Abstract
Pectin methylesterases (PMEs) catalyse the demethylation of pectin within plant cell walls, releasing methanol (MeOH) in the process. Thus far, PMEs have been found to be involved in diverse processes such as plant growth and development and defence responses against pathogens. Herbivore attack increases PME expression and activity and MeOH emissions in several plant species. To gain further insights into the role of PMEs in defence responses against herbivores, the expression of a Manduca sexta oral secretion (OS)-inducible PME in Nicotiana attenuata (NaPME1) was silenced by RNA interference (RNAi)-mediated gene silencing. Silenced lines (ir-pme) showed 50% reduced PME activity in leaves and 70% reduced MeOH emissions after OS elicitation compared with the wild type (WT), demonstrating that the herbivore-induced MeOH emissions originate from the demethylation of pectin by PME. In the initial phase of the OS-induced jasmonic acid (JA) burst (first 30 min), ir-pme lines produced WT levels of this hormone and of jasmonyl-isoleucine (JA-Ile); however, these levels were significantly reduced in the later phase (60–120 min) of the burst. Similarly, suppressed levels of the salicylic acid (SA) burst induced by OS elicitation were observed in ir-pme lines even though wounded ir-pme leaves contained slightly increased amounts of SA. This genotype also presented reduced levels of OS-induced trypsin proteinase inhibitor activity in leaves and consistently increased M. sexta larvae performance compared with WT plants. These latter responses could not be recovered by application of exogenous MeOH. Together, these results indicated that PME contributes, probably indirectly by affecting cell wall properties, to the induction of anti-herbivore responses.
Keywords: Defence, herbivory, jasmonic acid, Manduca, methanol, Nicotiana, pectin methylesterase, proteinase inhibitor
Introduction
Pectin methylesterases (PMEs, EC 3.1.1.11) are important cell wall enzymes involved in modelling the pectin matrix. Pectin is a major compound of plant cell walls and accounts for about one-third of all the macromolecules in dicotyledonous species. Different types of polysaccharides that are rich in galacturonic acid form the backbone of pectin: homoglacturonan (HG), xylogalacturonan (XG), rhamnogalacturonan I (RGI), and rhamnogalacturonan II (RGII) (Willats et al., 2001; Vorwerk et al., 2004). HG is a linear polymer of 1,4-linked α-D-galacturonic acid that is synthesized in the Golgi apparatus and deposited in the cell wall, with most (70–80%) of its galacturonic acid units methylated at the C-6 carboxyl group (O'Neill et al., 1990). PMEs catalyse the C-6 demethylation of HG within plant cell walls. In this process, methanol (MeOH) is released and negatively charged carboxyl groups are created which can associate with other HG chains by calcium cross-links, making the cell wall more rigid. However, PME activity can also contribute to cell wall loosening and degradation by promoting the activity of cell wall hydrolases such as endopolygalacturonases (Micheli, 2001; Willats et al., 2001). Thus, PMEs can regulate the texture and mechanical properties of plant cell walls in opposite ways.
PMEs belong to large gene families, for example, in Arabidopsis thaliana, 66 PME genes have been predicted (Pelloux et al., 2007). PMEs are involved in diverse processes associated with growth and development, such as cell wall remodelling, root tip elongation, seed germination, fruit ripening, and pollen tube growth (reviewed in Pelloux et al., 2007). Moreover, PMEs play a role in plant defence against many pathogenic bacteria and fungi. For example, in Solanum tuberosum, differences in susceptibility to Erwinia carotovora correlate with the degree of methylation (DM) of pectin, with resistant genotypes having a higher DM of pectin than the susceptible genotypes (Marty et al., 1997). Likewise, A. thaliana plants with reduced PME activity have a higher DM of pectin and are more resistant to fungal infection by Botrytis cinerea (Lionetti et al., 2007). PMEs are also involved in different processes during viral infection. Nicotiana tabacum PMEs promote the movement of tobacco mosaic virus by binding to the virus-encoded movement proteins necessary for the dispersal of the virus (Dorokhov et al., 1999; Chen et al., 2000; Chen and Citovsky, 2003).
There are indications that PMEs may also play a role in defence against herbivores. In several plant species, PME mRNA and protein levels are up-regulated after herbivore attack (Divol et al., 2005; Schmidt et al., 2005; Giri et al., 2006; von Dahl et al., 2006). In addition, emissions of MeOH are increased (Peñuelas et al., 2005; von Dahl et al., 2006) and demethylation of pectin by PMEs was suggested to be the source of the emitted MeOH during herbivory (von Dahl et al., 2006). Based on these observations, it was postulated that PME may participate in the induction of defence responses during insect herbivory either by generating MeOH as a signal molecule or via changes in the structural properties of the cell wall. To test this hypothesis, a herbivore-inducible PME gene (NaPME1) was silenced in N. attenuata plants and diverse plant responses to insect herbivores were studied.
Materials and methods
Plant growth and generation of ir-pme lines
Seeds of an inbred line of N. attenuata Torr. ex. Wats. and of the transformed ir-pme lines were germinated on Petri dishes containing Gamborg's B5 medium as described by Krügel et al. (2002). Plates were incubated in a growth chamber (Percival, Perry, IA, USA) at 26 °C/16 h light (155 μmol s−1 m−2)–24 °C/8 h dark. After 10 d, seedlings were planted into Teku pots (Waalwijk, The Netherlands) and transferred into 1.0 l pots after an additional period of 10 d. Plants were grown in the greenhouse under high-pressure sodium lamps (800–1000 μmol s−1 m−2) with a day/night ratio of 16 h (26–28 °C)/8 h (22–24 °C) and 45–55% humidity. Experiments were conducted when plants were at the rosette stage, shortly before stem elongation.
Transgenic N. attenuata (ir-pme) lines silenced in the expression of PME were constructed using the vector pRESC5PME1 (Supplementary Fig. S1A available at JXB online). This vector contains two 431 bp fragments of NaPME1 (accession no. DQ115979; von Dahl et al., 2006) which were PCR amplified using the primer pairs PME3-31 (5′-GCGGCGGAGCTCGGTTCGCAAAGCTTTGCTG-3′) and PME4-31 (5′-GCGGCGCTCGAGCTTTCACATTCCTACTTCC-3′), and PME5-19 (5′-GGTTCGCAAAGCTTTGCTG-3′) and PME6-31 (5′-GCGGCGCCATGGCTTTCACATTCCTACTTCC-3′). This 431 bp fragment corresponded to the pectinesterase superfamily domain in plant PMEs. Transgenic plants were generated via Agrobacterium-mediated transformation as described by Krügel et al. (2002). Transformants were selected on Gamborg's B5 plates supplemented with hygromycin (0.025 mg ml−1).
Elicitation treatments
To mimic herbivore feeding, the youngest or second youngest fully expanded rosette leaves were wounded with a fabric pattern wheel, causing four rows of punctured wounds on each side of the midrib. The wounds were immediately treated with 20 μl of water (w+w) or 20 μl of Manduca sexta oral secretions (OS) diluted 1:1 (v/v) with water (w+OS). Control plants remained untreated. Manduca sexta OS were collected from larvae reared on N. attenuata wild-type (WT) plants. Depending on the experiment, leaves were harvested at different time points after the treatments and immediately frozen in liquid nitrogen. Leaf samples were stored at –80 °C until further processing.
The average amount of MeOH emitted after M. sexta OS elicitation from a WT leaf was 0.58 μl min−1 and the treatment leads to an immediate increase of MeOH emissions. Hence, ∼8.7 μl of MeOH are released per leaf within the first 15 min after elicitation, when MeOH emissions are known to be at their maximum (von Dahl et al., 2006). Therefore, the amount of methanol (9 μl) applied to the wounds of one leaf (w+OS+MeOH) represents realistic exposure.
For the induction of defence compounds in systemic leaves, one freshly hatched M. sexta larva was placed on the youngest, fully expanded rosette leaf, and clip cages were attached to restrict larval movement. After 5 d of feeding, the youngest, fully expanded leaf adjacent to the attacked leaf was harvested and analysed.
Southern and northern blot analysis
Genomic DNA of the WT and ir-pme lines was isolated by the cetyltrimethylammonium bromide method (CTAB) as described in Rogers and Bendich (1994). DNA samples (10 μg) were digested with different enzymes (see below) overnight and separated on a 0.8% (w/v) agarose gel using standard conditions. DNA was blotted onto Gene Screen Plus Hybridisation Transfer membranes (Perkin Elmer Life and Analytical Sciences, Boston, MA, USA) using the capillary transfer method. RNA was extracted using the Trizol reagent (Sigma, Taufkirchen, Germany) according to the manufacturer's instructions. RNA samples (15 μg) were denatured in loading buffer at 68 °C for 10 min, separated on a 1.2% (w/v) formaldehyde–agarose gel, and blotted onto nylon membranes following standard conditions. Equal loading was controlled by staining with ethidium bromide.
For Southern blot analysis, gene-specific probes for NaPME1 and the hygromycin resistance gene hptII were generated by PCR using the primer pairs PME3-31 (5′-GCGGCGGAGCTCGGTTCGCAAAGCTTTGCTG-3′) and PME4-31 (5′-GCGGCGCTCGAGCTTTCACATTCCTACTTCC-3′), and HYG1-18 (5′-CCGGATCGGACGATTGCG-3′) and HYG3-20 (5′-CGTCTGTCGAGAAGTTTCTG-3′), respectively. To determine the number of T-DNA insertions, genomic DNA from ir-pme lines was digested with EcoRV and hybridized with the hptII probe. In addition, genomic DNA from WT plants was digested with DraI, SspI, HindIII, and EcoRI, and hybridized with the PME1 probe to determine the copy number of PME1 in the N. attenuata genome.
For northern blot analysis, the probe to detect PME expression was generated using the primer pair PME1_F (5′-GGGCCTCAGTTGCACTTGTAAT-3′) and PME1_R (5′-GGCAATGCTGCTGCTGTTTTAC-3′). DNA probes were labelled with [α-32P]dCTP (Perkin Elmer Life and Analytical Sciences) using a random prime labelling kit (Rediprime II, Amersham Pharmacia) according to the manufacturer's instructions.
PME and trypsin proteinase inhibitor (TPI) activity assays
PME activity was measured using a gel diffusion assay as described in Downie et al. (1998) and modified by Bourgault and Bewley (2002). TPI activity was measured using a gel diffusion assay as described in van Dam et al.(2001).
Methanol analysis
MeOH emissions of single leaves were measured with a proton transfer reaction mass spectrometer (PTR-MS; IONICON GmbH, Innsbruck, Austria) as described in von Dahl et al. (2006). The youngest rosette leaf was cut off at the petiole and inserted into a glass flow chamber. MeOH emissions were measured for 5–10 min to determine control emissions. Leaves were then wounded and MeOH release recorded for 10 min. Afterwards wounds were treated with 1:1 diluted M. sexta OS, and MeOH emissions were measured for an additional 10 min. The amount of MeOH released was calculated from the integrated areas of the two measurement periods after wounding and after application of OS. The calibration curve was obtained by repeated injections of 0.25, 0.35, 0.5, and 1 μl of MeOH into the flow chamber. MeOH emissions are expressed as μmol MeOH min−1 cm−2 leaf area. Leaf area was determined using Sigma Scan Pro 5 (SPSS) on scans of leaves.
Protein kinase activity assay
Protein kinase activity assays were performed as described by Zhang and Klessig (1997) and modified by Wu et al. (2007).
Phytohormone and nicotine analysis
For jasmonic acid (JA), jasmonyl-isoleucine (JA-Ile), and salicylic acid (SA) quantification, 200 mg of leaf material was extracted with 1 ml of ethylacetate containing 200 ng of [13C2]JA, [D4]SA, and [13C6]JA-Ile as internal standards. After centrifugation at 13 000 rpm for 20 min at 4 °C, the supernatant was transferred into a fresh tube and the sample was re-extracted with 1 ml of ethylacetate. The combined supernatants were dried and samples dissolved in 0.5 ml of 70% (v/v) MeOH. A 0.2 ml aliquot of the supernatant was transferred into glass vials and analysed by HPLC-(ESI)-MS/MS as described in Wang et al. (2007).
To quantify ethylene emissions, the second youngest, fully expanded rosette leaf was wounded and treated with 1:1 diluted M. sexta OS. After the treatment, the leaf was cut off at the petiole and inserted into a 250 ml glass vessel. Ethylene was allowed to accumulate for 5 h. The headspace of the vessel was flushed into a laser photo-acoustic spectrometer (INVIVO, Adelzhausen, Germany) and ethylene concentration measured as described in von Dahl et al. (2007). Ethylene emissions are expressed as nl h−1 g−1 fresh mass. Nicotine content of leaves was determined as described in Keinänen et al. (2001).
Manduca sexta performance assays
One M. sexta neonate was put on each plant and caterpillars were weighed after 3, 5, 7, 9, and 11 d with a microbalance. In a second experiment, caterpillars were kept in small plastic containers and were fed with leaf material from plants that had been sprayed twice a day with a 5% (v/v) MeOH/H2O solution. Spraying started 4 d before the start of the experiment and was continued until the end of the experiment. Each spraying delivered 0.1 ml of MeOH, the amount released in 6 h by an attacked N. attenuata leaf (von Dahl et al., 2006). Control plants were sprayed with water. Leaf material without midribs was excised from each plant from each treatment group, weighed, and placed into small plastic containers (10.5×8×4 cm). One neonate was put into each container, which was then closed with a perforated lid. All boxes contained a moist filter paper and were placed in an incubator and maintained at 75% humidity at 26 °C with a 16:8 h light:dark cycle. Leaf material was first replaced after 3 d and then changed every 2 d. At the end of day 9, total dry mass of larvae and leaves was recorded after drying at 65 °C for 3 d. The efficiency of conversion of ingested food (ECI) [(larval mass gain)/(leaf mass consumed)] was calculated according to Waldbauer (1968). Total protein content in leaves was quantified according to Bradford (1976).
Statistics
Data were analysed with the software StatView (Abacus Concepts, Inc., Berkeley, CA, USA). Analysis of variance (ANOVA) with Bonferroni corrected post hoc test or t-tests was used. To ensure homogeneity of variances, data were log transformed if necessary. Multiple plants were used in single experiments, and genotype and treatment were included as factors in the ANOVA.
Results
Generation of transgenic plants silenced in NaPME1
A putative PME gene from N. attenuata (NaPME1) previously described (von Dahl et al., 2006) was identified based on its strong up-regulation at the mRNA level after M. sexta attack and M. sexta OS elicitation (Schmidt et al., 2005; von Dahl et al., 2006). The NaPME1 mRNA starts to accumulate immediately after M. sexta OS elicitation and remains at higher than control levels for at least 24 h (von Dahl et al., 2006). To study the function of this gene in anti-insect responses in N. attenuata, a 431 bp fragment of the NaPME1 cDNA was cloned in an inverted orientation and used to generate stable transformed lines (ir-pme) by inverted repeat gene silencing (see Materials and methods). Two homozygous independently transformed ir-pme lines (434-7 and 457-8) were selected and used for all experiments. To determine the number of T-DNA insertions, genomic DNA from ir-pme lines was digested with EcoRV and hybridized with a probe specific for the hygromycin phosphotransferase gene hptII. Line 457-8 contained a single T-DNA insertion and, in line 434-7, a left border over-read occurred, producing a single band with the same size (2.4 kb) as the pRESC5PME1 plasmid DNA fragment (Supplementary Fig. S1B at JXB online). After EcoRI digestion and hybridization with a probe specific for NaPME1, line 434-7 presented three bands: one corresponding to the endogenous gene and two to the transgene, suggesting that the T-DNA had been inserted twice (in a tandem array) at a single site in the plant genome (Supplementary Fig. S1C). Southern blot analysis was also used to determine the copy number of PME1. The blot revealed that PME1 is a single copy gene in the genome of N. attenuata (Supplementary Fig. S1C).
The morphology and development of ir-pme lines were similar to those of the WT at all stages of the plant's life cycle (Supplementary Fig. S2 at JXB online, and data not shown).
PME activity and MeOH emissions are reduced in ir-pme lines
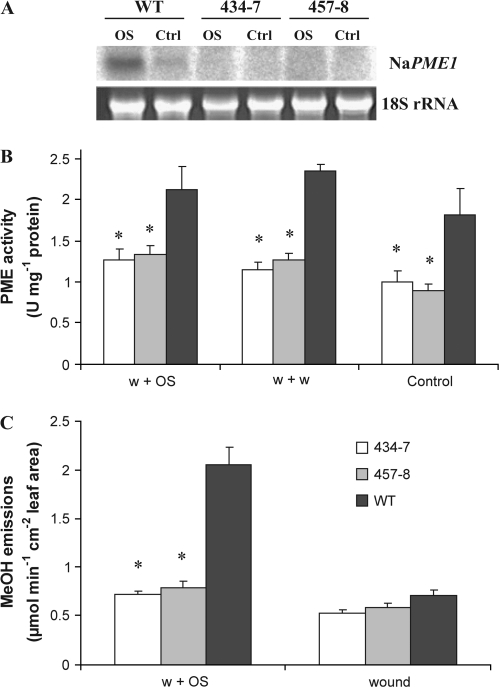
It has been previously shown that N. attenuata plants emit large amounts of MeOH when attacked by M. sexta larvae and it has been proposed that the source of this MeOH is the result of PME activation (von Dahl et al., 2006). Thus, the PME1 transcript levels, PME activity, and the release of MeOH in ir-pme lines were determined after they had been elicited with M. sexta OS. Control plants were left untreated. Transcript levels were measured in leaves of WT and ir-pme lines using northern blot analysis (Fig. 1A). In WT plants, NaPME1 transcripts were detectable in both control and treated leaves. As expected, OS treatment increased PME1 transcript levels several fold after 6 h. In contrast, in leaves of ir-pme lines, no NaPME1 transcripts were detectable in either control or treated plants (Fig. 1A).
Fig. 1.
PME1 transcript levels, PME activity, and MeOH emissions are reduced in ir-pme lines. (A) Northern blot analysis of NaPME1 mRNA in WT plants and ir-pme lines (434-7, 457-8) 6 h after wounding+OS treatment (OS) and in control leaves (Ctrl). (B) Mean (±SE) PME activity in N. attenuata leaves of WT plants and ir-pme lines 4 h after elicitation. Leaves were wounded and the wounds treated with either water (w+w) or 1:1 diluted M. sexta oral secretions (w+OS). Control plants remained untreated (n=4–5). (C) Mean (±SE) methanol emissions of single N. attenuata leaves of WT plants and ir-pme lines after wounding alone (wound) or subsequent application of 1:1 diluted M. sexta OS (w+OS) (n=3–5). Asterisks represent significant differences from the WT (ANOVA).
Secondly, PME activity was quantified in control, wounded, and OS-elicited WT and ir-pme lines. PME activity was significantly reduced by 40–50% in ir-pme lines compared with WT plants (Fig. 1B, two-way ANOVA, F2,35=26.34, P <0.001) in non-induced plants, 4 h after wounding and 4 h after OS elicitation. The two wound treatments slightly increased PME activity in all genotypes compared with non-induced plants (Fig. 1B). PME activity was also quantified at 40 min after the treatments and differences between genotypes similar to those observed at 4 h were detected (data not shown).
Finally, MeOH emissions of single leaves from WT and ir-pme lines were quantified using PTR-MS. MeOH emissions were recorded both after wounding and after applying OS to the wounds. As expected, treating the leaves of WT plants with OS caused a higher release of MeOH than wounding alone (Fig. 1C, paired t-test, P <0.001). In contrast, no MeOH burst occurred in ir-pme lines after either wounding or application of OS. On average, WT plants emitted three times more MeOH than ir-pme lines (Fig. 1C, ANOVA, F2,8=51.49, P <0.001).
Silencing of NaPME1 affects phytohormone dynamics after OS elicitation
To evaluate the influence of NaPME1 on direct defences against herbivores, local induction of different defence and defence-related responses (early and late signalling events) was compared in leaves of WT and ir-pme lines.
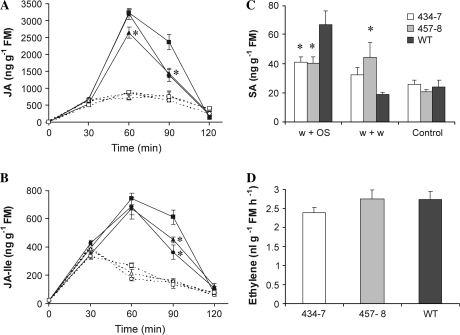
Manduca sexta larvae attack or OS elicitation causes a transient increase in JA and the amino acid conjugate JA-Ile which is larger than the increase caused by wounding alone (Schittko et al., 2000; Kang et al., 2006). The levels of these phytohormones were quantified in control and treated leaves (30, 60, 90, and 120 min after wounding or OS elicitation). In line 457-8, JA accumulated at significantly reduced levels (Fig. 2A, ANOVA, F2,11=5.49, P=0.02) after 30 min of induction, and in line 434-7 JA accumulated at significantly reduced levels after 60 min compared with the WT. At 90 min after OS treatment, leaves of both ir-pme lines contained 40% less JA than WT leaves (Fig. 2A, ANOVA, F2,11=8.31, P <0.01). A similar profile was observed for JA-Ile accumulation, with lower levels at 90 min in both ir-pme lines compared with WT (Fig. 2B, ANOVA, F2,11=8.27, P <0.01). In contrast, the accumulation of both JA and JA-Ile did not differ between WT and ir-pme lines after wounding alone (Fig. 2A, B, two-way ANOVA: JA, F2,59=0.29, P=0.75; JA-Ile, F2,59=0.34, P=0.72).
Fig. 2.
Silencing NaPME1 affects phytohormone dynamics after OS elicitation. Mean (±SE) JA (A) and JA-Ile (B) levels in N. attenuata leaves of WT plants (squares) and ir-pme lines 434-7 (circles) and 457-8 (triangles) 30, 60, 90, and 120 min after elicitation. Leaves were wounded and the wounds treated with either water (w+w, dashed lines) or 1:1 diluted M. sexta oral secretions (w+OS, solid lines). Control plants remained untreated (t=0 min) (n=4–5). (C) Mean (±SE) SA levels of WT plants and ir-pme lines 120 min after elicitation (n=5). (D) Mean (±SE) ethylene emissions of single leaves after wounding and application of OS (n=5). Asterisks represent significant differences from the WT (ANOVA).
Additional phytohormones such as ethylene (ET) and SA are known to tune defence responses against herbivores. Therefore, the levels of SA were quantified in leaves 30, 60, 90, and 120 min after wounding and OS elicitation, and in leaves of untreated plants. SA content in leaves harvested 30, 60, and 90 min after wounding and application of either water or OS did not differ from constitutive levels in WT and ir-pme lines (data not shown). However, 120 min after elicitation, SA levels varied among genotypes and treatments. Levels of SA were higher in wounded ir-pme lines compared with the WT (Fig. 2C, ANOVA, F2,12=4.84, P=0.03) and OS elicitation caused an increase in SA levels in WT plants but not in ir-pme lines (ANOVA, F2,12=5.81, P=0.02). In the latter, SA levels remained similar to the levels in wounded leaves.
ET emissions were also measured in WT and ir-pme lines 5 h after OS treatment using a photo-acoustic spectrometer. No differences were observed between genotypes (Fig. 2D, ANOVA, F2,12=1.03, P=0.39).
One of the earliest responses to herbivore attack in N. attenuata is the activation of mitogen-activated protein kinases (MAPK) such as SA-induced protein kinase (SIPK) (Wu et al., 2007). To test SIPK activity, leaves were harvested 10 min after OS elicitation corresponding to the peak of SIPK activity in N. attenuata (Wu et al., 2007). SIPK activity increased dramatically after OS treatment in all genotypes; however, no differences between WT and ir-pme lines were detected (Supplementary Fig. S3 at JXB online).
TPI activity is reduced in ir-pme lines after OS induction
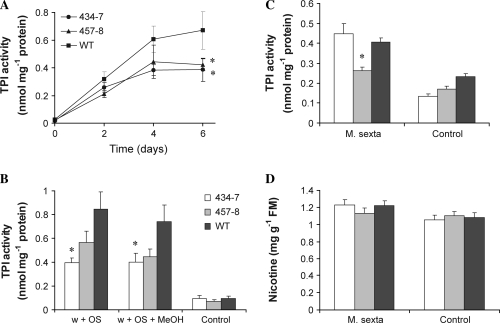
The increase in JA and JA-related compounds after herbivore attack or OS elicitation leads to the production of defence compounds such as TPIs within a few days (van Dam et al., 2001; Halitschke and Baldwin, 2005). TPI activity was monitored over several days after OS elicitation and in untreated leaves (day 0, Fig. 3A). TPI levels in ir-pme lines were reduced at 2 d and 4 d, and amounted only to 60% of WT levels after 6 d (Fig. 3A, two-way ANOVA, F2,48=5.96, P <0.01).
Fig. 3.
TPI activity is reduced in ir-pme lines after OS elicitation. (A) Mean (±SE) TPI activity in N. attenuata leaves of WT plants (squares) and ir-pme lines 434-7 (circles) and 457-8 (triangles) 2, 4, and 6 d after elicitation. Leaves were wounded and the wounds treated with 1:1 diluted M. sexta oral secretions. Control plants remained untreated (t=0 d) (n=5). (B) Mean (±SE) TPI activity in leaves of WT plants and ir-pme lines 4 d after the treatment. Leaves were wounded and the wounds treated with M. sexta OS diluted 1:1 in either water (w+OS) or methanol (w+OS+MeOH). Control plants remained untreated (n=9–10). Mean (±SE) TPI activity (C) and nicotine levels (D) in systemic leaves 5 d after M. sexta larva feeding on an adjacent leaf (M. sexta). Control plants were not attacked (n=19–20). Asterisks represent significant differences from the WT (ANOVA).
As described above, M. sexta attack or M. sexta OS elicitation immediately increases MeOH emissions in WT plants but not in ir-pme lines. To test whether the observed differences in the induction of TPI in ir-pme lines were caused by the reduced MeOH production in this genotype, TPI activity was quantified in OS-elicited leaves of WT and ir-pme lines supplemented with exogenous MeOH. The amount of MeOH added to the wounds represented the amount of MeOH released by a single WT leaf after OS treatment within the first 15 min (see Materials and methods). MeOH emissions are known to be highest during this interval (von Dahl et al., 2006). Therefore, the conditions used for these experiments reflect physiological MeOH amounts generated by the plant. MeOH treatment did not restore TPI activity to WT levels in ir-pme lines (Fig. 3B, two-way ANOVA, F1,53=2.24, P=0.14).
To test whether PME is involved in systemic signalling of defence responses, TPI activity and levels of nicotine were also measured in systemic leaves of WT and ir-pme lines. Leaves were harvested 5 d after a M. sexta larva had started feeding on the adjacent leaf. TPI activity was slightly reduced in systemic leaves of line 457-8 but not in line 434-7 compared with the WT (Fig. 3C, ANOVA, F2,55=10.02, P <0.001). Nicotine levels of systemic leaves did not differ between lines after M. sexta attack (Fig. 3D, ANOVA, F2,56=0.88, P=0.42).
Silencing of NaPME1 enhances M. sexta performance
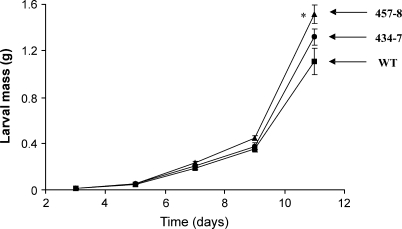
To test whether the observed differences in the production of jasmonates and SA and in the induction of TPI activity affect the performance of M. sexta larva, caterpillar performance bioassays were conducted in the glasshouse. Neonates were put on either WT plants or ir-pme lines and their weight was recorded every 2–3 d for a total of 11 d. Manduca sexta larvae feeding on ir-pme lines gained more body mass after 11 d than those feeding on WT plants (Fig. 4, ANOVA, F2,23=5.23, P=0.01).
Fig. 4.
Silencing of NaPME1 enhances M. sexta performance. Mean (±SE) larval mass of M. sexta larvae feeding on either the WT (squares) or ir-pme lines 434-7 (circles) and 457-8 (triangles) (n=8–9). Asterisks represent significant differences from the WT (ANOVA).
A second experiment to assess caterpillar performance was done with M. sexta larvae and leaf material in closed cages (Waldbauer, 1968). To test the effect of MeOH on M. sexta performance, plants were sprayed twice a day with either water or a 5% MeOH solution delivering 100 μl of MeOH at each spraying, the amount released in 6 h by an attacked plant (von Dahl et al., 2006). Supplementing ir-pme lines with exogenous MeOH did not affect larval growth or ECI compared with water-treated ir-pme lines (Supplementary Fig. S4 at JXB online). However, larvae feeding on ir-pme lines were more efficient in utilizing food than those feeding on the WT (Supplementary Fig. S4B, two-way ANOVA, F2,69=4.18, P=0.02). Total protein content of leaves, which influences caterpillar performance, did not differ between WT and ir-pme lines (data not shown).
Discussion
The observation that M. sexta OS induces the transcript levels of NaPME1 raised the question of whether this enzyme is involved in anti-herbivore defence responses, via either MeOH production, modification of the food ingested by the caterpillar, and/or changes in the cell wall structure and phytohormone production.
Plants release a considerable amount of MeOH into the atmosphere which is mainly produced during cell wall modification by the demethylation of pectin catalysed by PMEs (Fall and Benson, 1996; Seco et al., 2007). Interestingly, herbivore attack causes an increase in MeOH emissions when compared with non-induced plants (Peñuelas et al., 2005; von Dahl et al., 2006). For example, N. attenuata increases MeOH emissions when attacked by M. sexta larvae or elicited with M. sexta OS. It was previously postulated that this enhanced MeOH emission originates from the demethylation of pectin by the action of PMEs (von Dahl et al., 2006). By silencing NaPME1, an OS-inducible PME in N. attenuata, it is here demonstrated that this enzyme participates in the enhanced production of MeOH after M. sexta OS elicitation. Because NaPME1 expression was silenced using a cDNA fragment corresponding to the conserved pectinesterase domain, the possibility could not be ruled out that other members of the NaPME family also contribute to this reaction and accounted therefore for a fraction of the reduced MeOH emitted in ir-pme plants after elicitation and the ∼50% decrease in PME activity in the same genotype. It has been postulated that induced MeOH production during insect herbivory may mediate defence responses and interactions between plants and insects (Peñuelas et al., 2005; von Dahl et al., 2006). Moreover, studies performed with A. thaliana showed that MeOH affects the expression of hundreds of genes including stress- and defence-related genes (Downie et al., 2004). Thus, the generation of transgenic plants partially impaired in MeOH emission provides a very good system to test the above-mentioned hypotheses. Transgenic ir-pme lines showed no alterations in growth or morphology compared with the WT both in the greenhouse and in field trials in their native habitat in Utah, suggesting that NaPME1 is not involved in growth or developmental processes and may be specific for defence.
When analysed by a gel diffusion assay, total PME activity in leaves of WT plants was not enhanced by OS elicitation compared with wounding (after 40 min and 4 h), whereas MeOH emission increased several fold within 10 min. These results suggested that PME activity was rapidly and differentially affected by OS treatment and that this control occurred at the post-translational level. The total PME activity quantified after protein extraction in a gel diffusion assay may not reflect this rapid and probably transient activation of PME but rather the total activity induced at later time points by the transcriptional activation of NaPME1 and perhaps other PME family members.
In ir-pme lines, TPI activity in M. sexta OS-induced leaves was reduced by ∼40% compared with the WT after 6 d of the treatment. Supplying the wounds of ir-pme leaves with MeOH in quantities that mimicked the MeOH released from an attacked WT leaf did not restore TPI activity to WT levels. These results suggested that MeOH as a signal molecule does not directly participate in the induction of TPI in M. sexta OS-elicited leaves. It is possible, however, that supplementation of the wounds with exogenous MeOH does not efficiently mimic the release of MeOH by PME activity in attacked leaves which occurs in small quantities for several hours.
In addition to functioning in within-plant signalling, MeOH could also mediate interactions between plants and insects (Peñuelas et al., 2005; von Dahl et al., 2006). Other volatile organic compounds (VOCs) released by plants after herbivore attack such as green leaf volatiles (GLVs) and terpenoid volatiles have been extensively studied and play important roles in plant–herbivore interactions (Kessler and Baldwin, 2001; Halitschke et al., 2004). Whether MeOH could act in a similar manner needs further investigation. Little is known about the perception of MeOH by insects in this context, but it has been shown, for example, that bark beetles are attracted by alcohols such as ethanol (EtOH) and MeOH, which are used in host location (Byers, 1992). Manduca sexta larvae feeding on ir-pme lines gained more body mass than those feeding on WT plants. Supplementing ir-pme lines with exogenous MeOH did not reduce larval growth compared with untreated ir-pme lines, suggesting that the better performance of the M. sexta larvae on this genotype is independent of MeOH signalling. Changes in the kinetics of accumulation of jasmonates and SA but not ET were observed after M. sexta OS induction in ir-pme lines compared with the WT. Both JA and JA-Ile levels were significantly reduced in the late phase of the jasmonate burst after elicitation but not before. Manduca sexta OS-induced SA levels were significantly reduced by 40% in both ir-pme lines compared with WT SA levels 120 min after the treatment. Interestingly, wounded ir-pme leaves contained 2-fold higher levels of SA than WT leaves. In A. thaliana, perturbation of the cell wall structure or homeostasis by mutations in cellulose synthase genes leads to deregulation of JA biosynthesis and thereby defence responses (Ellis et al., 2002; Ko et al., 2006). These previous studies established a strong link between cell wall structure/homeostasis and JA biosynthesis and stress responses. In Arabidopsis and poplar (Populus trichocarpa), wounding and JA also up-regulate the transcript levels of several PME genes (Cheong et al., 2002; Pelloux et al., 2007), and induced changes in the physical properties of plant cell walls may affect elicitor perception or early signalling events in the plant's defence responses (Vorwerk et al., 2004; Pelloux et al., 2007). The actual mechanisms underlying the changes in phytohormone dynamics in ir-pme lines remain unknown at present.
Lower JA and JA-Ile levels could partially contribute to the mechanisms underlying the reduced levels of inducible TPI activity in this genotype, but additional mechanisms may also operate. These mechanisms may be diverse. For example, PME can contribute to the generation of oligogalacturonides (OGAs) by promoting the activity of endopolygalacturonases, which degrade pectin. These small pectin fragments are known to elicit defence responses in plants against pathogens (Cote and Hahn, 1994; Shibuya and Minami, 2001) and are involved in the induction of proteinase inhibitors in tomato (Bishop et al., 1981; Doares et al., 1995). Moreover, in the wild strawberry Fragaria vesca the ability of OGAs to induce plant defences depends on their DM, which is reduced by PME activity (Osorio et al., 2008). Therefore, reduced PME activity in ir-pme lines could affect the structure of OGAs, making them less efficient in mediating defence responses. A second possible scenario could be related to the production of free negatively charged carboxyl groups by induced PME activity. It is known that cations such as Ca2+ bind to these free groups, and silencing the expression of PME in Solanum tuberosum changes the accumulation and partitioning of Ca2+. Cell walls of these plants bind less Ca2+ than WT cell walls and the total content of Ca2+ is reduced in leaves of these transgenic plants (Pilling et al., 2004). Extracellular Ca2+ influx into the cytosol is generally observed as one of the earliest responses upon herbivore attack (Maffei et al., 2004) and therefore it is plausible that changes in cell wall-associated Ca2+ pools in ir-pme lines could diminish the induction of defence responses in N. attenuata. Additionally, PMEs may also play a role in the metabolism of the larva's ingested food, thereby negatively influencing its performance. This is supported by the observation that M. sexta larva's feeding on ir-pme lines were more efficient in utilizing food than those feeding on the WT. Finally, changes in the structure of pectin in the cell wall of ir-pme plants should also be considered as one factor potentially influencing the efficiency of food utilization by M. sexta caterpillars. Future investigations will focus on the elucidation of the actual mechanisms affected in ir-pme lines and leading to reduced responses to insect herbivores.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Plant transformation vector pRESC5PME1 and Southern blot analysis of ir-pme lines and the WT.
Fig. S2. Growth parameters of WT and ir-pme lines.
Fig. S3. In-gel MAPK activity assay of WT and ir-pme lines.
Fig. S4. Nutritional indices of M. sexta larvae feeding on WT and ir-pme lines.
Acknowledgments
We thank BESSY (Berliner Elektronenspeicheringgesellschaft für Synchrotronstrahlung) for use of their facilities during the PTR-MS measurements, and Dr K Gase, E Rothe, and Dr M Schöttner for helpful technical advice.
Glossary
Abbreviations
- JA
jasmonic acid
- JA-Ile
jasmonyl-isoleucine
- MeOH
methanol
- OS
M. sexta oral secretions
- PME
pectin methylesterase
- SA
salicylic acid
- TPI
trypsin proteinase inhibitor
References
- Bishop PD, Makus DJ, Pearce G, Ryan CA. Proteinase inhibitor-inducing factor activity in tomato leaves resides in oligosaccharides enzymically released from cell-walls. Proceedings of the National Academy of Sciences, USA. 1981;78:3536–3540. doi: 10.1073/pnas.78.6.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgault R, Bewley JD. Gel diffusion assays for endo-beta-mannanase and pectin methylesterase can underestimate enzyme activity due to proteolytic degradation: a remedy. Analytical Biochemistry. 2002;300:87–93. doi: 10.1006/abio.2001.5450. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byers JA. Attraction of bark beetles, Tomicus piniperda, Hylurgops palliatus, and Trypodendron domesticum and other insects to short-chain alcohols and monoterpenes. Journal of Chemical Ecology. 1992;18:2385–2402. doi: 10.1007/BF00984957. [DOI] [PubMed] [Google Scholar]
- Chen MH, Sheng JS, Hind G, Handa AK, Citovsky V. Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO Journal. 2000;19:913–920. doi: 10.1093/emboj/19.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Citovsky V. Systemic movement of a tobamovirus requires host cell pectin methylesterase. The Plant Journal. 2003;35:386–392. doi: 10.1046/j.1365-313x.2003.01818.x. [DOI] [PubMed] [Google Scholar]
- Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiology. 2002;129:661–677. doi: 10.1104/pp.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Hahn MG. Oligosaccharins—structures and signal transduction. Plant Molecular Biology. 1994;26:1379–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- Divol F, Vilaine F, Thibivilliers S, Amselem J, Palauqui JC, Kusiak C, Dinant S. Systemic response to aphid infestation by Myzus persicae in the phloem of Apium graveolens. Plant Molecular Biology. 2005;57:517–540. doi: 10.1007/s11103-005-0338-z. [DOI] [PubMed] [Google Scholar]
- Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proceedings of the National Academy of Sciences, USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorokhov YL, Makinen K, Frolova OY, Merits A, Saarinen J, Kalkkinen N, Atabekov JG, Saarma M. A novel function for a ubiquitous plant enzyme pectin methylesterase: the host-cell receptor for the tobacco mosaic virus movement protein. FEBS Letters. 1999;461:223–228. doi: 10.1016/s0014-5793(99)01447-7. [DOI] [PubMed] [Google Scholar]
- Downie B, Dirk LMA, Hadfield KA, Wilkins TA, Bennett AB, Bradford KJ. A gel diffusion assay for quantification of pectin methylesterase activity. Analytical Biochemistry. 1998;264:149–157. doi: 10.1006/abio.1998.2847. [DOI] [PubMed] [Google Scholar]
- Downie A, Miyazaki S, Bohnert H, John P, Coleman J, Parry M, Haslam R. Expression profiling of the response of Arabidopsis thaliana to methanol stimulation. Phytochemistry. 2004;65:2305–2316. doi: 10.1016/j.phytochem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. The Plant Cell. 2002;14:1557–1566. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R, Benson AA. Leaf methanol—the simplest natural product from plants. Trends in Plant Science. 1996;1:296–301. [Google Scholar]
- Giri AP, Wünsche H, Mitra S, Zavala JA, Muck A, Svatos A, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant's proteome. Plant Physiology. 2006;142:1621–1641. doi: 10.1104/pp.106.088781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Baldwin IT. Jasmonates and related compounds in plant–insect interactions. Journal of Plant Growth Regulation. 2005;23:238–245. [Google Scholar]
- Halitschke R, Ziegler J, Keinänen M, Baldwin IT. Silencing of hydroperoxide lyase and allene oxide synthase reveals substrate and defense signaling crosstalk in Nicotiana attenuata. The Plant Journal. 2004;40:35–46. doi: 10.1111/j.1365-313X.2004.02185.x. [DOI] [PubMed] [Google Scholar]
- Kang JH, Wang L, Giri A, Baldwin IT. Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell. 2006;18:3303–3320. doi: 10.1105/tpc.106.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen M, Oldham NJ, Baldwin IT. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. Journal of Agricultural and Food Chemistry. 2001;49:3553–3558. doi: 10.1021/jf010200+. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Ko JH, Kim JH, Jayanty SS, Howe GA, Han KH. Loss of function of COBRA, a determinant of oriented cell expansion, invokes cellular defence responses in Arabidopsis thaliana. Journal of Experimental Botany. 2006;57:2923–2936. doi: 10.1093/jxb/erl052. [DOI] [PubMed] [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12:177–183. [Google Scholar]
- Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiology. 2007;143:1871–1880. doi: 10.1104/pp.106.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiology. 2004;134:1752–1762. doi: 10.1104/pp.103.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty P, Jouan B, Bertheau Y, Vian B, Goldberg R. Charge density in stem cell walls of Solanum tuberosum genotypes and susceptibility to blackleg. Phytochemistry. 1997;44:1435–1441. [Google Scholar]
- Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends in Plant Science. 2001;6:414–419. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Albersheim P, Darvill A. The pectic polysaccharides of primary cell walls. In: Dey PM, editor. Methods in Plant Biochemistry, Vol. 2. London: Academic Press; 1990. pp. 415–441. [Google Scholar]
- Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca) The Plant Journal. 2008;54:43–55. doi: 10.1111/j.1365-313X.2007.03398.x. [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rusterucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends in Plant Science. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Filella I, Stefanescu C, Llusia J. Caterpillars of Euphydryas aurinia (Lepidoptera: Nymphalidae) feeding on Succisa pratensis leaves induce large foliar emissions of methanol. New Phytologist. 2005;167:851–857. doi: 10.1111/j.1469-8137.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- Pilling J, Willmitzer L, Bucking H, Fisahn J. Inhibition of a ubiquitously expressed pectin methyl esterase in Solanum tuberosum L. affects plant growth, leaf growth polarity, and ion partitioning. Planta. 2004;219:32–40. doi: 10.1007/s00425-004-1204-y. [DOI] [PubMed] [Google Scholar]
- Schittko U, Preston CA, Baldwin IT. Eating the evidence? Manduca sexta larvae can not disrupt specific jasmonate induction in Nicotiana attenuata by rapid consumption. Planta. 2000;210:343–346. doi: 10.1007/PL00008143. [DOI] [PubMed] [Google Scholar]
- Schmidt DD, Voelckel C, Hartl M, Schmidt S, Baldwin IT. Specificity in ecological interactions. Attack from the same lepidopteran herbivore results in species-specific transcriptional responses in two solanaceous host plants. Plant Physiology. 2005;138:1763–1773. doi: 10.1104/pp.105.061192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seco R, Penuelas J, Filella I. Short-chain oxygenated VOCs: emission and uptake by plants and atmospheric sources, sinks, and concentrations. Atmospheric Environment. 2007;41:2477–2499. [Google Scholar]
- Shibuya N, Minami E. Oligosaccharide signalling for defence responses in plant. Physiological and Molecular Plant Pathology. 2001;59:223–233. [Google Scholar]
- van Dam NM, Horn M, Mares M, Baldwin IT. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. Journal of Chemical Ecology. 2001;27:547–568. doi: 10.1023/a:1010341022761. [DOI] [PubMed] [Google Scholar]
- von Dahl CC, Havecker M, Schlögl R, Baldwin IT. Caterpillar-elicited methanol emission: a new signal in plant–herbivore interactions? The Plant Journal. 2006;46:948–960. doi: 10.1111/j.1365-313X.2006.02760.x. [DOI] [PubMed] [Google Scholar]
- von Dahl CC, Winz RA, Halitschke R, Kühnemann F, Gase K, Baldwin IT. Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. The Plant Journal. 2007;51:293–307. doi: 10.1111/j.1365-313X.2007.03142.x. [DOI] [PubMed] [Google Scholar]
- Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends in Plant Science. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Waldbauer GP. The consumption and utilization of food by insects. Advanced Insect Physiology. 1968;5:229–288. [Google Scholar]
- Wang L, Halitschke R, Kang JH, Berg A, Harnisch F, Baldwin IT. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta. 2007;226:159–167. doi: 10.1007/s00425-007-0477-3. [DOI] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology. 2001;47:9–27. [PubMed] [Google Scholar]
- Wu JQ, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. The Plant Cell. 2007;19:1096–1122. doi: 10.1105/tpc.106.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SQ, Klessig DF. Salicylic acid activates a 48-kD MAP kinase in tobacco. The Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.