hybrid assay. 1, sspg1d/IPG-1 interaction; 2, positive control (pGBKT7-53/ pGADT7-RecT interaction); 3, negtive control (pGBKT7-Lam/pGADT7-RecT interaction); 4, 5, sspg1d/IPG-1-delN interaction; 6, sspg1d/IPG-1-delC interaction. Yeast cells transformed with combinations of various AD and BD constructs were subjected to β-galactosidase overlay activity assay. The blue colour of the yeast cells and yeast growth on SD/-Ade/-His/-Leu/-Trp media indicate the activation of reporter genes and therefore a positive protein–protein interaction.
Verification of sspg1d–IPG-1 interaction in living plant cells
BiFC was used to test weather sspg1d and IPG-1 can associate in plant cells. sspg1d and IPG-1 was fused with the N-terminal 154 amino acid or C-terminal 84 amino acids of yellow fluorescent protein (YFP), respectively, driven by the CaMV 35S promoter. The two constructs were co-bombarded into onion epidermal cells. YFP fluorescence was monitored using laser confocal scanning microscopy.
As shown in Fig. 3, YFP fluorescence could be detected in onion epidermal cells co-transformed with YFPN–sspg1d and YFPC–IPG-1. No YFP fluorescence was detected in the negative controls (i.e. transformed with YFPC–IPG-1/YFPN, YFPN–IPG-1/YFPC) (data not shown). These results confirm that sspg1d interacts with IPG-1 in living plant cells.
Fig. 3.
Bimolecular fluorescence complementation assays in onion epidermal cells. The reconstitute YFP signals show that IPG-1 and sspg1d can associate in plant cells.
Dynamic subcellular localization of IPG-1 in response to Ca2+
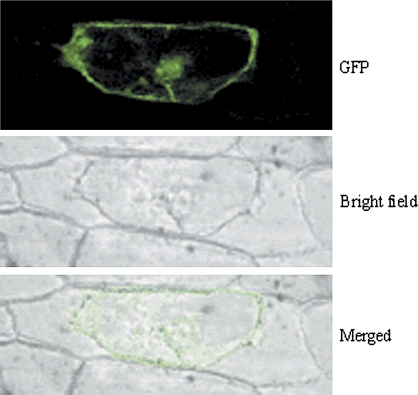
It is known that C2 domain proteins play a role in Ca2+-dependent spatio-temporal targeting in different regulatory signal transduction chains (Evans et al., 2004). Furthermore, it has been shown that the small rice C2 domain protein OsERG1 is translocated to the plasma membrane of plant cells in a Ca2+-dependent manner (Kim et al., 2003). To determine whether the newly identified C2 domain protein IPG-1 also exhibits calcium-dependent subcellular localization, the IPG-1–GFP construct driven by the CaMV 35S promoter was introduced into onion epidermal cells by bombardment for transient expression. As shown in Fig. 4, IPG-1 protein is mainly targeted to the plasma membrane and nucleus. After treatment with ionomycin, a calcium ionophore, for 5 h, the IPG-1–GFP signals was observed distributing throughout the cytosol (Fig. 5). It means that the Ca2+ ionophore treatment induced translocation of the green fluorescence signal emitted from the IPG-1-GFP from the plasma membrane and the nucleus to the cytosol.
Fig. 4.
Localization of IPG-1-GFP fusion in onion epidermal cell. Plant cell wall and membrane were separated by treatment with 20% sugar.
Fig. 5.
Subcellular localization of IPG-1-GFP fusion transiently expressed in onion epidermal cells before (A) and after (B) treatment with Ca2+ ionophore.
IPG-1 is highly induced by Sclerotinia sclerotiorum
Semi-quantitative PCR revealed that IPG-1 was highly induced by Sclerotinia sclerotiorum in canola leaf and stem (Wang et al., 2008). Here, real-time PCR was used to analyse the expression of IPG-1 in leaf, stem, and flower organs of canola following inoculation with Sclerotinia sclerotiorum. As shown in Fig. 6, the expression of IPG-1 in flowers is about three times higher than that in leaves and stems before inoculating with Sclerotinia sclerotiorum, whereas the expression level of IPG-1 in leaves and stems was about 2–3 times higher than in flowers following inoculation with Sclerotinia sclerotiorum. The experiment again provided evidence that IPG-1 is significantly induced in leaves and stems by Sclerotinia sclerotiorum.
Fig. 6.
Expression of IPG-1 in different organs of canola analysed by real-time PCR with eukaryotic elongation factor 1-α (EF-1α) gene as internal control. (A) Without inoculation with Sclerotinia sclerotiorum. (B) Inoculation with Sclerotinia sclerotiorum.
Determine the copy numbers of IPG-1 in the canola genome
The copy numbers of IPG-1 in the canola genome were analysed by Southern blot of canola genomic DNA probed with IPG-1 cDNA (Fig. 7). The results showed that IPG-1 has about 2–5 copies in the canola genome, which means that the canola genome may have a C2 domain gene family. To test whether other members in the gene family interact with sspg1d, an additional two genes in the gene family were isolated by RT-PCR and designated as BnC2d1 and BnC2d2. BLAST analysis indicated that the two genes also contain the C2 domain. Sequence comparison of the two genes with IPG-1 revealed that their identity is as high as 95% in amino acids (Fig. 8). The coding sequences of BnC2d1 and BnC2d2 were individually fused into the GAL4 activating domain of the pGADT7 vector. Yeast mating was used to test the interaction between sspg1d and the two genes. To our surprise, the two genes did not interact with sspg1d, although the experiment was replicated several times. This means that the two IPG-1 homologues may have evolved functions different from IPG-1. This may explain why only one gene that interacts with sspg1d in the canola cDNA library was isolated by yeast two-hybrid screening.
Fig. 7.
Southern blot of canola genomic DNA using 32P labelled IPG-1 cDNA as probe. Genomic DNA was digested with BamHI (1), EcoRI (2), EcoRV (3), HindIII (4), and XbaI (5). M, molecular weight marker.
Fig. 8.
Amino acid sequence alignment of IPG-1 with its homologues BnC2d1 and BnC2d2. (This figure is available in colour at JXB online.)
Discussion
PGs produced by fungi belong to cell wall-degrading enzymes and some of them from several necrotrophic fungal pathogens have been implicated as potential virulence factors unrelated to their enzyme activity (Shieh et al., 1997; Have et al., 1998; Poinssot et al. 2003; Kikot et al., 2008). They can cause Ca2+ elevation in the cell cytosol and subsequent cell death (Zuppini et al., 2005). In order to identify host factors involved in PG signalling, sspg1d, one of the important PGs secreted by Sclerotinia sclerotiorum during its early development and the plant infection process, was used as bait to screen PG-interacting proteins in the canola cDNA expression library by using a yeast two-hybrid technique. A C2 domain protein was identified that interacts with sspg1d, and which was further confirmed in plant cells by BiFC. The C2 domain was first described in protein kinase C (PKC), representing a large family comprising the most studied of all protein kinases in animals. Animal PKC isoforms include 3–4 conserved domains, C1–C4, representing catalytic and regulatory modules. Domains C1, C3, and C4 are present in all PKC isoforms, whereas the C2 domain is unique to the Ca2+-dependent isoforms PKCα, PKCβ, and PKCγ, thus identifying the C2 domain as a potential Ca2+-regulatory motif (Hug and Sarre, 1993). C2 domains interact with phospholipids in a Ca2+-dependent manner and thereby modulate a diverse range of cellular actions. They mediate the Ca2+-dependent translocation of soluble proteins to membranes, the Ca2+- and phospholipid-dependent activation of enzymes, Ca2+- and phospholipid-dependent interaction between proteins, or promote Ca2+-triggered self-association (Kopka et al., 1998). The C2 domain proteins found in human and animals generally consist of 1–3 C2 domains (Nalefski and Falke, 1996). Only a few C2 domain proteins are discovered in plants, and many of these proteins contain only single C2 domain, known as small C2 domain protein. The functions of already-described plant small C2 domain proteins are not yet very clear. In pumpkin, a small C2 domain protein has been reported to increase the size of mesophyll plasmodesmata to enable transport of cellular materials, including RNA molecules, from cell to cell (Xoconostle-Cazares et al., 1999). In Arabidopsis, the C2-domain protein BAPl negatively regulates defence responses (Yang et al., 2006). In rice, two small C2-domain rice proteins named OsERG1a and OsERG1b, are significantly induced by a fungal elicitor (Kim et al., 2003), implicating a functional role in defence signalling systems in plant cells. The HvC2d, a C2-domain protein identified in barley (Hordeum vulgare L.) was induced by exposure to different heavy metals and its mRNA was accumulated during leaf senescence (Ouelhadj et al., 2006). In this study, the novel C2 domain protein identified, IPG-1, was first shown to interact with sspg1d, a fungal effector of Sclerotinia sclerotiorum. It is closely related in sequence to a C2-domain protein in Arabidopsis (NP198590) with unknown functions (Wang et al., 2008). The expression studies (Fig. 6) showed that the mRNA of this gene was highly induced by Sclerotinia sclerotiorum inoculation. The subcellular localization of IPG-1–GFP fusion protein is dynamic in response to Ca2+ elevation. These results implicate a role of IPG-1 in Ca2+-dependent defence signalling.
Localization of proteins to distinct subcellular compartments, including membranes, is a critical event in multiple cellular pathways. The C2 domain has been identified in many cellular proteins involved in signal transduction or membrane trafficking. A majority of C2 domains bind the membrane in a Ca2+-dependent manner and thereby play an important role in Ca2+-dependent membrane targeting (Nalefski and Falke, 1996; Stahlen and Cho, 2001). Analyses of mammalian C2 proteins, for example, phospholipases, synaptotagmin I and protein kinase C, also showed that these proteins migrate after binding of Ca2+ from the cytosol to the plasma membrane and thus are able to transduce foreign signals into the cell (Pepio and Sossin, 2001; Ananthanarayanan et al., 2002; Teruel and Meyer, 2002). By using GFP constructs, Kim et al. (2003) showed that the small C2 domain protein OsERG1 is translocated to the plasma membrane of plant cells by treatment with a Ca2+ ionophore and also by a fungal elicitor. Immunocytochemical analyses with the other known small C2 domain protein from pumpkin (CmPP16-1) also suggested an association with the plasma membrane (Xoconostle-Cazares et al., 1999). Interestingly, there are several reports showing that such calcium-binding proteins are not only localized in the cytoplasm but also in the nucleus. Among these proteins with nuclear localization are calcium-dependent protein kinases (Dammann et al., 2003; Chehab et al., 2004), a novel calmodulin-binding protein (Perruc et al., 2004), and a novel C2 domain protein HvC2d1 found in barley (Ouelhadj et al., 2006). In addition, the calcium-dependent protein kinase McCPK1 from the ice plant was shown to undergo a reversible change in subcellular localization from the plasma membrane to the nucleus, endoplasmic reticulum, and actin filaments of the cytoskeleton in response to environmental stimuli (Chehab et al., 2004). In the present study, another novel protein was identified with a calcium-binding C2 domain-like motif that was shown to be located in a calcium-dependent manner in the cytosol and also in the nucleus. Our data indicate that IPG-1 plays a role in cytosolic and nuclear localized calcium signalling processes in response to external stressors.
It has been reported that the Arabidopsis C2 domain proteins, BAP1 and BAP2, are general inhibitors of programmed cell death (PCD) (Yang et al., 2007). Overexpression of BAP1 or BAP2, with their partner BON1, inhibits PCD induced by pathogens. Thus, the BAP genes function as general negative regulators of PCD induced by biotic stimuli. The BAP1 and BON1 molecules might become targets of pathogen effector proteins because of their ancestral role in cell death control during the evolution of plant innate immune system (Jones and Dangl, 2006). Considering that an endo-PG of Sclerotinia sclerotiorum could induce PCD in plant cells (Zuppini et al., 2005), and here, IPG-1 is shown to be the target of an endo-PG of Sclerotinia sclerotiorum, implicating IPG-1 in the PCD process in plant cells. It has been suggested that effective defence against biotrophic pathogens is largely due to PCD in the host, whereas necrotrophic pathogens benefit from host cell death; they can utilize dead tissue and are not limited by cell death (Govrin and Lexine, 2000; Glazebrook, 2005). Plants expressing animal PCD inhibitor genes, such as the human Bcl-2 and Bcl-xl and the nematode CED-9, confer resistance to several necrotrophic fungal pathogens including Sclerotinia sclerotiorum (Dickman et al., 2001). Considering that the plant material used here is susceptible to the fungus, it is postulated that the PG/IPG-1 interaction may interfere with the binding of IPG-1 with Ca2+ and the subsequent Ca2+-dependent signal transduction might be involved in PCD processes in the host. Overexpression of IPG-1 might inhibit PCD and produce resistance to Sclerotinia sclerotiorum.
At present, there are two models explaining pathogen perception by plants: the Guard Model and the recently proposed Decoy Model (van der Hoorn and Kamoun, 2008). The Guard Model proposes that pathogen effectors may have a common target; the plant R-protein indirectly perceives the presence of the pathogen effector by monitoring the state of the effector target that associates with the R-protein. In the absence of the R-protein, the effector target will enhance pathogen fitness in plants. The Decoy Model implies that some host targets of fungal effectors have evolved to act as a ‘decoy’ to trap the pathogen into a recognition event. These decoys only function in the perception of pathogen effectors without contributing to pathogen fitness in the absence of its cognate R-protein in plant. What types of effector target the IPG-1 belongs to is an interesting question to be explored. Whether IPG-1 associates with plant R-proteins remains to be investigated.
Nowadays, numerous laboratories are determining the enzymatic functions of pathogen effectors as well as their host targets (Chisholm et al., 2006), which will benefit elucidation of the molecular basis of plant resistance or susceptibility. These works are of great importance for both basic and applied research. This study lays the foundation for further investigation of the functions of PG, IPG-1, and the roles of PG–IPG-1 interaction in plant defence responses, which may also involve other factors such as calcium, phospholipids, and other proteins.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (30571153). The experiments were performed at the Institute of Cotton Genome Research, the State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University. We thank Professor Tianzhen Zhang for providing instruments and some reagents for the experiments.
Glossary
Abbreviations
- BiFC
biomolecular fluorescence complementation
- PG
polygalacturonase
- endo-PG
endo-polygalacturonase
- GFP
green fluorescent protein
- YFP
yellow fluorescent protein
- CaMV
cauliflower mosaic virus
References
- Ananthanarayanan B, Das S, Rhee SG, Murray D, Cho W. Membrane targeting of C2 domains of phospholipase C-δ isoforms. Journal of Biological Chemistry. 2002;277:3568–3575. doi: 10.1074/jbc.M109705200. [DOI] [PubMed] [Google Scholar]
- Boland GJ, Hall R. Index of plant hosts of Sclerotinia sclerotiorum. Canadian Journal of Plant Pathology. 1994;16:94–108. [Google Scholar]
- Chehab EW, Patharkar OR, Hegeman AD, Taybi T, Cushman JC. Autophosphorylation and subcellular localisation dynamics of a salt- and water deficit-induced calcium-dependent protein kinase from ice plant. Plant Physiology. 2004;135:1430–1446. doi: 10.1104/pp.103.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Zhang JF, Wu YM, Hou QS, Zhou YJ, Han H. Studies on virus and Sclerotinia sclerotiorum resistance of rapeseed germplasm in Brassica genus. Chinese Journal of Oil Crop Science. 1993;15:4–7. (in Chinese with English abstract) [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host–microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Dammann D, Ichida A, Hong B, Romanowsky S, Hrabak EM, Harmon AC, Pickard BG, Harper JF. Subcellular targeting of nine calcium dependent protein kinase isoforms from Arabidopsis. Plant Physiology. 2003;132:1840–1848. doi: 10.1104/pp.103.020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Südhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. Journal of Biological Chemistry. 1993;268:26386–26390. [PubMed] [Google Scholar]
- Dicman MB, Park YK, Oltersdorf T, Li W, Clemente T, French R. Abrogation of disease development in plants expressing animal antiapoptotic genes. Proceedings of the National Academy of Sciences, USA. 2001;98:6957–6962. doi: 10.1073/pnas.091108998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AS, Newton AC. Regulation of protein kinase Cβ II by its C2 domain. Biochemistry. 1997;36:15615–15623. doi: 10.1021/bi9718752. [DOI] [PubMed] [Google Scholar]
- Evans JH, Gerber SH, Murray D, Leslie CC. The calcium binding loops of the cytosolic phospholipase A2, C2 domain specify targeting to Golgi and ER in live cells. Molecular Biology of the Cell. 2004;15:371–383. doi: 10.1091/mbc.E03-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Govrin EM, Lexine A. The hypersensitive response facilitates plant infection by the necrothophic pathogen Botrytis cinerea. Current Biology. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- Have AT, Mulder W, Visser J, Jan AL, Kan V. The endo-polygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Molecular Plant–Microbe Interactions. 1998;11:1009–1016. doi: 10.1094/MPMI.1998.11.10.1009. [DOI] [PubMed] [Google Scholar]
- Hegedus DD, Rimer SR. Sclerotinia sclerotiorum: when ‘to be or not to be’ a pathogen? FEMS Microbiology Letters. 2005;251:177–184. doi: 10.1016/j.femsle.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochemical Journal. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambunathan N, McNellis TW. Regulation of Arabidopsis COPINE 1 gene expression in response to pathogens and abiotic stimuli. Plant Physiology. 2003;132:1370–1381. doi: 10.1104/pp.103.022970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kikot GE, Hours RA, Alconada TM. Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: a review. Journal of Basic Microbiology. 2008;48:1–11. doi: 10.1002/jobm.200800231. [DOI] [PubMed] [Google Scholar]
- Kim CY, Koo YD, Jin JB, et al. Rice C2 domain proteins are induced and translocated to the plasma membrane in response to a fungal elicitor. Biochemistry. 2003;42:11625–11631. doi: 10.1021/bi034576n. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kim JE, Lee JH, Lee MH, Jung HW, Bahk YY, Hwang BK, Hwang I, Kim WT. The Vr-PLC3 gene encodes a putative plasma membrane-localized phosphoinositide-speciWc phospholipase C whose expression is induced by abiotic stress in mung bean (Vigna radiata L.) FEBS Letters. 2004;556:127–136. doi: 10.1016/s0014-5793(03)01388-7. [DOI] [PubMed] [Google Scholar]
- Kopka J, Pical C, Hetherington AM, Müller-Röber B. Ca2+/phospholipids-binding (C2) domain in multiple plant proteins: novel components of the calcium-sensing apparatus. Plant Molecular Biology. 1998;36:627–637. doi: 10.1023/a:1005915020760. [DOI] [PubMed] [Google Scholar]
- Kretsinger RH. Structure and evolution of calcium-modulated proteins. Critical Reviews in Biochemistry. 1980;8:119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Riet BT, Verdonk JC, Parigi L, Tameling WI, Vossen J, Haring M, Musgrave A, Munnik T. Characterization of five tomato phospholipase D cDNAs: rapid and specific expression of LePLDβ1 on elicitation with xylanase. The Plant Journal. 2001;26:237–247. doi: 10.1046/j.1365-313x.2001.01023.x. [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Munnik T. Phospholipid signalling in plant defense. Current Opinion in Plant Biology. 2002;5:332–338. doi: 10.1016/s1369-5266(02)00268-6. [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. Calcium in plant defence-signalling pathways. New Phytologist. 2006;171:249–269. doi: 10.1111/j.1469-8137.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Li RG, Rimmer R, Buchwalolt L, Sharpe AG, Seguin-Swartz G, Hegedus DD. Interaction of Sclerotinia sclerotiorum with Brassica napus: cloning and characterization of endo- and exo-polygalacturonase expressed during saprophytic and parasitic mode. Fungal Genetics and Biology. 2004;41:754–765. doi: 10.1016/j.fgb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Liu CQ, Du DZ, Huang YJ, Wang CH. Study on tolerance to Sclerotinia sclerotiorum and the hereditary properties in B. napus. Agricultural Sciences in China. 1991;24:43–49. (in Chinese with English abstracts) [Google Scholar]
- Lomasney JW, Cheng HF, Roffler SR, King K. Activation of phospholipase Cδ1 through C2 domain by a Ca2+-enzyme-phosphatidylserine ternary complex. Journal of Biological Chemistry. 1999;274:21995–22001. doi: 10.1074/jbc.274.31.21995. [DOI] [PubMed] [Google Scholar]
- Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structure and functional diversity. Protein Science. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouelhadj A, Kusch KP, Humbeck K. Heavy metal stress and leaf senescence induce the barley gene HvC2d 1 encoding a calcium-dependent novel C2 domain-like protein. New Phytologist. 2006;170:261–273. doi: 10.1111/j.1469-8137.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- Perruc E, Charpenteau M, Ramirez BC, Jauneau A, Galaud JP, Ranjeva R, Ranty B. A novel calmodulin-binding protein functions as a negative regulator of osmotic stress tolerance in Arabidopsis thaliana seedlings. The Plant Journal. 2004;38:410–420. doi: 10.1111/j.1365-313X.2004.02062.x. [DOI] [PubMed] [Google Scholar]
- Pepio AM, Sossin WS. Membrane translocation of novel protein kinase Cs is regulated by phosphorylation of the C2 domain. Journal of Biological Chemistry. 2001;276:3846–3855. doi: 10.1074/jbc.M006339200. [DOI] [PubMed] [Google Scholar]
- Poinssot B, Vandelle E, Bentéjac M, Adrian M, Levis C, Brygoo Y, Garin J, Sicilia F, Coutos-Thévenot P, Pugin A. The endopolygalacturonase 1 from Botrytis cinerea activates grapevine defense reactions unrelated to its enzymatic activity. Molecular Plant–Microbe Interaction. 2003;16:553–564. doi: 10.1094/MPMI.2003.16.6.553. [DOI] [PubMed] [Google Scholar]
- Reddy ASN. Calcium: silver bullet in signaling. Plant Science. 2001;160:381–404. doi: 10.1016/s0168-9452(00)00386-1. [DOI] [PubMed] [Google Scholar]
- Rizo J, Südhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. Journal of Biological Chemistry. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- Shieh MT, Brown RL, Whitehead MP, Cary JW, Cotty PJ, Cleveland TE, Dean RA. Molecular genetic evidence for the involvement of a specific polygalacturonase, P2c, in the invasion and spread of Aspergillus flavus in cotton bolls. Applied and Environmental Microbiology. 1997;63:3548–3552. doi: 10.1128/aem.63.9.3548-3552.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlen RV, Cho W. Roles of calcium in the membrane binding of C2 domains. Biochemical Journal. 2001;359:679–685. doi: 10.1042/0264-6021:3590679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruel MN, Meyer T. Parallel single-cell monitoring of receptor-triggered membrane translocation of a calcium-sensing protein module. Science. 2002;295:1910–1912. doi: 10.1126/science.1065028. [DOI] [PubMed] [Google Scholar]
- van der Hoorn RAL, Kamoun S. From guard to decoy: a new model for perception of plant pathogen effectors. The Plant Cell. 2008;20:2009–2017. doi: 10.1105/tpc.108.060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schutze K, et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. The Plant Journal. 2004;40:428–438. doi: 10.1111/j.1365-313X.2004.02219.x. [DOI] [PubMed] [Google Scholar]
- Wang XY, Yang ED, Qi CK, Chen S, Zhang JF, Yang Q. Construction of cDNA expression library of oilseed rape and identification of the interaction partner of PG, a virulence factor from Sclerotinia sclerotiorum. Acta Agronomica Sinica. 2008;34:192–197. [Google Scholar]
- Xoconostle-Cazares B, Xiang Y, Ruiz-Medrano R, Wang HL, Monzer J, Yoo BC, McFarland KC, Franceschi VR, Lucas WJ. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science. 1999;283:94–98. doi: 10.1126/science.283.5398.94. [DOI] [PubMed] [Google Scholar]
- Yang HY, Li YQ, Hua J. The C2 domain protein BAP 1 negatively regulates defense response in Arabidopsis. The Plant Journal. 2006;48:238–248. doi: 10.1111/j.1365-313X.2006.02869.x. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Yang SH, Li YQ, Hua J. The Arabidopsis BAP1 and BAP2 genes are general inhibitors of programmed cell death. Plant Physiology. 2007;145:135–146. doi: 10.1104/pp.107.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SA, Wang X, Leach JE. Changes in the plasma membrane distribution of rice phospholipase D during resistant interactions with Xanthomonas oryzae pv. oryzae. The Plant Cell. 1996;8:1079–1090. doi: 10.1105/tpc.8.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuppini A, Navazio L, Sella L, Castiglioni C, Favaron F, Marianni P. An endopolygalacturonase from Selerotinia sclerotiorum induces calcium-mediated signaling and programmed cell death in soybean cells. Molecular Plant–Microbe Interaction. 2005;18:849–855. doi: 10.1094/MPMI-18-0849. [DOI] [PubMed] [Google Scholar]